Pancreatic panniculitis as the first presentation of pancreatic ductal adenocarcinoma
Wei-Fang Zhu,Shan Fang,Jian-Jun Qiao
Department of Dermatology, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China
TotheEditor:
Pancreatic panniculitis,also known as pancreatic fat necrosis or enzymatic panniculitis,is a rare type of panniculitis that occurs in 0.3%-3% of patients with pancreatic disease such as acute or chronic pancreatitis and pancreatic carcinoma,especially acinar cell carcinoma [1,2].The clinical manifestations are nonspecific erythema tender nodules,which need to be distinguished from other types of panniculitis.Subcutaneous fat necrosis may present before the diagnosis of pancreatic disease in up to 45% of patients [2].In addition,nearly half of pancreatic panniculitis is associated with malignancy and is considered a marker of poor prognosis [3].Therefore,these nodules serve as an early and valuable marker for discovering related pancreatic diseases which leads to skin biopsy,serum pancreatic enzyme,tumor marker detection,and imaging examination.We herein report a case of pancreatic panniculitis in both lower limbs presented in the context of pancreatic ductal adenocarcinoma (PDAC) with distant metastasis,and the skin lesions subsided after FOLFIRINOX (a combination of 5-fluorouracil,leucovorin,irinotecan,and oxaliplatin) chemotherapy.
A 73-year-old female had no family history of specific diseases or genetic disorders,except for a 10-year history of hypertension.The patient had developed multiple dark red,painful nodular-like lesions on bilateral calves for 5 months,without fever,chills,joint swelling.The lesions were not significantly relieved by the topical application of a strong corticosteroid cream,but gradually increased and fused.In addition,in the past 3 months,the patient repeatedly felt a dull pain in the upper abdomen,accompanied by poor taste and appetite.The body weight was decreased by nearly 8 kg.Physical examination showed disseminated dark red nodules,pigeon eggs to palm size,partially fused,with poorly defined borders on both calves (Fig.1).The surface of the lesions was slightly warm and painful on pressure.A deep tissue biopsy of a protrusive nodule on the medial side of the left calf was performed.Histopathology revealed a widening of the subcutaneous adipose space,large necrotic areas of adipose tissue with “ghost cells” and foamlike cells,and infiltration of lymphocytes,neutrophils,and eosinophils in the adipose lobules and around the blood vessels(Fig.2).Laboratory tests showed elevated serum amylase (535 U/L),lipase (727 U/L),carbohydrate antigen 19–9 (CA19–9,584.8 U/mL),and ferritin (644.4 ng/mL).Abdominal computed tomography (CT)revealed occupancy of the pancreatic head and uncinate process with enhancement scan strengthening,and pancreatic duct dilatation (Fig.3).Multiple enlarged lymph nodes around the pancreas,retroperitoneum,and mesenteric root were markedly enhanced on the enhancement scan.The volume of the pancreatic body and tail was decreased significantly.Multiple low signals were detected in the liver with a marked enhancement of the edges on the enhancement scan.In addition,CT angiography showed that the uncinate process of the pancreas was occupied and a cancer thrombus was formed in the superior mesenteric vein.Intrahepatic metastasis,peritoneal implantation metastasis,and peritoneal lymph node metastasis were suspected.Magnetic resonance cholangiopancreatography showed abnormal signals in the pancreatic head with dilatation of the pancreatic duct and common bile duct.All these images suggested pancreatic cancer with distant metastasis.To further confirm the diagnosis,we did pancreatic ultrasound-guide fine needle biopsy,and the histology showed heterogeneous glands visible in the fibrous tissue with infiltrative growth.The nuclei were enlarged with an irregular nuclear pattern and nucleoli were visible (Fig.4).The diagnosis was PDAC stage IV.

Fig.1. Multiple dark red subcutaneous nodules were scattered in both lower limbs,with pigeon eggs (white arrows) to palm size,partially fused,and unclear boundaries.A and B: before treatment;C and D: after treatment,all lesions were almost invisible.
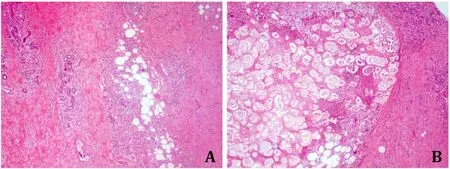
Fig.2. Histopathology of subcutaneous nodules (hematoxylin-eosin staining,original magnification×40).A: Lobular panniculitis.B: Fat necrosis was characterized by the presence of “ghost cells”: anucleated cells containing intracytoplasmic fine basophilic granulations.Fat lobules and around blood vessels were infiltrated by lymphocytes,neutrophils,and eosinophils.

Fig.3. Abdominal contrast-enhanced computed tomography.A: A plain scan showed a slightly low-density mass.B: The arterial phase of the enhanced scan showed mild uneven enhancement of the mass.C and D: The portal and equilibrium phases showed that the progressive enhancement of the mass was not obvious.

Fig.4. Pancreatic puncture histopathology (hematoxylin-eosin staining,original magnification×20).Heteromorphic gonadal bodies were observed in the fibrous tissue,showing infiltrating growth.The nuclei were enlarged with irregular nuclear patterns and nucleoli were visible.
After assessing the patient’s health status,we administered the first-line chemotherapy regimen FOLFIRINOX.As a result,serum amylase,lipase,and CA19–9 returned to normal levels in the patient after 2,3,and 4 sessions of chemotherapy,respectively.At the same time,pancreatic panniculitis lesions also gradually subsided.Abdominal CT reexamination after 4 rounds of chemotherapy revealed significant shrinkage of the pancreatic tumor and peripancreatic,retroperitoneal,and mesenteric lymph nodes.Meanwhile,multiple metastases in the liver disappeared.At present,the patient was still maintaining the chemotherapy regimen.
Panniculitis,clinically manifested as non-specific erythema tender nodules,is a histopathological description characterized by subcutaneous adipose tissue inflammation [4].It may be a primary disease,an underlying infection,a drug reaction,an autoimmune disease,a metabolic disorder,or a malignant tumor manifestation [5].Moreover,considerable clinical and histopathological overlap exists,requiring diagnosis in conjunction with history and other auxiliary tests.In our case,pancreatic tumor and pancreatic enzyme elevation were found after the diagnosis of panniculitis,and the lesion was resolved after tumor treatment.Therefore,pancreatic panniculitis is the most appropriate diagnosis.
The pathogenesis of pancreatic panniculitis has not been fully elucidated.One possible mechanism is that pancreatic enzymes,including trypsin,lipase,and amylase,are released in large quantities into the blood leading to increased vascular permeability with consequent excess accumulation of free fatty acids,and ultimately fat necrosis and inflammation [6,7].It has been demonstrated that amylase and lipase by themselves are not capable of leading to panniculitis [7],while free fatty acids can induce systemic inflammatory responses related to adipose tissue by activating macrophages and protein kinase C (PKC)-NF-κB axis,increasing the release of proinflammatory factors such as interleukin-6 and tumor necrosis factor-α[8].Another possibility is that some patients may have inherent enzyme deficiencies that prevent individuals from degrading pancreatic enzymes,such asα-1 antitrypsin deficiency-associated panniculitis [9].In addition,the deposition of antigen-antibody immune complexes has also been proposed to participate in the pathogenesis [10].
The subcutaneous adipose tissue necrosis appears as disseminated,erythematous tender nodules that may spontaneously resolve or ulcerate,expelling an oily,brown,sterile,viscous substance [2].They are usually located in the lower extremities,occasionally in the scalp,upper extremities,trunk,or even throughout the body,and can occur before,during,or after pancreatic disease [1].Clinically,it should be distinguished from various panniculitis,especially erythema nodosum,nodular vasculitis,lupus panniculitis,and sarcoidosis-associated panniculitis.Therefore,the diagnosis must be based on a deep skin biopsy,necessary laboratory tests,and imaging examinations.
The typical histopathological feature is characterized by lobular panniculitis without vasculitis.“Ghost cells” are formed when pancreatic enzymes cause coagulative necrosis of adipocytes followed by loss of their nuclei,which is a specific manifestation of pancreatic lipomatosis [1,11].Moreover,due to dystrophic calcification,small basophilic particles are often seen in the “ghost cells” [1].Laboratory workup usually reveals serum pancreatic hyperenzymemia and sometimes elevated tumor markers,especially carcinoembryonic antigens [11].Notably,other pancreatic enzymes,such as amylase and trypsin,are not necessarily abnormal,but lipase is significantly elevated in all cases [12].The retrospective analysis identified a lipase concentration of 4414 U/L as the optimal threshold,providing 73% sensitivity and 82% specificity for the diagnosis of neoplastic disease [3].These sensitivity and specificity values are comparable to those of CA19–9 for distinguishing PDAC from benign pancreatic disease [3].These findings suggest that patients with pancreatic panniculitis accompanied by highlevel serum lipase should be routinely screened for neoplastic disease.Imaging examination helps to discover potential pancreatic diseases,especially pancreatic tumors.
Treatment focuses on the etiology of the underlying pancreatic disease.Non-steroidal anti-inflammatory drugs and corticosteroids are considered a purely symptom-relieving treatment,and there is no evidence that they improve prognosis [7].Tetracycline may be a potential option for localized infection in panniculitis,as tetracycline has been shown to reduce the secretion of lipase and promote the regression of subcutaneous lesions [13].Plasma exchange can effectively reduce the level of pancreatic enzyme in serum,and subcutaneous injection of octreotide (a synthetic somatostatin-like polypeptide) can inhibit the production of pancreatic enzyme,but both only show a benefit for few patients [6].Correct and timely treatment can significantly improve remission rates,but in most cases,such as the present case,pancreatic disease was not detected until 5 months after panniculitis,resulting in delayed treatment.Unfortunately,the majority of PDACs are at advanced stage at diagnosis,with distant metastasis occurring in over 50% of cases,and the most common metastatic site is liver [14].
The preferred treatment for advanced PDAC is palliative chemotherapy,and the recommended first-line chemotherapy regimens are FOLFIRINOX or Gem-nabP (gemcitabine plus nabpaclitaxel).A phase 3,multicenter trial of FOLFIRINOX versus gemcitabine in the treatment of metastatic pancreatic cancer showed a median overall survival of 11.1 months in the FOLFIRINOX group versus 6.8 months in the gemcitabine group [15].Another phase 3 clinical trial showed that the Gem-nabP group had better median overall survival (8.5 months vs.6.7 months),median progressionfree survival (5.5 months vs.3.7 months),and response rate (23%vs.7%) than the gemcitabine alone group [16].However,there are currently no randomized controlled trial to compare FOLFIRINOX and Gem-nabP.In addition,targeted therapies seem to offer some new hope,such as olaparib (a PARP inhibitor) maintenance therapy,which is now approved for PDAC patients withBRCAmutations,and drugs targeting certainKRASmutations are being diligently studied [17].
Although rare,pancreatic panniculitis can lead to early diagnosis of underlying diseases and even predict the severity of neoplastic diseases.Therefore,skin pathology,serum trypsin testing,and imaging examination should be performed as soon as possible.In addition,pancreatic panniculitis should be considered a facultative paraneoplastic disease,and tumor screening should always be included in diagnostic examinations,especially for pancreatic tumors in elderly patients.Clinicians should be aware of these skin signs in pancreatic disease to recognize them and guide treatment appropriately.
Acknowledgments
None.
CRediT authorship contribution statement
Wei-Fang Zhu:Conceptualization,Data curation,Funding acquisition,Investigation,Writing– original draft.Shan Fang:Data curation,Validation.Jian-Jun Qiao:Conceptualization,Supervision,Writing– review &editing.
Funding
The study was supported by a grant from Zhejiang Provincial Natural Science Foundation (LGF21H190004).
Ethical approval
Written informed consent for publication was obtained from the patient.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
 Hepatobiliary & Pancreatic Diseases International2024年1期
Hepatobiliary & Pancreatic Diseases International2024年1期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Pre-MASLD: Should it be defined separately?
- Comment on “Robotic surgery and liver transplantation: A single-center experience of 501 robotic donor hepatectomies”
- Benign stricture of bilioenteric anastomosis after Whipple with synthetic polypropylene suture
- A robust genomic-based prognostic model for the assessment of cancer stemness and survival for patients with hepatocellular carcinoma
- Endoscopic hemostasis using self-expandable metal stent combined with PuraStat ® for patient with high risk of post-endoscopic sphincterotomy bleeding (with video)
- Post-fontan circulation hepatocellular carcinoma: Open and laparoscopic hepatectomy
