Biophotoelectrochemistry:An emerging frontier for channeling photoelectric effect into darkness zone of soils and sediments
Solar energy captured by photosynthetic plants in the photic zone is recognized as the main driver for the formation of organic matter utilized by soil communities.However,the contribution of organic transformation to the linkage of solar energy and microbial metabolism of soils is reduced when the vadose zone is saturated.In contrast to the conventional biophotoelectrochemistryviaphotosynthesis with phytoplankton during the periodic saturation of soils,recent studies suggest that non-phototrophic microorganisms in soils and sediments are able to conduct light-dependent metabolism to sustain their functionality with photosensitizers under illumination.These interactions and processes utilize long-distance electron transfer networks to interconnect diverse electron transfer chains that channel photoexcited electrons into the opaque zone for soil communities.Such an emerging process not only allows for a better understanding of biogeochemical processes such as soil carbon sequestration and mitigation,but also shows great potential for environmental treatment such as the bioremediation of contaminated soils.Therefore,we suggest that biophotoelectrochemistryviaphotoelectric effect can have significant,heretofore unappreciated,theoretical and practical values.
“What drives life is a little electric current,kept up by the sunshine”-Albert Szent-Györgyi,Nobel Prize Laureate in Physiology or Medicine(1937).
There are many types of soils,and they often change their characteristics as they oscillate between times of water saturation and unsaturation.As such,they constitute one of the most diverse and complicated set of ecosystems on the planet and as a whole are thought to significantly influence the biogeochemical cycling of elements and global climate change.The energy captured by plant photosynthesis is recognized as the main driver for the formation of organic matter utilized by microorganisms in soils,particularly when the vadose zone is unsaturated.However,periodic saturation results in soil morphology and chemistry changes and decreases plant contribution due to diffusion limitations.Under such conditions,the alternative pathway for solar capture and its interaction with soil microorganisms remain a mystery.Recent studies,along with the rapid development of electromicrobiology,suggest that non-phototrophic microorganisms can harvest extracellular electrons (Lovley and Holmes,2022;Wanget al.,2022),including the photoexcited electrons from photosensitizers,such as minerals and photosynthetic bacteria,for self-organization and sustained functionality under illumination.This discovery challenges the established theoretical frameworks for microbial metabolism and geochemical processes in saturated soils and sediments.
Conventional biophotoelectrochemistryvia photosynthesis with phytoplankton.Solar energy can drive geochemical processes through various pathways such as photosynthesis.When the upper layer of the vadose zone becomes saturated(either permanently or seasonally),soils are much like sediments.A reduction in net plant photosynthesis occurs immediately due to stomatal closure,reduced activity of major photosynthetic enzymes,and disruption of photosynthate transport.Under these conditions,the phytoplankton,a diverse polyphyletic group of photosynthetic prokaryotic and eukaryotic microorganisms,regulate the biogeochemical cyclesviathe uptake,incorporation,or transformation of numerous elements during photosynthesis,including,but not limited to,carbon,nitrogen,phosphorus,sulphur,and iron(Luet al.,2022).However,the phytoplankton die continuously and are replaced once a week,which may exceed phytoplankton growth by as much as 50%(Bidle and Falkowski,2004).Dead phytoplankton cells then serve as the primary energy source for soils and sediments,because the diverse assemblages of heterotrophic bacteria in soils and sediments usually lack the required photosensitive cellular compounds and they not only utilize dissolved matter exuded from living phytoplankton cells,but also colonize and rapidly degrade dying cells with specialized hydrolytic enzymes,that is,the conventional biophotoelectrochemistryviaphotosynthesis with phytoplankton(Fig.1a).
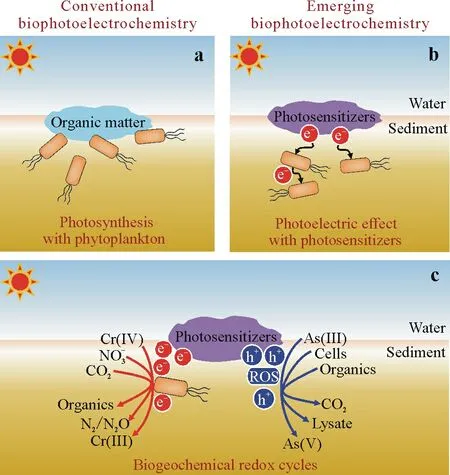
Fig.1 Schematic illustration of proposed biophotoelectrochemical processes in saturated soils and sediments:conventional biophotoelectrochemistry via photosynthesis with phytoplankton(a),emerging biophotoelectrochemistry via photoelectric effect with photosensitizers(b),and biogeochemical redox cycles driven by the emerging biophotoelectrochemistry.ROS=reactive oxygen species.
Emerging biophotoelectrochemistry via photoelectric effect with photosensitizers.A major breakthrough in our understanding of geochemical processes occurred with the proposal of emerging biophotoelectrochemistryviaphotoelectric effect with photosensitizers.This phenomenon was first reported by Luet al.(2012),who demonstrated the growth ofAlcaligenes faecalis,one of the most prevalent species of non-phototrophic microorganisms in natural soils,using solar energy with the assistance of minerals.Subsequently,similar results of what appeared to be an uninterrupted stream were reported(Sakimotoet al.,2016;Yeet al.,2019,2021,2022a,b;Huet al.,2022),which shed light on the biogeochemical processes in saturated soils and sediments.In other words,the photoexcited electrons from photosensitizers under illumination can be harvested by electroactive microorganisms in the opaque zone(Fig.1b).Such a process may significantly influence the ecological functions of soils and sediments such as carbon dioxide and nitrate reduction and pollution control.Meanwhile,the simultaneously produced oxidative species (e.g.,reactive oxygen species)also drive biogeochemical processes,such as the oxidation of organic compounds and heavy metals,and cell inactivation(Fig.1c).For instance,a recent study demonstrated that microplastic oxidation could be conductedviathe attack of photo-induced reactive species with aMethanosarcina barkeri-polymeric carbon nitride hybrid system,along with a sustainable conversion of microplastics to methane with ultrahigh selectivity(Yeet al.,2022b).
The unique physicochemical properties of saturated soils and sediments drive the emerging biophotoelectrochemical process.On the one hand,various mineral nanoparticles with marked photochemical activities,including birnessite,rutile,hematite,and sphalerite (Fig.2a),are widely distributed in the water layer and water-sediment interface due to the hydraulic disturbance during water flooding(Yeet al.,2021).Meanwhile,natural organic matter and dead microalgae also possess adequate photosensitive properties,particularly after covalent/noncovalent interactions with ions or molecules to form nanoscale clusters(Bushawet al.,1996;Zhanget al.,2006).On the other hand,the phylogenetically diverse electroactive microorganisms with extracellular electron transfer capacity,such asGeobacter sulfurreducensandShewanella oneidensis(Fig.2b),are enriched in anaerobic saturated soils and sediments(Lovley and Holmes,2022).These electroactive microorganisms,particularly electrotrophs that consume electrons from the extracellular electron donors,have the potential to establish outer-surface electrical contacts with photosensitizers.Then the formed photosensitizer-microbe complexes govern the diversity of biophysical microenvironments in soils and sediments.
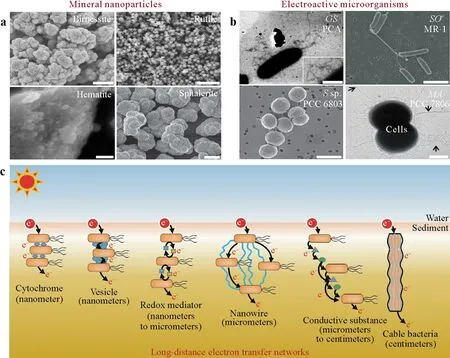
Fig.2 Schematic illustration of emerging biophotoelectrochemistry in the opaque zone of saturated soils and sediments:representative semiconductor minerals(Li et al.,2006;Cheney et al.,2008;Das et al.,2011;Voegelin et al.,2011)(a),representative electroactive microorganisms(Reguera et al.,2005;Gorby et al.,2006;Sure et al.,2015)(b),and proposed long-distance electron transfer networks(c).GS=Geobacter sulfurreducens;SO=Shewanella oneidensis;S=Synechocystis;MA=Microcystis aeruginosa.
A key question that arises is:how do these electroactive microorganisms in the opaque zone of the saturated soils and sediments harvest the photoexcited electrons for the water layer or water-sediment interface?The long-distance electron transfer networks may provide clues to answer this question(Fig.2c).Besides the extracellular electron transferviadirect contact of outer membrane cytochromes or vesicles on the cell surface or on conductive extensions(Hartshorneet al.,2009;Liuet al.,2020),the redox-active mediators from the natural environment and microbial metabolism can enhance electron transfer at a distance of nanometers to micrometers from the electron acceptors(e.g.,cell and minerals).A great breakthrough in long-distance electron transfer has been achieved with nanowires (metallic-like conductivity,micrometers long),which provide a flexible,web-like electronic network that significantly expands the prospects for electrical transferviaunique cell-surface and cell-cell interactions(Regueraet al.,2005).In addition,the latest discovery of cable bacteria takes electromicrobiology of soils and sediments to a whole new dimension,which shows that cable bacteria can be involved in the electron transfer with long conductive cables over a distance of centimeters(Pfefferet al.,2012).These diverse electron transfer chains interconnect with each other,thereby providing efficient pathways to channel more photoexcited electrons into the opaque zone of saturated soils and sediments than previously recognized.
Research prospects and future considerations.With vast swaths of the planet covered by soils and sediments,emerging biophotoelectrochemistryviaphotoelectric effect has attracted great interest.Unfortunately,this novel biogeochemical process has been ignored for a long time,and key knowledge about it is still in its infancy.Fundamentally,for instance,the electrical interplay between photosensitizers and electroactive microorganisms remains unclear,such as the response of the complex energy transfer networks in electroactive microorganisms to extracellularly photoexcited electrons.Technologically,more work is warranted,such as exploring the potential of biophotoelectrochemistry for bioremediation of contaminated soils.In addition,the biophotoelectrochemical process improves the growth and metabolism of other photic and chemotrophic autotrophic microbesviaelectron transfer and substrate supply.Therefore,more undiscovered,novel microbial mutualismsviabiophotoelectrochemistry can be investigated,and their roles in carbon sequestration and mitigation in soils need to be reevaluated,which will lead us into an exciting new decade of biophotoelectrochemistry and open up several new directions for future research.
ACKNOWLEDGEMENT
This work was supported by the National Science Foundation for Distinguished Young Scholars of China (No.41925028)and the National Natural Science Foundation of China(No.42177206).
- Pedosphere的其它文章
- Developing the new soil science-Advice for early-career soil scientists
- Balancing machine learning and artificial intelligence in soil science with human perspective and experience
- Soils in extraterrestrial space:Need for studies under microgravity
- Role of biochar in raising blue carbon stock capacity of salt marshes
- Long-term fertilizer nitrogen management-Soil health conundrum
- Partnering crops with root-associated microbes for soil health and agricultural sustainability

