Adsorption of trichlorphon on phyllosilicate minerals:Effect of low-molecular-weight organic acids
Hongfeng CHEN ,Zhouyang HE ,Mingxia HOU ,Cilai TANG,3 and Yonghong WU
1College of Hydraulic&Environmental Engineering,China Three Gorges University,Yichang 443002(China)
2Hubei Field Observation and Scientific Research Stations for Water Ecosystem in Three Gorges Reservoir,China Three Gorges University,Yichang 443002(China)
3Engineering Research Center of Eco-Environment in Three Gorges Reservoir Region,China Three Gorges University,Yichang 443002(China)
4State Key Laboratory of Soi and Sustainable Agriculture,Institute of Soil Sciences,Chinese Academy of Sciences,Nanjing 210008(China)
ABSTRACT The use of trichlorphon in large quantities causes a large number of organic pollutants to enter water,sediments,and soils.Phyllosilicate minerals are considered effective adsorbents for organic pollutants.However,the adsorption behavior of organic pollutants on soil minerals affected by low-molecular-weight organic acids(LMWOAs)is not fully understood.In this study,the effect of LMWOAs on the adsorption behavior of trichlorphon on phyllosilicate minerals was investigated using a combination of adsorption measurements and molecular spectroscopic techniques(attenuated total reflection-Fourier transform infrared spectroscopy(ATR-FTIR)and X-ray photoelectron spectroscopy(XPS)).The adsorption of trichlorphon onto kaolinite(KAO)and montmorillonite(MON)was suppressed by increasing pH,indicating that electrostatic interaction played a key role in adsorption,especially at low pH.In the presence of citric acid(CA),there was an obvious promotion of trichlorphon adsorption on KAO and MON.In the presence of oxalic acid(OA),the adsorption of trichlorphon on KAO was promoted,whereas the adsorption on MON was inhibited,especially at pH 4.0.The presence of CA and OA increased the adsorption by increasing the exposure of hydrophobic sites of KAO and MON.The results from ATR-FTIR and XPS also indicated that the hydrophobic Si-O sites of phyllosilicate minerals were the preferred adsorption sites for trichlorphon in the presence of CA and OA,probably driven by the hydrophobic effect.However,the weak effect of OA on MON caused an increase in the electrostatic repulsion between MON and trichlorphon molecules,thus inhibiting adsorption.This study is significant for a deeper understanding of self-purification of soil and sediment systems in the presence of organic pollutants.
Key Words: adsorption behavior,citric acid,hydrophobic effect,organic pesticide,organic pollutant,organochlorine,oxalic acid
INTRODUCTION
To improve agricultural products,organic pesticides are widely used to control diseases and pests (Lópezet al.,2011;Yadavet al.,2015).More than 70%of these pesticides are present in soil or water system (Yadavet al.,2015;Kalathooret al.,2021)with only 30%of pesticides being effectively used.Due to the complex chemical structures and bonds,most pesticides such as organochlorine and organophosphorus compounds are refractory(Swackhamer and Hites,1988;Samuelet al.,2010).Bioaccumulation of these pesticides occurs easily in animal fatty tissues,breast milk,and even human blood through the food chain(Ntowet al.,2008).According to World Health Organization and United Nations Environment Programme,approximately 3 million people are at risk of organic pesticide poisoning every year worldwide,with approximately 200 thousand people dying due to organic pesticide poisoning.More than 95%of these cases are from developing nations(FAO/WHO,2014).
Trichlorphon(dimethyl-2,2,2-trichlorphonchloro-l-hydroxyethyl phosphonate),an insecticide applied through contact and ingestion,has been recommended for forest and agricultural use against fruit flies,lepidopterous and coleopterous larvae,household pests,and ectoparasites in domestic animals(Antón and Ariz,1994).Trichlorphon is widely employed as insecticide and disinfectant in water production and forests(Karami-Mohajeriet al.,2014)owing to its high efficiency,low toxicity,and low residue production.However,under alkaline conditions,it easily decomposes into dichlorvos,reinforcing the toxicity by 10 times(Wenet al.,2012).Trichlorphon is also responsible for inducing an imbalance in cellular calcium homeostasis,excitatory response of the intracellular plasmic reticulum,and apoptosis(Liuet al.,2009).Moreover,the use of trichlorphon in large quantity threatens the environment due to enhanced risk of non-point source pollution,causing a large number of organic pollutants to enter water,sediments,and soils.Owing to its leaching potential and moderate water solubility in soils,trichlorphon is frequently detected in groundwater,water (Samuelet al.,2010),and sediments (Wanget al.,2020).The toxicity of organic pollutants is often reduced to a certain extent because of the binding of organic pollutants in sediments and soils with active components,such as minerals,organic matter,anions,and cationic forming organic-inorganic complexes(Martinset al.,2018;Chenget al.,2021).
Soil minerals,owing to their large charges and active sites on their surfaces,have extensive significance in the fate of organic pollutants(Lee and Hur,2020;Chenget al.,2021).Therefore,phyllosilicate minerals are considered effective adsorbents for organic pollutants.The contribution of soil mineral components towards the adsorption and/or fixation of organic pesticides has received wide attention.Yuanet al.(2014)demonstrated that soil minerals,especially phyllosilicate and Fe/Al/Mn oxides,are important sorbents for organic pollutants.Renet al.(2018)studied the adsorption behavior of persistent organic pollutants on soil components.Martinset al.(2018)reported the key roles played by organic carbon(C) and quartz content of soil samples in the sorption of atrazine on soil.Xuet al.(2020)concluded that electrostatic interaction,cation exchange,hydrophobic effect/hydrogen bonding,and ligand exchange are primarily responsible for the adsorption of organic pollutants on soil.
Organic acids widely exist in soil and soil solution,such as carboxylic acids (e.g.,oxalic,citric,and malic acids),substituted benzoic and cinnamic acids,etc.(Strobel,2001;Huanget al.,2020).Low-molecular-weight organic acids(LMWOAs),distinguished by the presence of-COOH functional group,such as citric acid(CA)and oxalic acid(OA)are the most abundant organic acids in soil and soil solution(Huanget al.,2016;Lazoet al.,2017).Owing to microbial processes and root exudates,the concentration of LMWOAs can be much higher than 1 mmol(Parsons,1983).Moreover,the interaction of LMWOAs with soil mineral surface has great impact on the bioavailability and mobility of nutrients and contaminants.The carbonyl and hydroxyl functional groups in LMWOA structures promote the formation of complexes with ligands in soil(Konget al.,2014).Heet al.(2014)demonstrated that organic matter directly or indirectly influences the physical conformation of soil minerals,which in turn affects the adsorption of butachlor on minerals.Furthermore,because of the decreased partitioning of organic pollutants on minerals as a consequence of occupying the adsorption sites of minerals by organic matter,the adsorption of organic pollutants is indirectly reduced(Charleset al.,2006;Awadet al.,2019).The adsorption of enzyme on goethite,kaolinite,and two colloids from soil decreased with increase in LMWOA concentration in the following sequence:tartrate>oxalate>acetate(Huanget al.,2003).Zhenget al.(2019)found that LMWOA aging slightly increases chlorpyrifos sorption onto biochar and that the sorption first increases and then decreases with increasing CA concentration.Therefore,the presence of organic acids,especially LMWOAs,can enhance the competition between anions and organic ligands for adsorption sites on soil minerals.
Despite numerous reports on the sorption of organic pollutants,the adsorption behavior of organic pollutants on soil minerals affected by LMWOAs is not yet fully understood.Therefore,our aim was to further explore the effects of OA and CA on the adsorption behaviors and mechanisms of trichlorphon on phyllosilicate minerals in soil.Two typical and common phyllosilicate minerals with distinct structural differences,kaolinite(KAO)and montmorillonite(MON),were selected as adsorbents.
MATERIALS AND METHODS
Trichlorphon and minerals
All reagents used in this study were of analytical grade.All solutions were prepared using ultrapure water with a resistivity of 18.45 MΩ cm,which was obtained from an ultrapure water system(SYZ-10 L).
Trichlorphon (>99% purity) used in this study was purchased from Aladdin Reagent Company (China) and stored at 4°C.The octanol-water partition coefficient of trichlorphon was 0.43 and its solubility in water was 120 g L-1.Figure 1 shows the structure of trichlorphon.In aqueous solution,trichlorphon can dissociate protons and become slightly negatively charged.
The samples of KAO and MON were purchased from Sigma-Aldrich(Germany).The fractions of KAO and MON(<2 μm)were separated by wet sedimentation,as described in our previous study (Chenet al.,2017).Wet sediments were dried,ground,and stored in a desiccator.The specific surface areas of KAO and MON were 9.09 and 85.21 m2g-1,respectively,as obtained using the BET (Brunauer-Emmet-Teller)-N2method.The distributions of KAO and MON are different in different soil types.For example,KAO is mainly distributed in tropical and subthermal soils in southern China,while MON is mainly found in the northern soil of China,which is located in temperate,humid,and subhumid regions(Huanget al.,2000).Kaolinite(1:1)has one tetrahedral silica sheet(T-plane)fused to one octahedral alumina sheet(O-plane),which leads to Si-O and Al-O sites on the T-plane and Al-O sites on the O-plane.In comparison,MON(2:1)has one O-plane and two T-planes,which leads to only Si-O sites on the T-planes and Al-O sites on the O-plane(van Olphen,1963;Tombácz and Szekeres,2006).For both KAO and MON,due to isomorphic substitution and their hydrophobic nature,the Si-O sites are permanently negatively charged,while the Al-O sites are amphoteric and hydrophilic.Thus,these sites are conditionally charged and pH-dependent (Tombácz and Szekeres,2006;Gupta and Miller,2010;Wanget al.,2013;Chenet al.,2017).Other properties,including elemental analysis(Table SI),X-ray diffraction patterns (Fig.S1),and surface charge analysis of KAO and MON(Fig.S2)can be found in detail in the Supplementary Material.
Adsorption isotherms
Batch equilibration studies were performed to measure the adsorption isotherms in the presence/absence of LMWOAs at a given pH.Two LMWOAs,OA and CA,were used in this study and their structures are shown in Fig.1.To prepare suspensions and ensure the complete dispersion of phyllosilicate mineral sites,phyllosilicate minerals(10.0 g L-1)were continuous stirred in 0.01 mol L-1KCl for 48 h at the desired pH values.Before batch adsorption,the suspensions were purged for 30 min with N2gas to remove CO2.Each batch sample was prepared by transferring 1)an aliquot of the stock KAO or MON suspension,2) the background KCl solution(0.01 mol L-1),3)an amount of the stock OA or CA solution,and 4)an amount of the stock trichlorphon solution to a 50-mL polypropylene tube.The pH of the suspensions was adjusted to 4.0 and 6.0 using either 0.05 mol L-1HCl or KOH.The final concentration of KAO or MON in the tubes was maintained at 5.0 g L-1,whereas that of trichlorphon ranged from 0 to 45 mg L-1,and the total volume was maintained at 20 mL.In the presence of LOWAs,the final concentration of OA or CA was maintained at 10 mg L-1.The suspensions were equilibrated for 24 h at 25°C using an end-over-end rotation.The pH of the suspensions was checked 2-3 times and readjusted to ensure the desired values.Each suspension was maintained under N2atmosphere for 10 min during pH adjustment.After equilibration,each suspension was centrifuged at 8 000×gfor 15 min at 4°C and the supernatant was filtered through a 0.45-μm nylon membrane filter.

Fig.1 Structure of trichlorphon(Tri),oxalic acid(OA),and citric acid(CA).
The concentration of trichlorphon in the filtrate was analyzed using a UV-Vis spectrometer(UV-1900PC,AOELAB,China).Trichlorphon can interact with benzidine monohydrochloride to form a yellow complex,which can be detected by UV-Vis spectroscopy.The procedure can be described as follows:1)5 mL filtrate was placed in a 50-mL polypropylene cenfuge tube,2)2 mL benzidine monohydrochloride solution(1 mol L-1)was added to the centrifuge tube,3)2 mL sodium perborate solution(1 mol L-1)was then added to the tube,4)the mixture was shaken quickly for 20 min in a 70°C water bath,and 5) after heating,the solution was allowed to cool to 25°C for 30 min,after which the absorbance of the mixture was measured at 610 nm by a UV-1900PC spectrometer.
Attenuated total reflection-Fourier transform infrared spectroscopy
A Fourier transform infrared (FTIR) spectrometer(PerkinElmer,UK)using an attenuated total reflection(ATR)accessory equipped with a ZnSe ATR crystal was used to measure all the ATR-FTIR spectra at 25°C.The ATR-FTIR samples were KCl(0.01 mol L-1),KAO,MON,and KAO-/MON-trichlorphon complexes,which were prepared at pH 4.0,6.0,and 8.0 similar to the batch adsorption isotherms.The concentration of trichlorphon in the complexes was 45 mg g-1,which matched the maximum adsorption at the given pH.The suspensions were centrifuged at 12 000 r min-1for 15 min and the obtained precipitates were washed twice with 0.01 mol L-1KCl having the same pH as the suspension and stored at 4°C for ATR-FTIR analysis.The sample spectrum was measured after collecting the background spectrum(0.01 mol L-1KCl).Sample measurements were performed with small solid taken from the precipitate.At a randomly selected point of precipitation,a fixed amount of precipitate(0.05 g)was removed with a small medicine spoon and mounted on a round ZnSe crystal to collect the spectrum.This process was repeated three times for each sample and the samples with equal weight were used.The ATR crystal was carefully cleaned with ethanol to remove any residue from the previous sample prior to spectrum collection.The spectra were collected at 4 cm-1with 256 scans in the range of 700-4 000 cm-1.The spectra were measured in duplicate or triplicate to verify the reproducibility of the results.
The sample spectra were normalized,as described by Chenet al.(2017).The spectra of KAO and KAO-trichlorphon complex,as well as MON and MON-trichlorphon complex,were normalized using the band areas at 3 620 and 912 cm-1,respectively.
X-ray photoelectron spectroscopy
X-ray photoelectron spectroscopy (XPS) was used to study the composition of KAO,MON,and KAO-/MONtrichlorphon complexes.The XPS samples were prepared in the same manner as the ATR-FTIR samples.After centrifugation and washing,the precipitates were dried and ground through a 100-mesh sieve for XPS measurements.The loadings of trichlorphon fractions on phyllosilicate minerals were calculated based on the adsorption capacity obtained from the isotherm curves.The XPS spectra were collected using an AXIS Suprea X-ray photoelectron spectrometer(Japan),with the details of collecting and correcting the XPS spectra reported earlier(Chenet al.,2020).The C spectra were fitted using Thermo Avantage software(UK)with the Gaussian-Lorentzian mixed function(Gaussian/Lorentzian=20) after subtracting a Shirley baseline.To ensure the reliability of spectra,all the spectra were fitted with the least number of components.
RESULTS AND DISCUSSION
Trichlorphon adsorption in the absence of LMWOAs
The adsorption isotherms of trichlorphon on KAO and MON in the absence of LMWOAs at 0.01 mol L-1KCl and pH 4.0 and 6.0 are depicted in Fig.2a,d.With increasing concentration of added trichlorphon,there was a rapid increase in adsorption,which then levelled offwith further increase.Increasing pH resulted in the suppression of adsorption for phyllosilicate minerals,which could be ascribed to the decreased positive charges on the surface of phyllosilicate minerals(Tombácz and Szekeres,2006)and increased electrostatic repulsion between phyllosilicate minerals and dissolved trichlorphon molecules at high pH(Vermeeret al.,1998;Duet al.,2021).For both phyllosilicate minerals,the adsorption capacity at pH 4.0 was approximately 3 times that at pH 6.0.This indicates the significance of suspension pH on the adsorption of trichlorphon for both phyllosilicate minerals.Kaolinite showed a slightly stronger adsorption capacity of trichlorphon(0.85 mg g-1)compared to MON(0.78 mg g-1);however,at pH 6.0,both minerals showed similar adsorption capacities of approximately 0.29 mg g-1.Suspension pH had a similar effect on the adsorption of KAO and MON.Slightly steeper slopes of the isotherms at pH 4.0 and 6.0 for KAO in comparison to that of MON suggested that the affinity constant of trichlorphon was slightly larger for KAO than for MON.
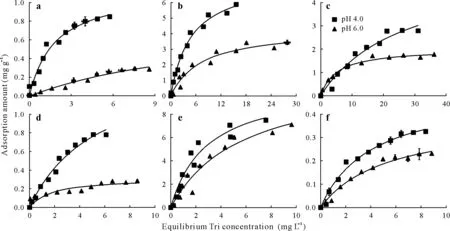
Fig.2 Adsorption isotherms(fitted by Langmuir equation)of trichlorphon(Tri)on kaolinite(a-c)and montmorillonite(d-f)at 0.01 mol L-1 KCl and pH 4.0 and 6.0 in the absence(a and d)and presence of low-molecular-weight organic acids,oxalic acid(b and e)and citric acid(c and f).Vertical bars indicate standard deviations of the means(n=2).
The adsorption data were fitted using the Langmuir equation:
whereq(mg g-1)is the amount of trichlorphon adsorbed per unit mass,qm(mg g-1) is the maximum amount of trichlorphon that can be adsorbed,KL(L mg-1) is the affinity constant of the adsorption between trichlorphon and phyllosilicate minerals,andC(mg L-1)is the equilibrium concentration of trichlorphon(Vermeeret al.,1998).The obtained values of the parameters are listed in Table I.The Langmuir model provided a good fit (R2>0.90) to the adsorption data.For a given pH,theKLvalues followed the order of KAO>MON,which indicates that KAO has a stronger adsorption affinity for trichlorphon than MON.A lower pH is more conducive for the adsorption of trichlorphon on mineral surfaces.
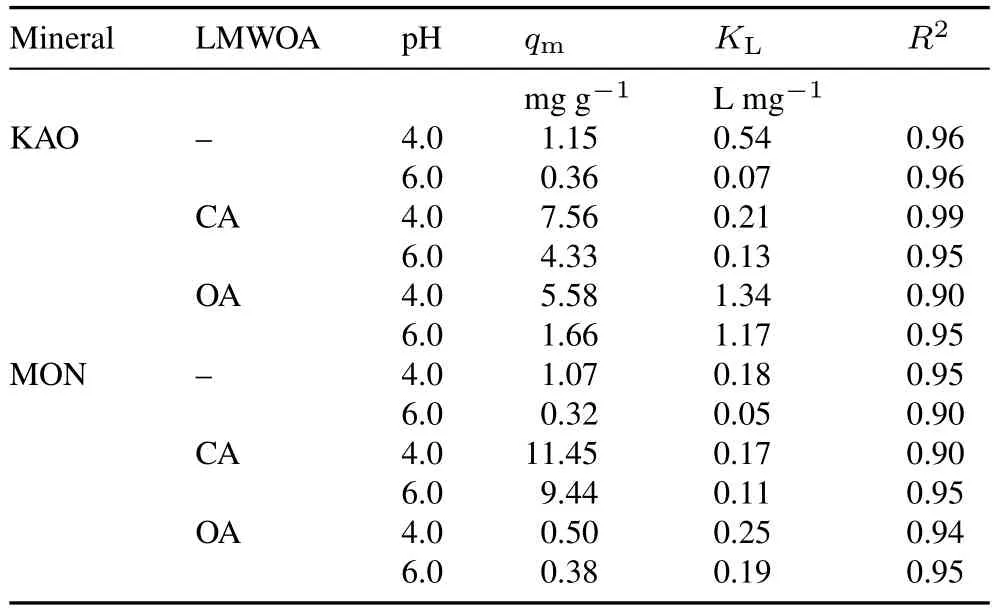
TABLE IParametersa)of Langmuir equation used to describe trichlorphon adsorption on kaolinite(KAO)and montmorillonite(MON)in the absence(-)and presence of low-molecular-weight organic acids(LMWOAs),citric acid(CA)and oxalic acid(OA),at two pH values
Trichlorphon adsorption in the presence of LMWOAs
Adsorption of trichlorphon on KAO and MON in the presence of LMWOAs at two different pH values and an ionic strength of 0.01 mol L-1KCl is depicted in Fig.2b,c,e,f.Similar to that observed in the absence of LMWOAs,increased pH resulted in decreased adsorption of trichlorphon by both phyllosilicate minerals in the presence of LMWOAs.The presence of CA was found to substantially increase trichlorphon adsorption(Fig.2b,e)for both phyllosilicate minerals.In particular,the promotion was more obvious at a higher pH in the presence of MON,which was proved by theqmvalues(Table I).The values of the fitting parameterKLat pH 6.0 were larger in the presence of CA than in its absence,which may be due to the reduced stability of phyllosilicate mineral-trichlorphon complexes in the presence of CA.Furthermore,the adsorption in the presence of CA was less pH-dependent than that in the absence of CA.This is generally attributed to the adsorption of polar organic ligands on mineral surfacesviainteractions with surface hydroxyl species(Ali and Dzombak,1996;Boilyet al.,2000;Angoveet al.,2002).The enhanced adsorption capacity due to the presence of CA may be,at least in part,due to the increased number of hydroxylated sites on the surface of phyllosilicate minerals.This means that the presence of CA enhances the uptake of trichlorphon by increasing positive charges and active sites of phyllosilicate minerals.Higher adsorption of trichlorphon by MON-CA system compared to that by KAO-CA system is simply due to the fact that MON has more hydroxylated sites(Tombácz and Szekeres,2006).
The presence of OA also enhances the adsorption of trichlorphon by KAO.The obtained adsorption capacity for KAO in the presence of OA at pH 4.0 and 6.0 were 5.58 and 1.66 mg g-1(Table I),respectively,which were about 5 times larger than that in the absence of OA.HigherKLvalues were obtained in the presence of OA.Furthermore,the adsorption profile,especially at a low equilibrium concentration of trichlorphon,showed near complete adsorption.The pH dependency of trichlorphon adsorption was less significant in the presence of OA than in the absence of OA.However,for MON,trichlorphon adsorption was inhibited in the presence of OA.The adsorption capacity at pH 4.0 obtained from the fitted line was 0.50 mg g-1,which was less than that in the absence of OA(1.07 mg g-1).At pH 6.0,trichlorphon adsorption was nearly identical in the absence and presence of OA.
Surface charges of minerals are affected by the presence of several LMWOAs.At low LMWOA concentrations(<1.0 mmol L-1),the negative surface charges of mineral particles increase owing to the adsorption of organic anions(Xuet al.,2004).Citric acid has a stronger effect than OA(Xuet al.,2003,2004).Moreover,at the same pH,KAO had more surface positive charges than MON(Fig.S2).Thus,the increased negative surface charges due to the LMWOAs followed the orders of phyllosilicate mineral-CA system>phyllosilicate mineral-OA system and KAO-LMWOA system>MON-LMWOA system.This resulted in weakening of the electrostatic attraction between trichlorphon and two phyllosilicate minerals.It implies that electrostatic attraction is not the main driving force for trichlorphon adsorption,which may be the reason for the decreased adsorption of trichlorphons on the surface of MON in the presence of OA.Despite this,the phyllosilicate mineral-CA system still showed the strongest capacity.The significant trichlorphon adsorption on phyllosilicate minerals observed even in the presence of strong electrostatic repulsion has often been considered as indirect evidence of hydrophobic interactions(Schlautman and Morgan,1993).It is plausible for trichlorphon because of the hydrophobic nature of trichloride and phosphonate groups(Dkmenet al.,2020).The Si-O sites on the T-plane of KAO and MON are generally considered to be hydrophobic in nature;however,isomorphic substitution increases the negative charge density,resulting in decreased hydrophobicity(Kampeerapappunet al.,2007).The hydrophobic sites on phyllosilicate minerals might be increased owing to the effect of carboxyl groups of CA and OA,which are considered hydrophilic(Bistriet al.,2007).Owing to the stronger effect of CA than that of OA(Xuet al.,2003,2004),more hydrophobic sites on phyllosilicate minerals were explored,thus enhancing trichlorphon adsorption in the presence of CA.Kaolinite has only one partially hydrophobic T-plane,and the basal O-plane is gibbsite-like with Al-O groups and is highly hydrophilic (Gupta and Miller,2010).Based on this,hydrophobic interaction can at least partly explain the higher adsorption of trichlorphon in the MON-CA system than in the KAO-CA system at pH 4.0 and 6.0;hydrophobic interaction is important for trichlorphon adsorption on Si-O sites.
Analysis of ATR-FTIR spectra
The ATR-FTIR spectra of KAO and KAO-trichlorphon complex in the presence of LMWOAs are shown in Fig.3.Owing to the low trichlorphon amounts present,the adsorption bands of trichlorphon were not detected.Therefore,the spectra of clay-trichlorphon complex only showed the bands corresponding to KAO.The characteristic frequencies and chemical identification to identify the absorption bands have been reported previously (Madejová and Komadel,2001;Chenet al.,2017).The changes in the FTIR adsorption peaks of KAO in the presence of trichlorphon were mainly observed in the wavenumber range of 750 to 1 200 cm-1and from 3 570 to 3 780 cm-1(Fig.3).The decrease in peak areas can be attributed to the adsorption of trichlorphon(Chenet al.,2017).Distinctly decreased peak areas were observed at 1 005,1 030,1 099,and 1 114 cm-1for KAO-trichlorphon complex at pH 4.0 and 6.0.These bands are ascribed to Si-O stretching.It has been proven that trichlorphon is preferentially adsorbed onto the hydrophobic sites of KAO through the hydrophobic effect.In the presence of LMWOAs,the decreased peaks at 911 and 940 cm-1indicated that trichlorphon was also adsorbed to the inner-surface hydroxyl groups of KAO.These results suggested that the presence of LMWOAs enhanced trichlorphon adsorption on the hydrophilic hydroxyl groups of KAO,which enhances trichlorphon adsorption on the surface of KAO.
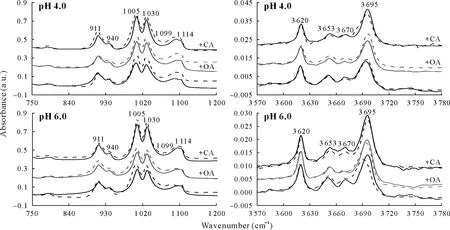
Fig.3 Attenuated total reflection-Fourier transform infrared spectroscopy spectra of kaolinite(KAO)and KAO-trichlorphon complex in the absence and presence(+)of low-molecular-weight organic acids,oxalic acid(OA)and citric acid(CA),at pH 4.0 and 6.0 in the wavenumber ranges of 750-1 200 and 3 570-3 780 cm-1.Solid curves represent KAO-trichlorphon complex,and dotted curves represent KAO.a.u.=absorbance unit.
The FTIR adsorption peaks of MON were measured in the wavenumber range of 700-1 200 cm-1(Fig.4).An obvious decrease in peak area was observed at 986 cm-1for MON-trichlorphon complex and the complexes in the presence of OA and CA at pH 4.0 and 6.0.This band was assigned to Si-O stretching.The absence or presence of fewer hydroxyl groups was due to the presence of less Al in the octahedral positions of MON.In the presence of LMWOAs,the band at 986 cm-1distinctly shifted to a lower wavenumber,especially at pH 6.0.This band was the coincident peak of two Si-O stretches located at 1 023 and 1 024 cm-1(Madejová,2003;Chenet al.,2017).The shift in the peak position resulted from the change in the peak areas of the two Si-O stretches.In summary,the absence or presence of fewer hydrophilic hydroxyl groups made trichlorphon adsorption on these sites impossible,especially in the presence of OA.

Fig.4 Attenuated total reflection-Fourier transform infrared spectroscopy spectra of montmorillonite (MON) and MON-trichlorphon complex in the absence and presence(+)of low-molecular-weight organic acids,oxalic acid(OA)and citric acid(CA),at pH 4.0 and 6.0 in the wavenumber range of 700 to 1 200 cm-1.Solid curves represent MON-trichlorphon complex,and dotted curves represent MON.a.u.=absorbance unit.
Structural profiles of adsorbed phyllosilicate mineral-trichlorphon complexes
The characteristic C 1s high-resolution XPS spectra obtained for KAO and MON along with their trichlorphon complexes in the presence and absence of LMWOAs at pH 4.0 and 0.01 mol L-1KCl are shown in Figs.5 and 6.The C 1s spectra of KAO(Fig.5a)and MON(Fig.6a)showed a peak at 284.8 eV,which was used as an internal reference to correct other peaks.The binding energy of 284.8 eV corresponded to aliphatic C atoms(C-C and/or C-H groups)(Singhet al.,2014).The second peak at approximately 286.2 eV was attributed to C in C-O single bonds.The third peak at approximately 288.7 eV corresponded to C in O-C=O groups(Leiet al.,2014).All the above peaks were also found to be present in the samples of KAO-and MON-trichlorphon complexes(Figs.5 and 6).However,a new peak observed at approximately 284.1 eV could be attributed to C in the trichlorphon molecule,which was probably bonded to chlorine(Dorenet al.,1994).This indicated the surface binding of trichlorphon to KAO and MON.Furthermore,in the presence of LMWOAs,the spectra of KAO-trichlorphon(in the prssence of CA)and MON-trichlorphon complexes showed the appearance of two additional peaks at higher binding energy(near 296.1 and 293.2 eV),which can be ascribed to the π-π*shakeup satellite(Monteil-Riveraet al.,2000).This result suggested that carboxylic groups interacted with the surface of phyllosilicate minerals,which was consistent with the ATR-FTIR results.

Fig.5 X-ray photoelectron C 1s spectra obtained for kaolinite(KAO)and KAO-trichlorphon(Tri)complex at pH 4.0 in the absence and presence(+)of low-molecular-weight organic acids,citric acid(CA)and oxalic acid(OA).The original spectrum is indicated by empty circles.The best fit curves and individual peaks are shown by solid lines.
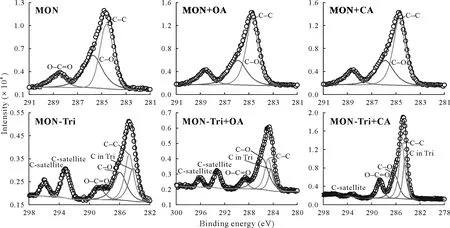
Fig.6 X-ray photoelectron C 1s spectra obtained for montmorillonite(MON)and MON-trichlorphon(Tri)complex at pH 4.0 in the absence and presence(+)of low-molecular-weight organic acids,citric acid(CA)and oxalic acid(OA).The original spectrum is indicated by empty circles.The best fit curves and individual peaks are shown by solid lines.
The binding energy peak positions and calculated surface atom percentages of C in the samples are summarized in Table SII(see Supplementary Material for Table SII).A slight shift(0.1-0.7 eV)in the C-O peak position was observed for the samples of phyllosilicate mineral-trichlorphon complexes.In general,the shift of the peak toward a higher binding energy indicates the loss of relative electronic density around the atom owing to the effect of its nearest neighbors(Biaet al.,2017).In this case,it can be inferred that the shift may be due to the presence of adsorbed trichlorphon.Furthermore,the new contributions of C in trichlorphon and two C-satellites were the evidence of the presence of LMWOAs near the surface region of phyllosilicate minerals.The interactions between phyllosilicate minerals and trichlorphon affected by LMWOAs could be deduced from changes in the atom percentages of C.The minimum atom percentages of C in trichlorphon and C-satellites of MON-trichlorphon complex in the presence of OA were consistent with the lowest adsorption capacity of trichlorphon on MON in the presence of OA.
CONCLUSIONS
The adsorption of trichlorphon on phyllosilicate minerals decreased with increasing pH.The effect of pH was stronger in the absence of LMWOAs than in their presence.The adsorption of trichlorphon on phyllosilicate minerals was promoted by the presence of CA.In contrast,trichlorphon adsorption on MON was inhibited by the presence of OA,especially at pH 4.0.The ATR-FTIR and XPS results indicated that Si-O sites of phyllosilicate minerals were the preferred adsorption sites of trichlorphon and were probably driven by the hydrophobic effect.The presence of LMWOAs increased the exposure of hydrophobic sites of phyllosilicate minerals and thus increased trichlorphon adsorption.The inhibition of trichlorphon adsorption on MON by OA may be due to the weak effect of OA and increased electrostatic repulsion between MON and trichlorphon molecules.These findings indicated that soil minerals with hydrophobic sites can be used as adsorbents for organochlorine contaminants.Organic acids,particularly LMWOAs,in soils had a significant effect on the behavior of organochlorine contaminants.The results not only help to better understand the behavior of organic pesticides in soils and sediments,but also provide a way to remediate organic pesticide-contaminated soils and sediments.
ACKNOWLEDGEMENTS
This study was financially supported by the National Natural Science Foundation of China(Nos.41825021,42007020,and 21876097)and the National Natural Science Foundation Project of International Cooperation and Exchange,China(No.41961144010).The authors would like to thank all the reviewers who participated in the review,as well as MJEditor(www.mjeditor.com)for providing English editing services during the preparation of this manuscript.
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
- Pedosphere的其它文章
- Developing the new soil science-Advice for early-career soil scientists
- Biophotoelectrochemistry:An emerging frontier for channeling photoelectric effect into darkness zone of soils and sediments
- Balancing machine learning and artificial intelligence in soil science with human perspective and experience
- Soils in extraterrestrial space:Need for studies under microgravity
- Role of biochar in raising blue carbon stock capacity of salt marshes
- Long-term fertilizer nitrogen management-Soil health conundrum

