Patterns and drivers of seasonal water sources for artificial sand-fixing plants in the northeastern Mu Us sandy land,Northwest China
Yanwu PEI ,Laiming HUANG ,Ming’an SHAO,3 ,Jiao WANG and Yinglong ZHANG
1Yellow River Delta Modern Agricultural Engineering Laboratory,Institute of Geographic Sciences and Natural Resources Research,Chinese Academy of Sciences,Beijing 100101(China)
2College of Natural Resources and Environment,Northwest Agriculture and Forestry University,Yangling 712100(China)
3State Key Laboratory of Urban and Regional Ecology,Research Center for Eco-Environmental Sciences of Chinese Academy of Sciences,Beijing 100085(China)
4College of Resources and Environment,University of Chinese Academy of Sciences,Beijing 100049(China)
5Shenmu Ecological Association,Shenmu 719399(China)
ABSTRACT Understanding plant water-use patterns is important for improving water-use efficiency and for sustainable vegetation restoration in arid and semi-arid regions.However,seasonal variations in water sources and their control by different sand-fixing plants in water-limited desert ecosystems remain poorly understood.In this study,stable isotopic ratios of hydrogen (δ2H) and oxygen (δ18O) in precipitation,soil water,groundwater,and xylem water were determined to document seasonal changes in water uptake by three representative plant species(Pinus sylvestris var.mongolica Litv.,Amygdalus pedunculata Pall.,and Salixpsammophila)in the northeastern Mu Us sandy land,Northwest China.Based on the depth distribution and temporal variation of measured gravimetric soil water content(SWC),the soil water profile of the three species stands was divided into active(0.01 g g-1 <SWC <0.08 g g-1,20%<coefficient of variation(CV)<45%),stable(0.02 g g-1 <SWC <0.05 g g-1,CV <20%),and moist(0.08 g g-1 <SWC <0.20 g g-1,CV >45%)layers.Annually,P.sylvestris,A.pedunculata,and S.psammophila obtained most water from deep(59.2%±9.7%,moist layer and groundwater),intermediate(57.4%±9.8%,stable and moist layers),and shallow(54.4%±10.5%,active and stable layers)sources,respectively.Seasonally,the three plant species absorbed more than 60%of their total water uptake from the moist layer and groundwater in the early(June)dry season;then,they switched to the active and stable layers in the rainy season(July-September)for water resources(50.1%-62.5%).In the late(October-November)dry season,P.sylvestris(54.5%-66.2%)and A.pedunculata(52.9%-63.6%)mainly used water from stable and moist layers,whereas S.psammophila(52.6%-70.7%)still extracted water predominantly from active and stable layers.Variations in the soil water profile induced by seasonal fluctuations in precipitation and groundwater levels and discrepancies in plant phenology,root distribution,and water demand are the main factors affecting the seasonal water-use patterns of artificial sand-fixing plants.Our study addresses the issue of plant water uptake with knowledge of proportional source-water use and reveals important implications for future vegetation restoration and water management in the Mu Us sandy land and similar desert regions around the world.
Key Words: desert ecosystem,MixSIAR model,plant water uptake,soil moisture,stable isotopes,vegetation restoration,water-use efficiency
INTRODUCTION
Plant water uptake is a key process in the global water cycle,which can provide critical insights into plant responses to changing environments(Jacksonet al.,2000;Arocaet al.,2012;Razaet al.,2020).Furthermore,it plays an important role in understanding,modeling,and regulating hydrological processes in the soil-plant-atmosphere continuum(Daiet al.,2020).Therefore,quantifying the sources and magnitude of plant water uptake and their spatial and temporal variability has received increasing attention over the past several decades(McCole and Stern,2007;Eggemeyeret al.,2009;Songet al.,2016a;Wuet al.,2019;Zhaoet al.,2020).
It is well established that root characteristics(e.g.,length,density,and depth distribution)strongly affect plant water sources (PWS) and the strategies for their use.Thus,for example,deep-rooted trees and shrubs mainly use deep soil water and/or underlying groundwater (e.g.,McCole and Stern,2007;Dinget al.,2018),whereas shallow-rooted plants,such as grasses and herbs,predominantly absorb shallow soil water(e.g.,Prechslet al.,2015;Priyadarshiniet al.,2016).Distinct water-use patterns among different species are attributed to hydrological niche segregation(HNS)(Silvertownet al.,2015),which makes it possible for various species to coexist in the same habitat (Eggemeyeret al.,2009;Wanget al.,2017).Specifically,HNS is defined as(i)partitioning of space on fine-scale soil-moisture gradients,(ii) partitioning of water as a resource,and/or (iii) partitioning of recruitment opportunities among years caused by species specializing in particular patterns of temporal variance of water supply.Changes in PWS occur not only among species,but across different growth stages in a single species as well(Songet al.,2016a;Wuet al.,2019).For instance,Songet al.(2016a) found that younger,i.e.,<22 years old,Mongolian pine trees(P.sylvestris)only used 0-100 cm soil water in a semi-arid sandy land of Northwest China.In contrast,olderP.sylvestris,i.e.,>30 years old,used both soil water and groundwater.Further,significantly contrasting water uptake patterns were observed inH.rhamnoidesspecimens of different ages.Particularly,seedlings ofH.rhamnoidesand juvenileH.rhamnoidesdepend on both shallow and middle soil water during the entire growing season,whereas water used by matureH.rhamnoidesindividuals shifts from shallow and middle soil water during the early growing season to groundwater during the peak growing season(Wuet al.,2019).The use of water by plants also varies with season and rainfall events.Similarly,Zhaoet al.(2020)reported a shift in water-use patterns byRobinia pseudoacaciafrom deep to shallow soil layers during the transition period between the dry and rainy seasons in the middle region of the Loess Plateau,Northwest China.This is owing to the dimorphic nature of plant root systems in water-limited regions,which enables them to switch between shallow and deep water sources depending on water availability (Prietoet al.,2010).The shift among water sources in dimorphic-rooted plant species plays a critical role in plant growth and survival given the ever-increasing frequency and severity of extreme drought events in arid and semi-arid regions around the world.Owing to the large variations in climate,plant,soil,and water conditions,plant water-use patterns are site-and species-specific,and ageand time-dependent.Thus,unraveling the water sources of different plant species and their depth(spatial)and temporal variations will not only enhance our understanding of plantwater relationships but provide data for eco-hydrological modeling and water management as well.
Stable isotopes have been extensively used as fingerprints for studying PWS by assuming that the isotopic composition of hydrogen and oxygen(δ2H andδ18O)in water does not change during root water uptake and its transport to shoots(Zimmermannet al.,1967;Rothfuss and Javaux,2017).By comparingδ2H orδ18O in xylem water to those of potential contributive water sources(e.g.,rain water,stream water,water from different soil layers or groundwater),studies have enabled the identification and quantification of the relative contribution of different water sources to overall plant water uptake.Further,the application of stable isotopes to investigate PWS has been reported in various ecosystems,such as forestlands(e.g.,Meissneret al.,2014),grasslands(e.g.,Changet al.,2019),and farmlands(e.g.,Pennaet al.,2020).Such studies have demonstrated that PWS are highly variable in space and time,depending on i)the ability of the root to extract water(characterized by the depth distribution and biomass of fine roots and their hydraulic properties),ii)soil water content and availability(characterized by the ability of soil to fulfill plant water demands),and iii)plant water demand and drought tolerance(characterized by the magnitude of transpirational water loss and plant adaptability to drought stress)(Ehleringer and Dawson,1992;Rothfuss and Javaux,2017).Despite extensive studies on PWS in various ecosystems,the source of water used by artificial sand-fixing plants in the water-limited desert ecosystem remains poorly understood.
The Mu Us sandy land,located in Northwest China,is a water-limited desert region with an area of 42 000 km2.Evaporation in this region largely exceeds precipitation(Huanget al.,2009).To prevent severe desertification,the Chinese government has implemented a series of ecological projects in this region,such as the Natural Forest Protection Plan,the“Grain for Green”Program,and the“Three-North”Shelterbelt Project.As a result,the vegetation coverage of forestland and grassland in the Mu Us sandy land has increased from 0.9% to more than 40% (Zhang and Wu,2020).However,unsustainable vegetation restoration in some areas,such as improper introduction of trees with high water demands,overplanting,and unbalanced proportions of plant species,has caused severe soil-water deficit conditions,a sharp decline in groundwater levels,and even degradation or dieback of the restored vegetation(Shiet al.,2019;Huanget al.,2021).Therefore,it is necessary to understand the patterns and drivers of seasonal water sources for typical restoration species in this region.Although studies have been conducted in similar sandy lands (e.g.,Wanget al.,2017),little is known about the changes and drivers of plant water sources in the region of the water-limited Mu Us sandy land.To fill this knowledge gap,δ2H andδ18O of precipitation,soil water,groundwater,and xylem water were measured every month over two consecutive growing seasons to investigate the sources of water used by three plant species,namely,Pinus sylvestrisvar.mongolicaLitv.,Amygdalus pedunculataPall.,andSalixpsammophila,in the northeastern Mu Us sandy land.The objectives of this study were i) to investigate the variations in the isotopic compositions of precipitation,soil water,groundwater,and xylem water,ii)to quantify the contribution of different water uses to overall plant water uptake using the MixSIAR model,and iii)to identify the factors affecting the water-use patterns of the three plant species.The two hypotheses proposed in this study are as follows:i)the water-use pattern differs among species growing in the same habitat and ii)variations in PWS are sensitive to seasonal changes in precipitation and groundwater levels.This research provides information for the effective protection of vulnerable sandy environments and for the rational reconstruction of vegetation in the Mu Us sandy land and similar desert regions.
MATERIALS AND METHODS
Study area
This study was conducted at the Gechougou watershed(38°11′-38°53′N,109°21′-110°03′E),located in the northeastern Mu Us sandy land,Northwest China(Fig.1).The watershed covers an area of 45 km2and has an altitude ranging from 1 145 to 1 263 m.The region lies within a semiarid continental climate zone with an annual mean temperature of 9.1°C and an annual mean precipitation of 375.4 mm over the past 16 years(2001-2016),respectively.The lowest(-16.7°C)and highest(27.6°C)temperatures generally occur in January and August,respectively.More than 70% of precipitation occurs during the rainy season from July to September (Xiuet al.,2018).Most of the sand dunes in the studied watershed are stable because of widespread vegetation restoration,which aims to prevent severe desertification and wind erosion.As a result,vegetation coverage has increased rapidly over the past several decades,as confirmed by the satellite-retrieved normalized difference vegetation index(NDVI)(Xiuet al.,2018).Typical plants used for vegetation restoration includeP.sylvestris,A.pedunculata,S.psammophila(Zhang and Wu,2020).The fixed aeolian sandy soils in the watershed are classified as Aridic Arenosols according to the World Reference Base for Soil Resources(FAO,2007)or Sandic Primosols according to Chinese Soil Taxonomy(CRGCST,2001).Soil particle size distribution shows a high sand content(>95%)and a low silt content(<5%)in clay-free sandy soil(Peiet al.,2021a).Field capacity and saturation water contents of the sandy soil of the region are 0.10-0.12 and 0.21-0.25 g g-1,respectively.The groundwater level in the studied watershed is shallow,with the depth of the shallowest water table being less than 1 m below the ground surface(Peiet al.,2021b),providing a potential water source for restored vegetation.
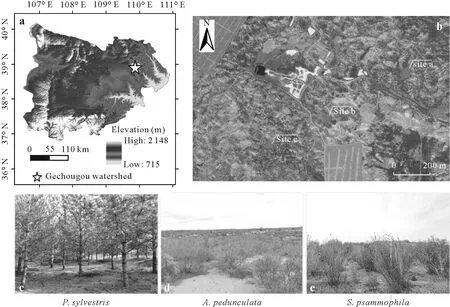
Fig.1 Location of the study area in the northeastern Mu Us sandy land,Northwest China(a),remote sensing image and sampling sites a-c(b),and the plant species Pinus sylvestris,Amygdalus pedunculata,and Salixpsammophila distributed at sites a,b,and c,respectively(c-d).
Experimental design and sample collection
Three widely distributed species in the Gechougou watershed,namely,P.sylvestris,A.pedunculata,andS.psammophila,were selected to study seasonal changes in plant water-use patterns.There were two reasons for selecting these particular species:i) the area occupied by the three plant species accounted for more than 90%of the total restored area in the Gechougou watershed and ii)the groundwater levels where the three species stands are located are less than 3 m deep into the soil profile,which makes groundwater a potential water source for plant water uptake.The distance between any two stands was less than 1.5 km under similar topographical conditions.Three plots (20 m× 20 m forP.sylvestrisand 10 m× 10 m forA.pedunculataandS.psammophila) were randomly selected in each stand.Basic information on the plant species under study is shown in Table I.Based on the mean diameter at breast height and average plant height in each stand,one representative plant was selected in each plot and tagged for sampling and determination of stable isotopic composition.A minimum of three replicates is generally used for statistical analysis and has been widely used to determine PWS (Nieet al.,2012).In addition,the sampling time in the rainy season(July-September) was 3-5 d after the precipitation event(>10 mm),based on the response of soil water dynamics to precipitation in the study region (Liuet al.,2016;Yuet al.,2018),such as to minimize the influence of hysteresis between plant water uptake and rainfall infiltration.The growing season in the Mu Us sandy land runs from late April to the end of November.There were many fallen leaves in theA.pedunculataandS.psammophilastands,but few in theP.sylvestrisstand during the late growing season(November).However,the leaves of shrubs are primordial in April and May due to the relatively cold weather (Wang G Pet al.,2015).Therefore,the sampling time in our study ranged from the mid-growing season (June) to the late growing season (November).Hence,soil,plant,precipitation,and groundwater samples were collected once a month from June to November in 2018 and 2019.
On each sampling date,twig xylem samples of the three species were collected between 09:00 and 14:00 hours when the plants were actively transpiring,and lignified twigs were cut from the base of the canopy of each sampled plant in the four cardinal directions(east,south,west,and north).Phloem tissues were removed to avoid contamination by isotopically enriched water in phloem tissues(Dawson and Pate,1996).The clipped twigs were immediately placed in brown glass vials with screw caps and sealed with a polyethylene parafilm.The collected xylem samples were placed in a cooler with ice for transportation to the laboratory,where they were frozen and stored(-20°C)for subsequent isotope analysis.The total number of plant xylem samples was 108(3 species×3 replicates per species×6 months per year×2 years).
Soil and plant samples were collected simultaneously on each sampling date.Three soil cores were taken from each plot of the three species at depth intervals of 20 and 30 cm above and below 60 cm,respectively,till groundwater level.The deepest sampling depths inP.sylvestris,A.pedunculata,andS.psammophilastands were different because of variations in groundwater levels,which were 80-100,160-180,and 260-280 cm,respectively,during the sampling period.Samples were collected using an auger and separated into two parts for stable isotope analysis of soil water and determination of gravimetric soil water content(SWC).Soil samples for stable isotope analysis were placed in brown glass vials with screw caps,wrapped in parafilm,and stored at-20°C in a freezer until water extraction.The total number of soil samples for stable isotope analysis was 270.Soil samples for the determination of SWC were placed in an aluminum box (8 cm in diameter and 5 cm high)and dried at 105°C for 24 h.In addition,root samples of the three species were collected at 10-cm intervals until groundwater level using a root auger (9 cm in diameter and 10 cm high)at the end of the growing season in 2019,according to the procedure described by Changet al.(2019).Root samples were collected using an auger at 50-,100-,and 150-cm positions from the tree trunks and perpendicular to the tree row.At each position,roots were collected at 10-cm depth intervals down the soil profile.Collected root samples were washed and dried to weigh the biomass of coarse roots(diameter>2 mm)and fine roots(diameter<2 mm).
Precipitation samples were collected using a funnel device immediately after each precipitation event(>10 mm)ended.The funnel device was placed in the open interspaces at the center of the stands of the three species.A total of 27 precipitation samples were collected during the study period.Groundwater samples were collected from a waterlevel observation well(10 cm in diameter)in each stand.The timing,frequency,and storage of groundwater samples were synchronized with the collection of the soil and plant samples.Air temperature and precipitation were recorded using a weather station(RGB M002,Onset,Bourne,USA)installed in the watershed.Groundwater levels were automatically recorded using a water level indicator(Onset HOBO®U20-001-04,Onset,Bourne,USA).
Isotope measurements of water samples
Water was extracted from the plant and soil samples using a cryogenic vacuum distillation system (LI-2000,LICA,Beijing,China) according to the procedure described by Westet al.(2010).The time used for extraction varied from 1.5 to 3 h,depending on the water content of the samples.Plant xylem and soil samples were weighed after extraction to ensure that the extraction efficiency exceeded 98%,i.e.,the extracted water content accounted for>98%of the measured plant water content or SWC(Westet al.,2010;Yanget al.,2015;Wanget al.,2017).Rainwater,groundwater,and extracted water were filtered using 0.44-μm aqueous phase pin-type filters to avoid impurities.

TABLE IBasic information of the studied plant species Pinus sylvestris,Amygdalus pedunculata,and Salixpsammophila
Both a liquid water isotope-analyzer and an isotope ratio mass spectrometer(IRMS)can be used to measureδ2H andδ18O (Brandet al.,2009;Guptaet al.,2009;Zhao and Wang,2021).In this study,δ2H andδ18O of xylem water were measured by an Isoprime-100 IRMS (Isoprime Ltd.Inc.,Cheadle,UK),because the instrument can effectively eliminate the effects of trace amount of soluble organic matter(Zhao and Wang,2021).The precision of measurements by IRMS was consistently±0.5‰forδ2H and±0.1‰forδ18O.Organic matter was not detected in precipitation,soil water,or groundwater.As a consequence,δ2H andδ18O of precipitation,groundwater,and soil water were measured using a liquid water isotope analyzer(L2130i,Picarro,Santa clara,USA) with analytical precision of±0.1‰forδ2H and±0.025‰forδ18O(Chenet al.,2021).The analytical precision and accuracy of the Isoprime-100 IRIS were similar to those of the liquid water isotope analyzer.Moreover,previous studies have shown that there are no discrepancies between the isotopic compositions of precipitation and soil water measured by these two instruments(Wanget al.,2017).Thus,it was expected that the isotopic ratios measured using the two instruments would not affect subsequent analysis.The isotopic compositions of all samples measured by the two instruments were normalized to the Vienna Standard Mean Ocean Water(V-SMOW)standard(Huanget al.,2023).
The calculation of isotopic ratio(δ,‰)was expressed as follows:
where X represents2H or18O,respectively,andRsampleandRstandardare the2H/1H or18O/16O values of the samples and standard(V-SMOW),respectively.Line-conditioned excess(lc-excess,‰)represents soil water evaporation intensity and is calculated as follows:
whereaandbrepresent the slope and intercept of the local meteoric water line(LMWL),respectively(Landwehr and Coplen,2006).
The weighed mean values of isotopic ratios(δ2H orδ18O)of precipitation per month(δXp,mean,‰)were calculated by the following align:
whereδiand PPTirepresent the isotopic composition of precipitation and precipitation amount in each monthi,respectively.
Determination of plant water sources
The Bayesian isotope mixing model MixSIAR(Stock and Semmens,2013) was used to identify the quantitative contributions of soil water and groundwater to plant water uptake.The MixSIAR model can estimate the proportion of source contributions to a mixture using options for fixed/random effects,source data types,priors,and error terms(Stock and Semmens,2013).In this study,δ2H andδ18O values of xylem water were used as mixture data inputs into the MixSIAR model.The mean values and standard errors ofδ2H andδ18O of groundwater and water from different soil layers were used as the“source”data inputs into the MixSIAR model.It is assumed that isotopic fractionation does not occur during plant water uptake,owing to the high isotopic similarity between soil water and xylem water(Zimmermannet al.,1967;Rothfuss and Javaux,2017).Furthermore,it has been reported that there is no fractionation of H and O isotopes during root water uptake in the semi-arid Loess Plateau(Wanget al.,2017).Therefore,the discrimination value was set to 0 for both theδ2H andδ18O.The run length of the Markov chain Monte Carlo(MCMC)was set to“long”(chain length=300 000,burn=200 000,thin=100,chains=3).The median values(50%quartiles)are shown as MixSIAR predictions.Water sources associated with different soil depths were combined into three layers(active,stable,and moist)to facilitate subsequent analysis and comparison.The three layers were identified based on measured SWC and its variability.
Statistical analysis
Statistical analysis was performed using SPSS software(version 16.0,SPSS Inc.,Chicago,USA).Seasonal changes in isotopic compositions of xylem water,soil water,and groundwater among different species(P.sylvestris,A.pedunculata,andS.psammophila)were analyzed using one-way analysis of variance(ANOVA).Temporal and spatial(depth)variations in SWC were also analyzed using ANOVA.Figures and Tables were elaborated using Origin 9.0(Origin Software Inc.,Fairview,USA)and Excel 2016,respectively.
RESULTS
Temporal variations in precipitation and isotopic compositions
The highest and lowest air temperatures and precipitation occurred in August and late January,respectively(Fig.2).Total precipitation in 2018 and 2019 was 401 and 270 mm,respectively,of which more than 75%occurred during the rainy season from July to September.Compared to the average precipitation(375 mm)over the past 16 years(2001-2016),2018 was considered a normal year,and 2019 was a dry year,with precipitation 32%lower than the preceding year.
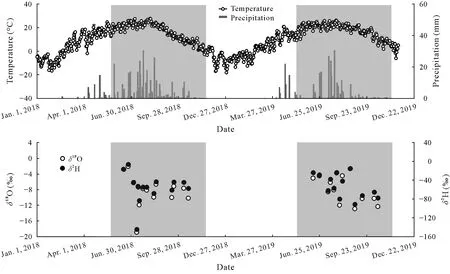
Fig.2 Temporal variations in air temperature,precipitation,and H and O isotopic ratios(δ2H and δ18O)in precipitation of 2018 and 2019.The shaded areas represent the sampling periods in 2018 and 2019.
Less heavy precipitation events in 2019 enriched heavier H and O isotopes.The weighted mean value ofδ2H andδ18O of precipitation(-52.27‰and-7.73‰)was higher in 2019 than that in 2018 (-55.74‰and-8.29‰).The local meteoric water line(LMWL)was fitted based on the precipitation data,expressing asδ2H=7.56δ18O+6.40 in 2018 andδ2H=6.87δ18O+0.88 in 2019(Fig.S1,See Supplementary Material for Fig.S1).The slope and intercept of LMWL were less than those of global meteoric water line(GMWL),expressing asδ2H=8δ18O+10(Craig,1961).This was attributed to the faster evaporation of precipitation in the Mu Us sandy land,particularly in 2019,as it was a particularly dry year.
Isotopic compositions of xylem water,soil water,and groundwater
Theδ2H values of xylem water forP.sylvestris,A.pedunculata,andS.psammophilaranged from-71.94‰to 51.29‰,from-70.84‰to 55.98‰,and from-82.41‰to 50.47‰,with average values of-63.18‰,-64.17‰,and-65.96‰,respectively(Table II).Theδ18O values of xylem water forP.sylvestris,A.pedunculata,andS.psammophilavaried from-9.80‰to-7.04‰,from-10.13‰to-7.30‰,and from-10.19‰to-7.04‰,with mean values of-8.41‰,-8.50‰,and-8.55‰,respectively.Theδ2H andδ18O values of xylem water showed a linear relationship for each plant species in both years(Fig.S1).The slope of plant xylem water line(PWL)forP.sylvestris,A.pedunculata,andS.psammophilaranged from 5.51 to 7.21,which were all lower than the slope of LMWL(Fig.S1).
The isotopic composition of soil water differed among species and changed over time(Table II).The average value ofδ2H of soil water in theP.sylvestris,A.pedunculata,andS.psammophilastands was-62.36‰,-66.82‰,and-70.31‰in 2018,and-65.20‰,-68.65‰,and-65.24‰in 2019,respectively.The corresponding average value ofδ18O of soil water in the three species stands was-8.35‰,-8.06‰,and-8.84‰in 2018,and-8.55‰,-8.86‰,and-8.61‰in 2019,respectively.Most soil water isotopes were distributed below the LMWL(Fig.S1),suggesting that soil water was partly replenished by precipitation and underwent an enrichment process due to evaporation.The soil water line(SWL)fitted by linear regression ofδ2H andδ18O showed different slopes,ranging from 5.73 to 6.49,all of which were lower than the slope of LMWL(Fig.S1).
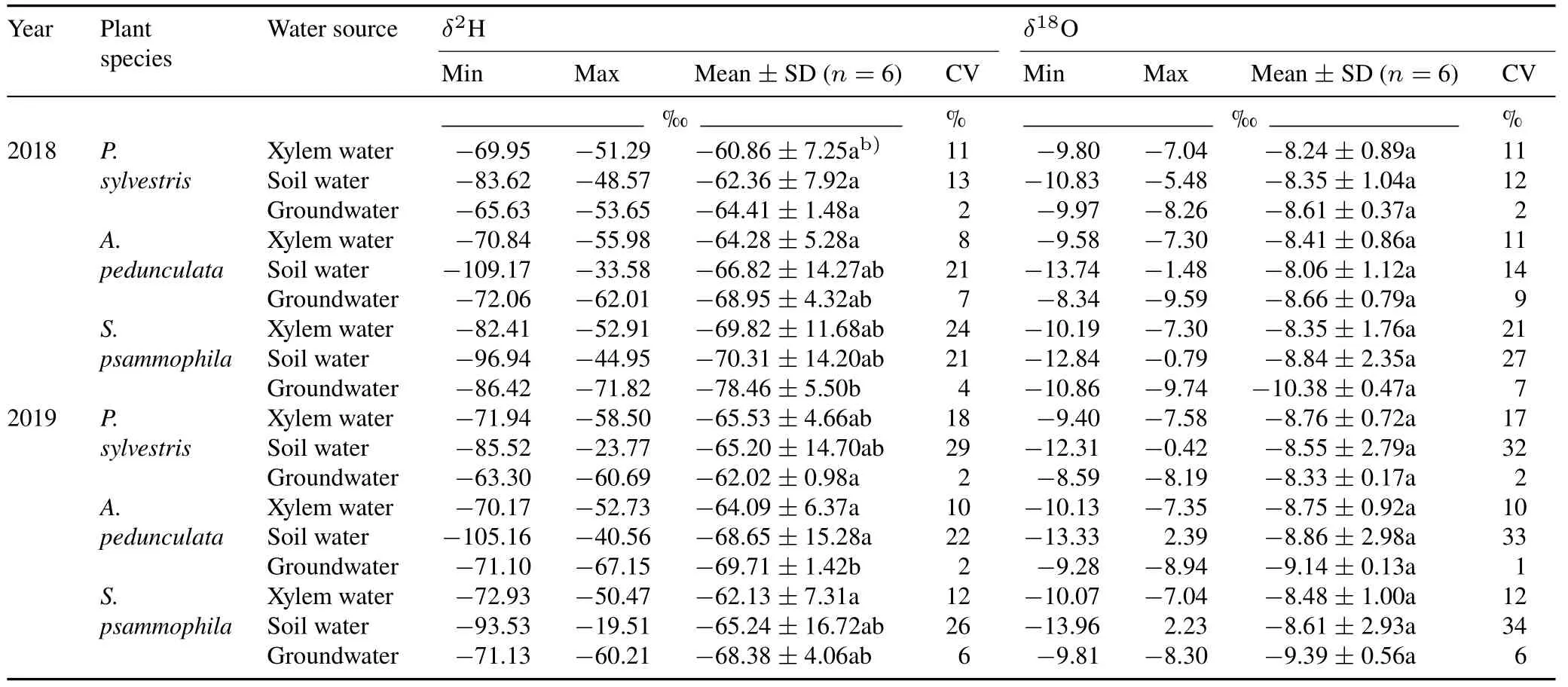
TABLE IIStatistical dataa) of H and O isotopic ratios(δ2H and δ18O)of xylem water,soil water,and groundwater in Pinus sylvestris,Amygdalus pedunculata,and Salixpsammophila stands during the sampling period of 2018 and 2019
Groundwater exhibited relatively steady isotope values in each stand during the measurement period,with a coefficient of variation(CV)of less than 10%(Table II).In turn,the average value ofδ2H of groundwater in theP.sylvestris,A.pedunculata,andS.psammophilastands was-63.22‰,-69.33‰,and-73.42‰,respectively.The corresponding average value ofδ18O in the three species stands was-8.47‰,-8.90‰,and-9.88‰,respectively.The slopes of the fitted groundwater water line(GWL)for theP.sylvestrisandA.pedunculatastands varied from 3.15 to 5.35(Fig.S1),which were lower than the slope of LMWL,suggesting groundwater recharge by precipitation.However,the slope of GWL for theS.psammophilastand was greater than that of LMWL (Fig.S1),suggesting an additional groundwater supply other than precipitation,as previously reported by Chenet al.(2012).
Depth distributions and temporal variations of SWC and isotopic compositions
The SWC inP.sylvestris,A.pedunculata,andS.psammophilastands showed similar depth distribution and temporal variation(Fig.3).For the vertical distribution,there was an“inflection point”of SWC in all studied stands owing to groundwater fluctuations.The average depth of“inflection point”inP.sylvestris,A.pedunculata,andS.psammophilastands was 60,120 and 200 cm,respectively.The SWC above the“inflection point”was lower than 0.08 g g-1and showed relatively small changes with depth in each month,but it was higher than 0.08 g g-1below the“inflection point”and showed larger variations with increasing depth(Fig.3).For temporal changes,the SWC above the depth of“inflection point”in the rainy season(July-September)was generally higher than that in the dry season,i.e.,June,October,and November (Fig.3).Based on the depth distribution and temporal variation of SWC,the soil water profile of the three stands can be divided into three layers,i.e.,active,stable,and moist layers(Table SI,see Supplementary Material for Table SI):i)the depth of the active layer inP.sylvestris,A.pedunculata,andS.psammophilastands was 0-30,0-60,and 0-100 cm,respectively,in which SWC varied greatly with season due to the impacts of precipitation and evaporation(0.01 g g-1<SWC<0.08 g g-1,20%<CV<45%);ii)the depth of the stable layer inP.sylvestris,A.pedunculata,andS.psammophilastands was 30-60,60-120,and 100-200 cm,respectively,in which SWC exhibited smaller temporal changes(0.02 g g-1<SWC<0.05 g g-1,CV<20%)than those shown by the active layer;iii)the depth of the moist layer inP.sylvestris,A.pedunculata,andS.psammophilastands was 60,120,and 200 cm below the groundwater level,respectively,in which SWC was much higher and showed the largest variations due to the recharge of soil water by fluctuating groundwater(0.08 g g-1<SWC<0.20 g g-1,CV>45%).

Fig.3 Depth distribution and temporal variation of gravimetric soil water content(SWC)in Pinus sylvestris,Amygdalus pedunculata,and Salixpsammophila stands during the sampling(once a month)periods of 2018 and 2019.The shaded area represents the average shallow groundwater level.
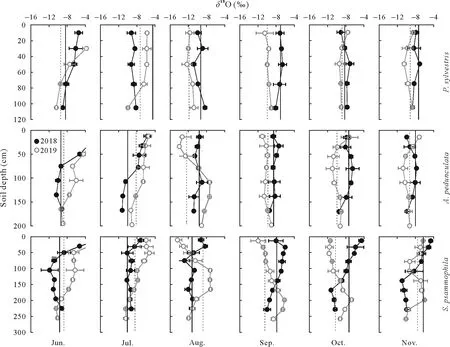
Fig.4 Depth distribution and temporal variation of O isotopic ratio(δ18O)in soil water in Pinus sylvestris,Amygdalus pedunculata,and Salixpsammophila stands during the sampling(once a month)periods of 2018 and 2019.Vertical full and dash lines represent the isotopic ratio of xylem water in 2018 and 2019,respectively.Error bars represent standard deviations of means(n=6).
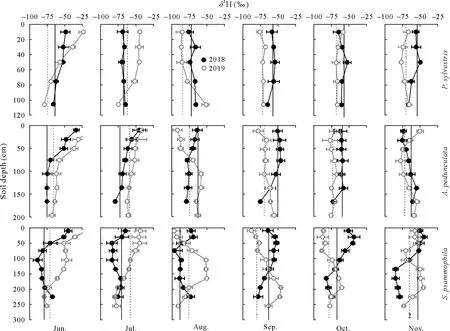
Fig.5 Depth distribution and temporal variation of H isotopic ratio(δ2H)of soil water in Pinus sylvestris,Amygdalus pedunculata,and Salixpsammophila stands during the sampling(once a month)periods of 2018 and 2019.Vertical full and dash lines represent the isotopic ratio of xylem water in 2018 and 2019,respectively.Error bars represent standard deviations of means(n=6).
Soil waterδ18O andδ2H values became more negative with increasing depth(Figs.4 and 5).The moist layer generally had lowestδ18O(from-10.86‰to-8.19‰)andδ2H (from-86.42‰to-53.65‰) and smallest CV (<10%)in each stand over the sampling period(Table SI).In contrast,the active layer showed the largest variation ofδ18O(from-13.96‰to 2.23‰)andδ2H(from-109.17‰to-14.76‰),with a CV ranging from 28%to 51%forδ18O and from 25%to 37%forδ2H(Table SI).As for temporal variations,bothδ18O andδ2H of soil water in the shallow active layer varied greatly in different months(Figs.4 and 5),thus reflecting rapid changes in soil evaporation and rainfall percolation.However,the isotopic composition of soil water in the stable and moist layers changed only slightly during the different months.
Variations in the contribution of different water sources to plant water uptake
The proportional contributions of the different water sources to the three species were quantified using the MixSIAR model (Fig.6).Annually,P.sylvestris,A.pedunculata,andS.psammophiladerived most of the water they used from the deep(59.2%±9.7%from moist layer and groundwater),intermediate(57.4%±9.8%from stable and moist layers),and shallow sources (54.4%±10.5%from active and stable layers) over the sampling period.Compared with the normal year of 2018,all three species increased their use of groundwater in the dry year of 2019,with water uptake fractions from groundwater increasing by 56.6%,5.1%,and 4.7% forP.sylvestris,A.pedunculata,andS.psammophila,respectively.Seasonally,the three plant species absorbed more than 60% of water from the moist layer and groundwater in the early(June)dry season;then,they switched to active and stable layers in the rainy season(July-September)for water sources(50.1%-62.5%).Notably,the water uptake fraction byP.sylvestris,A.pedunculata,andS.psammophilafrom the active layer in the rainy season increased by 112.4%-602.8%,234.5%-644.7%,and 142.6%-185.1%,respectively,compared with that of the early (June) dry season.In the late (October-November)dry season,P.sylvestris(54.5%-66.2%)andA.pedunculata(52.9%-63.6%)mainly used water from the stable and moist layers,whileS.psammophila(52.6%-70.7%)still extracted water predominantly from the active and stable layers.Compared to the rainy season,P.sylvestris,A.pedunculata,andS.psammophilaincreased their absorption of groundwater by 53.2%,49.9%,and 59.7%,respectively,in the dry season,suggesting the importance of groundwater for plant water uptake during drought.

Fig.6 Seasonal variations in the proportion of water used by plant species Pinus sylvestris,Amygdalus pedunculata,and Salixpsammophila from four soil layers using MixSIAR model during the sampling(once a month)periods of 2018 and 2019.
DISCUSSION
Variations and controls of the vertical distribution of soil water isotopic composition
The vertical isotopic gradient of soil water was primarily influenced by evaporation and infiltration.The isotopic ratios of soil water in the three stands showed similar vertical gradients(Figs.4 and 5),with the active layer enriched in heavier isotopes,compared to the stable or moist layer(Table SI).As a result,δ2H andδ18O of soil water became more depleted as soil depth increased,especially in the early (June) dry season(Figs.4 and 5)owing to less precipitation and higher evaporation from shallow soils in the dry season(Gazis and Feng,2004;Songet al.,2016a,b;Wanget al.,2017).Soil waterlc-excesscan directly reflect evaporation intensity(Allison and Barnes,1983;Zhao and Wang,2021).Accordingly,our results showed that thelc-excessvalue of soil water in the active layer was more negative than that in the stable or moist layer,suggesting a greater water evaporation rate(Eggemeyeret al.,2009;Songet al.,2016a,b;Zhao and Wang,2021).However,in the rainy season(July-September),the mixing of soil water with newly infiltrated precipitation dampened the evaporative isotopic enrichment of shallow soil layers(Figs.4 and 5),suggesting that precipitation infiltration was also an important factor controlling the isotopic compositions of soil water during the rainy season(Songet al.,2016a).This was confirmed by the positive correlations(0.71<R2<0.80,P <0.05)detected between the water isotopic ratios of the active layer and precipitation(Fig.S2,See Supplementary Material for Fig.S2).Due to the combined influence of soil evaporation and precipitation infiltration,the active layer generally exhibited larger variations in isotopic ratios(CV>25%)than the stable or the moist layer(CV<20%)(Table SI).Previous studies in other arid/semiarid ecosystems have reported that water isotopic compositions showed greater changes in topsoil layers than in deeper soil layers(Wanget al.,2017;Changet al.,2019;Zhaoet al.,2020).Therefore,future studies should sample the shallow soil layers at shorter intervals,as they are the most dynamic,and consider the seasonal variability of soil water isotopic compositions and their hydrometeorological drivers.
Soil waterδ2H andδ18O in the stable and moist layers showed few variations with both depths and sampling dates compared to those of the active layer(Figs.4 and 5).The isotopic ratios of the deeper soil water were primarily affected by precipitation infiltration and groundwater fluctuation,considering the limited effect of evaporation in deeper soil layers,as indicated by the higherlc-excess(Fig.S3,See Supplementary Material for Fig.S3).On the one hand,the infiltration and/or percolation of precipitation through preferential flow that bypassed superficial soils can lead to mixing of new (mobile) and old (stationary) water in the soil profile (Gazis and Feng,2004),resulting in vertical distribution of isotopic ratios.On the other hand,the upward movement of groundwater by capillary forces that recharged deep soils could affect soil water isotopic ratios at depths near the water table.This was confirmed by the positive correlations detected between the isotopic ratios of soil water in the moist layer and those of groundwater(Fig.S3).Our findings demonstrate the important role of groundwater in deep soil-water recharge (Rossattoet al.,2012;Songet al.,2016b).The various controls(e.g.,soil evaporation,precipitation infiltration,and groundwater fluctuation) on the isotopic composition of soil water emphasize the spatial in addition to the temporal variability inδ2H andδ18O of water within the vadose zone.
Seasonal changes and driving factors of xylem water sources
The stable isotope technique combined with the Mix-SIAR model provides an effective method for quantifying the contributions of different water sources to plant water uptake(Nieet al.,2012;Brinkmannet al.,2018;Zhaoet al.,2020).Recent studies have shown the limitation of using stable isotopes to distinguish plant water sources owing to the isotopic mismatch between xylem water and soil water(Barbetaet al.,2022;De La Casaet al.,2022).There are several reasons for such isotopic mismatch:i)the water extraction technique(Orlowskiet al.,2018) and ii) isotopic fractionation during plant water uptake,especially in loamy and clay soils with different types of soil water,i.e.,mobile water,plantavailable water,and bound water(Zhao and Wang,2021).In our study,the high extraction efficiency(>98%)and the use of IRMS to analyze the isotopic composition of xylem water effectively eliminated isotopic fractionation (Brandet al.,2009).Moreover,water in sandy soils with loose structure tended to be more mobile,avoiding the existence of“two-water world”(McDonnell,2014),selective extraction of soil water,and isotopic fractionation during root water uptake(Songet al.,2016a).Consequently,the study of plant water uptake using stable isotopes in the Mu Us sandy land is reliable,and the results provide important insights into the origin and proportional water use of different sources.
The results of MixSIAR analysis indicated that in the early(June)dry season,P.sylvestris,A.pedunculata,andS.psammophilamainly absorbed water from deep soil(i.e.,from the moist layer) and groundwater,with total proportional contributions of 83.1%,81.8%,and 61.1%,respectively.This was attributed to the low precipitation,high air temperature,and strong evaporation(Fig.2)resulting in low moisture content in shallow soils(Fig.3)that cannot meet plant water demands during this period(Songet al.,2020).Previous studies have shown that trees and shrubs in water-limited ecosystems rely primarily on water from the deep soil or on groundwater throughout the dry season(Songet al.,2016a;Brinkmannet al.,2018;Zhaoet al.,2020).However,the three plant species changed water source in the late (October-November) dry season (Fig.6),which is inconsistent with previous results.Compared to June,the three plant species showed a decreasing dependence on groundwater in October and November,with the proportional contributions decreasing by 20.2%-31.1%,17.8%-23.6%,and 11.0%-20.5%forP.sylvestris,A.pedunculata,andS.psammophila,respectively;accordingly,the contributions of water from the shallow soil,i.e.,the active layer,increased by 12.0%-20.8%,16.5%-23.5%,and 20.7%-45.3%,respectively.Two explanations have been put forward.i)Plant water demand decreases at the end of growing season due to decreasing air temperature and evapotranspiration(Fig.2)and ii)Plants tend to preferentially absorb water from shallow soil in order to reduce the energy requirements during the process of water transport from roots to leaves(Hasselquist and Allen,2009).
Therefore,there was a shift in water use from a predominantly deep-water source,i.e.,the moist layer and groundwater,during the early(June)dry season to a predominantly shallow-water source,i.e.,the active and stable layers,during the rainy season(July-September).Notably,the water uptake fraction byP.sylvestris,A.pedunculata,andS.psammophilafrom the active layer in the rainy season(July-September)increased by 1.1-6.0,2.3-6.4,and 1.4-1.9-fold,compared to the early (June) dry season.This was attributed to the dimorphic root systems of plants in water-limited regions,which enables them to switch between shallow and deep water sources in different seasons(Sperry and Hacke,2002;Prietoet al.,2010;Beyeret al.,2018).Many plants,e.g.,arbors and shrubs,in arid/semiarid regions have functionally dimorphic root systems(Dawson and Pate,1996;Hoekstraet al.,2014;Yanget al.,2015).Taproots can obtain deep soil water or groundwater when shallow soil water is deficient,as in the dry season,whereas lateral roots can absorb shallow soil water when it is frequently replenished by precipitation during the rainy season(Asbjornsenet al.,2008;Nieet al.,2012).The results of our investigation showed that more than 70%of the collected root biomass of the three plant species was distributed in the active layer of the stands and that more than half of the collected roots were fine roots(Fig.7),which enabled the plants under study to obtain shallow soil water during the rainy season.Furthermore,it is less energy demanding for plants to absorb shallow soil water because it is more accessible and easily restored by precipitation than deep soil water(Songet al.,2016a).In our study,the roots ofP.sylvestrisextended through the entire soil profile down to the groundwater,whereas the roots ofS.psammophilaandA.pedunculatawere mainly distributed in the active and stable layers (Fig.7).Particularly,P.sylvestrisshowed a higher water demand and transpiration thanS.psammophilaorA.pedunculata(Peiet al.,2020;Songet al.,2020).Differences in plant water demand and root distribution explain whyP.sylvestrishad a greater dependence on groundwater thanS.psammophilaandA.pedunculata(Fig.6).

Fig.7 Vertical distribution of fine roots(diameter(d)<2 mm)and coarse roots(d >2 mm)for plant species Pinus sylvestris,Amygdalus pedunculata,and Salixpsammophila.The black solid,dashed,and dotted lines represent the average groundwater level(GL)of P.sylvestris,A.pedunculata,and S.psammophila stands,respectively.
Implications for vegetation restoration and water management
Desertification is one of the most serious environmental problems in arid and semi-arid regions in the world (Liet al.,2013;Cherletet al.,2018).Balancing vegetation restoration and water use to maintain a sustainable vegetated ecosystem remains a significant challenge in desertification control (Asadallaet al.,2021).Large-scale afforestation using trees with high water demand occurs commonly in desert regions(Haliket al.,2019),because trees are more beneficial in controlling wind erosion and stabilizing desert sands than grasses(Sunet al.,2021).However,excessive afforestation,e.g.,over-planting trees at high density,can have negative effects on water resources,especially when water consumption is higher than water supply (Karnieliet al.,2008;Wang Y Qet al.,2015;Huang and Shao,2019).The key goal of studying plant water uptake in desert ecosystems is to provide a solid theoretical foundation for designing rational and sustainable vegetation restoration and water management strategies(Wanget al.,2017;Zangiabadiet al.,2020).The water-uptake patterns of different sandfixing plants,as estimated using the MixSIAR model,varied with the growing season.The three plant species,P.sylvestris,A.pedunculata,andS.psammophilamainly used water from the soil active and stable layers during the rainy season,whereas they switched to water sources into the deeper soil-moist layer and groundwater in the dry season(Fig.6).This suggests that these three plant species can adapt to seasonal drought by changing water sources.Furthermore,positive correlations between the contribution of water from the active layer and cumulative monthly precipitation(Fig.S4,See Supplementary Material for Fig.S4)indicated the importance of precipitation for shallow soil-water replenishment and subsequent plant water uptake.Consequently,reducing evaporative water loss from shallow soils,as,for example,by mulching(Flint and Childs,1987),is essential for increasing soil water storage and reducing drought stress.Other desert regions with limited precipitation could use similar practices to prevent evaporative water loss from the shallow soil layers.
In addition to soil water,groundwater is important for plant water uptake in arid desert regions because potential evapotranspiration largely exceeds precipitation (Karnieliet al.,2008;Liuet al.,2010).Our results indicated that all three species (P.sylvestris,A.pedunculata,andS.psammophila)showed the ability to absorb groundwater throughout the growing season,but the fraction of groundwater uptake varied with time and among species.The proportional contribution of groundwater to plant water uptake peaked in the early(June)dry season(Fig.6)and was the highest inP.sylvestris(54.8%),followed byA.pedunculata(38.8%)andS.psammophila(36.5%).Variation in water uptake patterns may lead to ecological niche separation and the complementary use of different water sources(Asbjornsenet al.,2008).This,in turn,facilitates species coexistence and improves water use efficiency.Nevertheless,improper(e.g.,introducing high-water-demand plants)and excessive(e.g.,overplanting)afforestation in desert regions can cause severe soil water deficits and groundwater depletion(Ohteet al.,2003;Luoet al.,2020).Our results demonstrate that the contribution of groundwater to plant water uptake is positively correlated with the depth of the groundwater level(Fig.S4),indicating that increasing plant water uptake can lead to a decline in groundwater.Given the increasing water shortage in desert regions,trees with high water consumption,such asP.sylvestris,are unsuitable for large-scale afforestation.Additionally,thinning is necessary in densely populatedP.sylvestrisstands with greater water consumption to alleviate severe soil desiccation and groundwater decline.As for shrubs,it is recommended that vegetation restoration and water use be balanced to maintain a sustainable vegetated ecosystem according to the re-vegetation threshold.
The stable isotope technique provided a quantitative interpretation of plant water use from various water sources.This method explicitly contributes to the field water cycle and provides useful insights for the design of optimal watermanagement practices.This study considered the influence of environmental factors(e.g.,soil evaporation,precipitation infiltration,and groundwater fluctuation)and plant ecological characteristics(e.g.,root distribution,dimorphic root systems,and plant water demands)on water use patterns.However,the possible effects of sampling time on the results have not been fully evaluated.In addition,recent work has shown that there is an apparent distinction between tightly bound and more mobile soil water(Sprengeret al.,2016),and that plants might favor one over the other(McDonnell,2014).Therefore,it is important to sample potential soil water sources held across the variability of soil water tensions and at multiple times under heavy precipitation events to clarify the effects of soil water heterogeneity on plant water uptake.Moreover,the stable isotope technique can be coupled with sap-flow measurements,water-energy balance,and model simulations to characterize plant water consumption and partition evapotranspiration.These studies can provide important insights into plant-soil water relationships,and are crucial for the improvement of water resources management.
CONCLUSIONS
This study used stable isotopes(δ2H andδ18O)to reveal the dynamic water-use patterns of three plant species during the growing seasons of 2018 and 2019 in the northeastern Mu Us sandy land,China.The three plant species had different water sources in the dry and rainy seasons.P.sylvestris,A.pedunculata,andS.psammophilaabsorbed more than 60%of water from the moist layer and groundwater in the early(June)dry season,and then switched to the active and stable layers for water(50.1%-62.5%)in the rainy season(July-September).From October to November in the late dry season,P.sylvestris(54.5%-6.2%)andA.pedunculata(52.9%-63.6%)mainly used water from the stable and moist layers,whileS.psammophila(52.6%-70.7%)still extracted water predominantly from the active and stable layers.Variations in the soil water profile induced by seasonal fluctuations in precipitation and groundwater level and discrepancies in plant root distribution and water requirement were the main factors affecting seasonal water-use patterns of the three plant species.This study provides a useful method for identifying plant water sources in desert ecosystems affected by seasonal fluctuations in precipitation and groundwater.Further,our findings are of great significance for future vegetation restoration and water management efforts towards sustainable development in arid regions.
ACKNOWLEDGEMENTS
This study was funded by the National Natural Science Foundation of China(No.42377302),the Youth Innovation Promotion Association of Chinese Academy of Sciences(No.2019052),the Bingwei Outstanding Young Talent Project from the Institute of Geographical Sciences and Natural Resources Research,China (2017RC203),and the Open Foundation of State Key Laboratory of Urban and Regional Ecology,China(SKLURE2023-2-2).We thank Mr.Ronglei Li for his kind assistance in field sampling and collection.Laiming Huang would like to thank his wife Libo Shen and his twin son and daughter Yaoren Huang and Yaoqi Huang for their powerful spiritual support.
SUPPLEMENTARY MATERIAL
The Supplementary Material for this article can be found in the online version.
- Pedosphere的其它文章
- Developing the new soil science-Advice for early-career soil scientists
- Biophotoelectrochemistry:An emerging frontier for channeling photoelectric effect into darkness zone of soils and sediments
- Balancing machine learning and artificial intelligence in soil science with human perspective and experience
- Soils in extraterrestrial space:Need for studies under microgravity
- Role of biochar in raising blue carbon stock capacity of salt marshes
- Long-term fertilizer nitrogen management-Soil health conundrum

