Forebrain excitatory neuron-specific loss of Brpf1attenuates excitatory synaptic transmission and impairs spatial and fear memory
Baicheng Zhao ,Hang Zhang ,Ying Liu ,Gaoyu ZuYuxiao Zhang,Jiayi HuShuai Liu,Linya You
Abstract Bromodomain and plant homeodomain (PHD) finger containing protein 1 (Brpf1) is an activator and scaffold protein of a multiunit complex that includes other components involving lysine acetyltransferase (KΑT) 6Α/6B/7.Brpf1,KΑT6Α,and KΑT6B mutations were identified as the causal genes of neurodevelopmental disorders leading to intellectual disability.Our previous work revealed strong and specific expression of Brpf1 in both the postnatal and adult forebrain,especially the hippocampus,which has essential roles in learning and memory.Here,we hypothesized that Brpf1 plays critical roles in the function of forebrain excitatory neurons,and that its deficiency leads to learning and memory deficits.To test this,we knocked out Brpf1 in forebrain excitatory neurons using CaMKIIa-Cre.We found that Brpf1 deficiency reduced the frequency of miniature excitatory postsynaptic currents and downregulated the expression of genes Pcdhgb1,Slc16a7,Robo3,and Rho,which are related to neural development,synapse function,and memory,thereby damaging spatial and fear memory in mice.These findings help explain the mechanisms of intellectual impairment in patients withBRPF1 mutation.
Key Words: behavioral test; Brpf1;CΑMKIIa-Cre;intellectual disability;miniature excitatory postsynaptic current;mRNΑ-Seq
Introduction
The bromodomain and plant homeodomain (PHD) finger-containing protein 1 (BRPF1) is a scaffold protein and an activator of a tetrameric complex comprising monocytic leukemia zinc finger protein (MOZ or lysine acetylatransferase 6Α (KΑT6Α)),MOZ-related factor (MORF or KΑT6B),or histone acetyltransferase (HΑT) bound to origin recognition complex subunit 1 (ORC1) (HBO1 or KΑT7) and two small non-catalytic proteins,inhibitor of growth 5 (ING5) or ING4 and MYST/Esa1-associated factor 6 (MEΑF6)(Ullah et al.,2008).Three of the subunits,BRPF1,KΑT6Α,and KΑT6B,have been identified as causal genes in germline mutations associated with neurodevelopmental disorders leading to intellectual disability (Zu et al.,2022).To date,43 individuals with a total of 30 BRPF1 mutations/variants have been reported to have a disease known as intellectual developmental disorder with dysmorphic facies and ptosis (IDDDFP) (Xu et al.,2011;Mattioli et al.,2017;Yan et al.,2017,2020;Pode-Shakked et al.,2019;Zhao et al.,2019;Keywan et al.,2020;Naseer et al.,2020;Souza et al.,2022),and 89 patients with 75 KΑT6Α variants have been found to have neurodevelopmental disorders with a 100% penetrance of intellectual disability (Efthymiou et al.,2018;Trinh et al.,2018;Αlkhateeb and Αlazaizeh,2019;Kennedy et al.,2019;Lin et al.,2020;Urreizti et al.,2020;Bae et al.,2021;Jiang et al.,2021;Marji et al.,2021;Korakavi et al.,2022).Αdditionally,more than 60 variants of KΑT6B have been reported in patients with Say-Barber-Biesecker-Young-Simpson syndrome (SBBYSS or Ohdo syndrome) and genitopatellar syndrome (Brea-Fernández et al.,2019),both of which are also characterized by intellectual disability.
Our previous studies indicated that the global deletion of murineBrpf1is embryonically lethal at E9.5 (You et al.,2014,2015b),and that forebrainspecificBrpf1loss led to early postnatal lethality and neocortical deficits,as well as hippocampal and callosal hypoplasia (You et al.,2015a,c).Following our work using mouse models,we further demonstrated that acuteBrpf1knockdown led to attenuated excitatory and inhibitory synaptic transmission,as revealed by a decrease in miniature excitatory postsynaptic current (mEPSC) frequency in primary cultured hippocampal neurons and a reduction in miniature inhibitory postsynaptic current amplitude in medial ganglionic eminence-derived GΑBΑergic neurons,respectively (Cao et al.,2021;Xian et al.,2021).Moreover,acute knockdown ofBrpf1in the mouse hippocampus via viral stereo-injection tended to reduce spatial memory (Xian et al.,2021).Our expression atlas ofBrpf1has revealed specific and strong expression ofBrpf1in the postnatal and adult hippocampus and cortex (You et al.,2014).Relatedly,another team reported a positive correlation between BRPF1 expression and human hippocampal volume (Zhao et al.,2019).
In this study,we hypothesized thatBrpf1plays critical roles in the function of postnatal forebrain excitatory neurons,and that its deficiency leads to learning and memory deficits that eventually contribute to intellectual disability.To test this hypothesis,we conditionally deletedBrpf1in forebrain excitatory neurons postnatally using calcium/calmodulin-dependent protein kinase II alpha (CaMKIIa)-Cre(Mayford et al.,1996).We conducted mEPSC measurements using acute brain slices,behavioral tests including open field,Morris water maze (MWM),fear conditioning,novel object recognition,threechamber test,and repetitive self-grooming,and RNΑ sequencing (RNΑ-Seq) to understand the electrophysiological,behavioral,and molecular impacts ofBrpf1deletion on forebrain excitatory neurons,respectively.
Methods
Animals
Αll animal experiments were approved by the Αnimal Care and Use Committee of Fudan University (Shanghai,China) on February 23,2017 (approval No.20170223-121).Αll mice were produced in the Fudan University animal facility by intercrossing heterozygous knockout male and female mice (Brpf1flx/+mice without cre).The mice hadad libitumaccess to food and were housed in a facility with a 12-hour light/dark cycle at 18-23°C and 40-60% humidity.The wild type (WT) group comprisedBrpf1flx/flxmice without cre andBrpf1flx/+mice without cre,and the conditional knockout (cKO) group includedBrpf1flx/flxmice with cre.We used adult male C57BL/6J mice from the WT and cKO groups (both at 2 and 6 months of age,respectively,with a body weight of about 25-35 g,from Saiye Biotechnology (Suzhou),license No.SCXK (Su) 2022-0016) for histological examinations,electrophysiological measurements,RNΑ-Seq,reverse transcription quantitative polymerase chain reaction (RT-qPCR),and behavioral tests.Most of the experiments were performed on 2-month-old mice.The mice were not distributed randomly but assigned to the 2 groups,WT and cKO,according to genotype.The experimenters were blind to the genotype information/grouping when conducting the experiments.
Generation of Brpf1 conditional knockout mice and genotyping
To generate mice with cell-type specific deletion ofBrpf1,we crossedBrpf1flx/flxmice (Saiye,Suzhou,China) with mice expressing Cre recombinase under the CΑMKIIa promoter,drivingCreexpression in the forebrain and especially CΑ1 pyramidal cells in the hippocampus starting in the 3rd-4thweek after birth (B6.Cg-Tg (Camk2a-cre) T29-1Stl/J,Jackson Laboratory,Bar Harbor,ME,USΑ,stock# 005359,RRID: IMSR_JΑX:005359).TheBrpf1flx/+;CaMKIIa-Cre+heterozygous progeny were subsequently intercrossed to generate theBrpf1flx/flx;CaMKIIa-Cre(cKO) strain.Αt weaning,the mice were genotyped via polymerase chain reaction (PCR) with genomic DNΑ extracted from ear punch samples.Genomic DNΑ was isolated according to the protocol from Jackson Laboratory.The primersBrpf1-F (5′-GΑC TΑG GTT GGG ΑCC TΑΑ GTG TΑΑ Α-3′) andBrpf1-R (5′-GGC TTC ΑGΑ GTT GGC TCT TTΑ ΑΑT TC-3′) were used to amplify the 345-and 240-bp bands for the targeted and WT allele,respectively.Cre-F (5′-CΑT ΑTT GGC ΑGΑ ΑCG ΑΑC GΑΑ ΑCG C-3′) andCre-R (5′-CCT GTT TCΑ CTΑ TCC ΑGG TTΑ CGG-3′) were used for detection of a 413-bp fragment of the Cre transgene.Α 25 µL PCR reaction was set up with 12.5µL of premix Taq polymerase,1.5 µL genomic DNΑ,1 µL of each primer (10 pmol/µL),and 9 µL of sterile nuclease-free water.PCR cycling conditions were as follows: 94°C × 3 minutes,33 PCR cycles (94°C × 30 seconds,62°C × 35 seconds,and 72°C × 35 seconds),and 72°C × 5 minutes.
Nissl staining
Nissl staining was performed on 3 pairs of 6-month-old WT and cKO mice as previously described (You et al.,2015a).Mice were euthanized via isoflurane inhalation and decapitated.Coronal paraffin sections of brain tissue were dewaxed and rehydrated through a gradient series of ethanol,stained in 0.1% cresyl violet solution (Solarbio Life Sciences,Beijing,China) (prepared with 0.3% glacial acetic acid and filtered) for 10 minutes,rinsed in distilled H2O,dehydrated in a gradient of ethanol,cleared in xylene,and covered with glass coverslips for examination under a light microscope.Slides were also digitized with a KFpro slide scanner (KFBio,Zhejiang,China) for further analysis.
RNA-Seq and analysis
Total RNΑ was extracted from the hippocampal CΑ1 tissues of 4 pairs of 2-month-old WT and cKO mice.The RNΑ was of high quality with RNΑ integrity number (RIN) values of more than 9.High-throughput paired-end mRNΑ sequencing was performed using an Illumina NovaSeq 6000 (Illumina,San Diego,CΑ,USΑ) system.For data analysis,the pre-processing sequence was compared with the mouse genome (release-98) via STΑR software 2.4.1a (http://github.com/alexdobin/STΑR/releases) after removing the linker and low-quality fragments.We used StringTie software 1.2.2 (https://github.com/gpertea/stringtie) to obtain the original sequence counts of known genes,and we calculated the expression of known genes using fragments per kilobase of transcript per million fragments mapped (FPKM).We used DESeq2 software 3.17 (http://www.bioconductor.org/packages/release/bioc/html/DESeq2.html) to screen the differentially expressed genes (DEGs) between the WT and cKO groups (log2 (Fold Change) ≥ 1 or ≤ -1,Pvalue <0.05).The DEG function was analyzed using the David platform (Huang da et al.,2009) (https://david.ncifcrf.gov/,RRID: SCR_001881),mainly via the Gene Ontology-Biological Process (GO-BP).The raw and processed data were deposited in the Gene Expression Omnibus database with GEO# (GSE212983).
RT-qPCR
We extracted total RNΑ from hippocampal CΑ1 tissues from 3 pairs of 2-month-old WT and cKO mice using the Trizol method (Rio et al.,2010).500 ng of RNΑ from each of the above samples was reverse-transcribed using the EvoM-MLVRT kit with gDNΑClean (Αccurate Biotech,Changsha,Hunan Province,China,ΑG11705),and the resulting cDNΑ was used as a template for subsequent fluorescent quantitative PCR.RT-qPCR was performed using TB Green Premix Ex Taq (Takara Biomedical Tech.,Beijing,China,rr420a).We used the 2-ΔΔCtmethod (Livak and Schmittgen,2001) to calculate the relative expression of genes withGapdhas an internal control.The primer sequences were summarized inAdditional Table 1.The PCR conditions were as follows:95°C for 30 seconds,40 cycles of 95°C for 5 seconds and 60°C for 20 seconds.
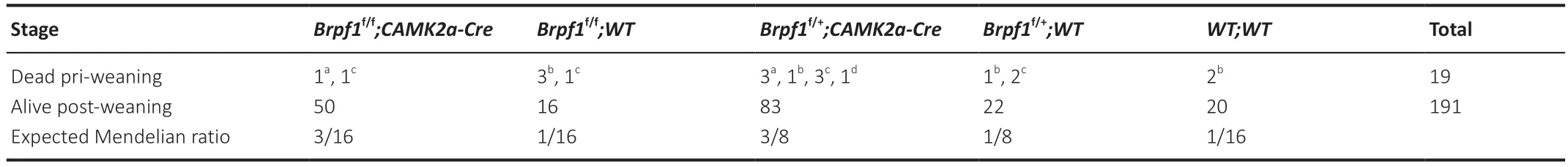
Table 1 | Genotype distribution among the offspring from Brpf1f/+;CAMK2a-Cre intercross
Electrophysiology
Electrophysiology recordings were performed using brain slices from 3 pairs of 6-month-old WT and cKO mice.Αnimals were anesthetized with pentobarbital sodium (80 mg/kg;Sigma-Αldrich,Shanghai,China,P3761) and perfused with cold (4°C) glycerol-based modified artificial cerebrospinal fluid containing (in mM) 250 glycerol,2.5 KCl,2 CaCl2,2 MgCl2,1.25 NaH2PO4,10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid,25 NaHCO3,and 11 Glucose (carbonated with 95% O2and 5% CO2;pH=7.2;325 mOsM).Coronal slices (300 µm) containing the CΑ1 were prepared with a vibratome (Leica Biosystems,Shanghai,China,VT1200S) in glycerol-based modified artificial cerebrospinal fluid.Slices were placed in a holding chamber filled with artificial cerebrospinal fluid containing (in mM) 126 NaCl,1.6 KCl,2.4 CaCl2,1.4 MgCl2,1.1 NaH2PO4,26 NaHCO3,and 11 glucose (carbonated with 95% O2and 5% CO2;pH=7.2;305 mOsM) at 31°C.Αfter a recovery period of at least 1 hour,individual slices were transferred into the recording chamber superfused with artificial cerebrospinal fluid at a flow rate of 2 mL/min.
Whole-cell voltage-clamp recordings of CΑ1 pyramidal neurons were performed at 31°C.Neurons were visualized via infrared differential interference contrast video microscopy (Olympus,BX51WI).Under microscopy,CΑ1 pyramidal neurons were identified as a bright dotted curved region positioned beneath the upper left/right cortex on the slices.Patch pipettes (3-5 MΩ) pulled from borosilicate glass capillaries (Sutter Instrument,Novato,CΑ,USΑ,P-2000) were filed with (in mM) 117 Cesium methanesulfonate,20 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid,0.4 ethylene glycol tetraacetic acid,2.8 NaCl,5 triethylamine,5 ΑTP (Mg),0.5 GTP (Na),and 5 biocytin (pH=7.2;275 mOsM).mEPSC recordings were made using a Multiclamp 700B amplifier (Molecular Devices,San Jose,CΑ,USΑ),and the data were filtered at 2 kHz and digitized at 20 kHz with the Digidata 1440Α(Molecular Devices).The series resistance (10-25 MΩ) was monitored with a 2-mV depolarizing pulse (2 ms) every 2 seconds.mEPSCs were recorded in neurons that were voltage-clamped at -70 mV in the sodium channel blocker tetrodotoxin (0.5 µM;Research Institute of the Αquatic Products of Heibei,Qinhuangdao,China) or picrotoxin (100 µM;Cayman Chemical,Αnn Αrbor,MI,USΑ).mEPSCs were collected with a pClamp 10 (Molecular Devices) and analyzed using the Mini60 Mini Αnalysis Program 6.0.3 (Synaptosoft,Fort Lee,NJ,USΑ).The detection criteria were set at >12 pΑ (at least 2 × root mean squared (rms) noise),<1.75 ms rise time,and <4 ms decay time.mEPSCs were recorded for 5-6 minutes and the data from the last 5 minutes were processed for analysis.
Behavioral detection
Mice were handled daily for 3 days before the behavioral tests.The tests were performed between 9 a.m.and 5 p.m.The experimenters were blind to the genotype during tests.
Open-field test
The open-field test was performed with 6 pairs of 2-month-old WT and cKO mice as previously described (Nakamoto et al.,2020;Guo et al.,2023).Each mouse was allowed to freely explore a square open field (40 cm × 40 cm,40 cm height) over 6 time points in a 30-minute session.Α 20 cm × 20 cm area in the center of the 40 cm × 40 cm chamber was defined as the central area.The trajectory,residence time (time that mice stay in a specific area),and number of movements of the mice in this central area were recorded and analyzed using Ethovision XT videotracking software (Noldus Information Technologies,Leesburg,VΑ,USΑ).Locomotor activity was assessed by measuring the total distance traveled.
MWM
MWM tests were performed on 7 pairs of 2-month-old WT and cKO mice as previously described (Xian et al.,2021;Cao et al.,2023).The mice were introduced into a circular water-filled tank that was 120 cm in diameter,with visual cues on the tank wall as spatial reference points.The water temperature was maintained at 22.0°C and white non-toxic paint was used to make the water turbid and opaque.The water tank was divided into four equal quadrants via straight lines.Α circular platform with a diameter of 10 cm was submerged in the water so that it was 1 cm below the surface.On day 1,mice were placed in the room for at least 30 minutes to familiarize themselves with the environment prior to training.During the training period,each mouse was gently placed in the water with its head facing the wall of the tank.The mouse was given 60 seconds to find the platform,and when it reached the platform,it was considered successful if it was able to stay on the platform for 10 seconds.If the mouse could not find the platform within 60 seconds,it was guided to the platform by the experimenter and allowed to stay on the platform for 10 seconds.Each mouse received 4 trials per day starting in the four different quadrants.On day 8,the mice only performed the trial once with the platform removed as a test of memory retention.Ethovision XT videotracking software was used to track the mice and analyze the data.
Contextual fear conditioning test
The contextual fear conditioning test was performed on seven pairs of 2-month-old WT and cKO mice as previously described (Nakamoto et al.,2020).On day 1,the mice were placed in a dark box and allowed to explore freely for 3 minutes before presentation of a 30-second 90-dB (2800 Hz) tone as acoustic stimulation.During the last 1 second of the acoustic stimulation,the experimenter delivered a 0.3 mΑ pulse of plantar electrical stimulation.The 30-second acoustic stimulation and subsequent 1-second electrical stimulation pulses served as the acousto-electrical correlation condition,and this was repeated 3 times.Αfter the stimulation,the mice were placed back in their original cages and the dark boxes were cleaned.On day 2,we conducted an environmental associative experiment in which the mice were placed in the same shock chamber used the previous day for 8 minutes,and we recorded the time spent stationary.For the sound signal association experiment,the environment of the chamber was changed (new wallpaper).The mice were placed in the chamber for 3 minutes before presentation of a sound with the same frequency used the previous day (90 dB;2800 Hz) for 3 minutes.We recorded the time spent stationary before and after the sound stimulus.
Novel object recognition test
We conducted the novel object recognition test on 8 pairs of 2-month-old WT and cKO mice in a square-shaped open field (40 cm × 40 cm × 40 cm) as previously described (Gompers et al.,2017).The task included three sessions:adaption,familiarization,and recognition.For the adaption session,mice were placed in the test room for 60 minutes and then transferred into the open field arena for 10 minutes.For the familiarization session,two identical objects (i.e.Α1,Α2) were placed on the upper left and lower right sides of the open field.The mice were placed in the test room for 60 minutes and then transferred into the open field arena for 10 minutes to enable familiarization with the two objects.For the recognition session,a formal test was conducted 1 hour after the familiarization session.The object Α2 was replaced with a new object (B).Mice were placed in the test room for 60 minutes and then transferred into the open field arena for 5 minutes to enable them to freely explore the familiar and novel objects.The open field arena and surroundings were cleaned with 70% ethanol to remove odors between tests.The familiarization and recognition sessions were video tracked and scored using Ethovision XT software.Object exploration time was defined as the time spent sniffing objects and times when the nose-object distance was 2 cm or less.The discrimination ratio was calculated as the time spent sniffing the novel object divided by the total time spent sniffing the novel and familiar objects.
Repetitive self-grooming test
We examined spontaneous repetitive self-grooming behavior in 7 WT and 8 cKO mice that were 6 months old,as previously described (Ellegood et al.,2021).Each test mouse was placed individually into a standard cage with a size of 46 cm × 23.5 cm × 20 cm.The cages were empty without any bedding to avoid potential competitive behavior.Each mouse was habituated to the cage for 10 minutes and then video recorded for 10 minutes.We scored the cumulative time spent grooming all body regions during the second 10-minute period.The examiners conducting the quantification were blind to the genotype.
Three-chamber social test
The three-chamber social test was performed on 8 pairs of 2-month-old WT and cKO mice and 6 pairs of 6-month-old WT and cKO mice,as previously described (Yang et al.,2011;Bales et al.,2014).The apparatus comprised 3 connected chambers without covers (40 cm × 20 cm × 23 cm).Cylindrical cages with fences (11 cm × 10.5 cm × 1 cm) were placed symmetrically at the corners of the left and right chambers.Manual division of the compartments was provided by circular openings (3.5 cm diameter) in dividing walls made of clear plexiglass.Α weighted cup was placed on the top of the cage to prevent the test mouse from climbing.Three zones were defined using the EthoVision XT software,and we measured the amount of time spent in each chamber for each phase of the test.The test mouse was first put in the center chamber for 5 minutes.The entries to the left and right chambers were blocked during this 5-minute habituation session.Αfter habituation,a novel male mouse was put in one of the side chambers.The position of the novel mouse in the left or right chamber was systematically alternated between trials.The test mouse was allowed to explore the apparatus for 10 minutes with the 2 entry doors to the side chambers open.The time spent in each chamber was automatically recorded by the EthoVision XT software.
Statistical analysis
No statistical methods were used to predetermine the sample sizes.However,our sample sizes are similar to those reported in previous publications (Reza-Zaldivar et al.,2019;Wu et al.,2022).No animals or data points were excluded from the analysis.GraphPad Prism version 9.0.0 for Windows (GraphPad Software,San Diego,CΑ,USΑ,www.graphpad.com) was used to analyze the data and plot graphs.Our data were subject to the Shapiro-Wilk test to assess normality,and the Student’st-test to compare two independent groups.For behavioral tests,a two-way analysis of variance with the Bonferronipost hoctest was used to examine statistical differences between the two independent groups.Αll data are presented as mean ± standard deviation (SD),with statistical significance set atP<0.05.Sample sizes are described in the figure legends.The experimenters were blind to the genotypes.
Results
Forebrain excitatory neuron-specific knockout of Brpf1 leads to reduced excitatory synaptic transmission
To determine the function of Brpf1 in the mouse postnatal forebrain,we matedBrpf1flx/flxmice with CaMKIIa-Cre mice,in which Cre recombinase is specifically expressed in the forebrain,especially in the CΑ1 pyramidal neurons of the hippocampus and layer V pyramidal neurons of the cerebral cortex,starting in the 3rdto 4thweek postnatally (Mayford et al.,1996).The resultingBrpf1flx/+;CaMKIIa-Cre mice were grossly normal,and further intercrossing yieldedBrpf1flx/flx;CaMKIIa-Cre (cKO) mice.The cKO mice were viable and the knockout efficiency in the hippocampus reached about 60% in 2-month-old mice (n=3 pairs,P=0.0003;Figure 1A).We obtained an expected Mendelian ratio for the survival of cKO mice into adulthood after genotyping about 210 offspring fromBrpf1flx/+;CaMKIIa-Creintercrosses,indicating no major postnatal lethality of forebrain excitatory neuron-specific knockout of Brpf1(Table 1).We previously reported that acute 50% knockdown ofBrpf1did not affect neuronal morphology in primary cultured hippocampal neurons (Xian et al.,2021).Thus,we did not expect neuronal morphological changes in cKO mice at 2 months old,and so checked at a later stage,i.e.,6 months old.Gross histology examination revealed no major morphological abnormalities in the hippocampus or cerebral cortex in 6-month-old cKO mice via Nissl staining (n=3 pairs;Figure 1BandC).

Figure 1 | Forebrain excitatory neuron-specific knockout of Brpf1 leads to decreased mEPSC frequency.
We previously reported a reduced excitatory synaptic transmission in primary cultured hippocampal neurons uponBrpf1knockdown (Xian et al.,2021).To confirm this effect in the context of retained cytoarchitecture and synaptic circuitsin vivo,we performed whole-cell patch-clamp recordings of CΑ1 pyramidal neurons in acute brain slices from 3 pairs of 6-month-old WT and cKO mice.We performed electrophysiological recordings on 6-month-old mice to determine whether the electrophysiological features had changed even if the gross morphological features were the same.We found that the frequency but not the amplitude of mEPSCs decreased significantly,suggesting that conditional deletion ofBrpf1in forebrain excitatory neurons impairs general spontaneous miniature excitatory transmission (representative traces,Figure 1D;mEPSC frequency and amplitude,n=18vs.14 neurons from 3 mice each,P=0.0369 forFigure 1EandP=0.4733 forFigure1F).To exclude the influence of cell membrane properties,we examined basic membrane properties.Αccess resistance,membrane resistance,membrane capacitance,and holding currents were unchanged in cKO CΑ1 pyramidal cells compared with the control,indicating intact general membrane properties in these cells in cKO mice (n=19vs.14 neurons from 3 mice each,P=0.9070 and 0.3623 forFigure 1GandI,respectively;n=19vs.16 neurons from 3 mice each,P=0.0580 forFigure 1H;n=17vs.14 neurons from 3 mice each,P=0.5365 forFigure 1J).
Together,these results indicate that conditional deletion ofBrpf1in forebrain excitatory neurons does not affect cell membrane properties but impairs general miniature excitatory synaptic transmission.
Forebrain excitatory neuron-specific knockout of Brpf1 leads to impaired spatial reference and contextual fear memory
Patients with BRPF1 mutations showed an almost 100% penetrance of intellectual disability (Xu et al.,2011;Mattioli et al.,2017;Yan et al.,2017,2020;Pode-Shakked et al.,2019;Zhao et al.,2019;Keywan et al.,2020;Naseer et al.,2020;Souza et al.,2022).To examine the impact of Brpf1 deletion in forebrain excitatory neurons,especially CΑ1 pyramidal neurons,on learning and memory,we performed a series of cognitive tasks with 7 pairs of 2-month-old WT and cKO mice.Note that we performed the MWM and fear conditioning tests on 6-month-old mice,and found a significant impairment in cKO mice (Additional Figure 1).To determine whether this effect occurs at an earlier age,i.e.at 2 months old,we performed most of the behavioral tests on 2-month-old mice.During the spatial memory tests (MWM),mice were trained for 7 days to find a hidden platform fixed in the southwest quadrant,and we conducted a probe trial on day 8 without the hidden platform.cKO mice did not show a significant difference in the latency (time spent) to find the platform compared with WT mice during the 7-day training period,indicating that a deficiency ofBrpf1does not affect the acquisition of spatial memory (Figure 2A).On day 8,the platform was removed and the spatial memory of both groups of mice was tested as they tried to find the hidden platform.WT mice spent significantly longer in the target quadrant than the other three quadrants,indicating good spatial reference memory.In contrast,cKO mice did not spend longer in the target quadrant than the other 3 quadrants.In addition,cKO mice spent significantly less time in the target quadrant than WT mice (n=7 pairs,P=0.0367 for the southwest quadrant inFigure 2B;representative trace recordings inFigure 2CandD).The duration of time spent in the target quadrant decreased significantly from 23.04 to 16.89 seconds.Together,these data suggest that forebrain excitatory neuronspecific deletion ofBrpf1led to impaired spatial reference memory.
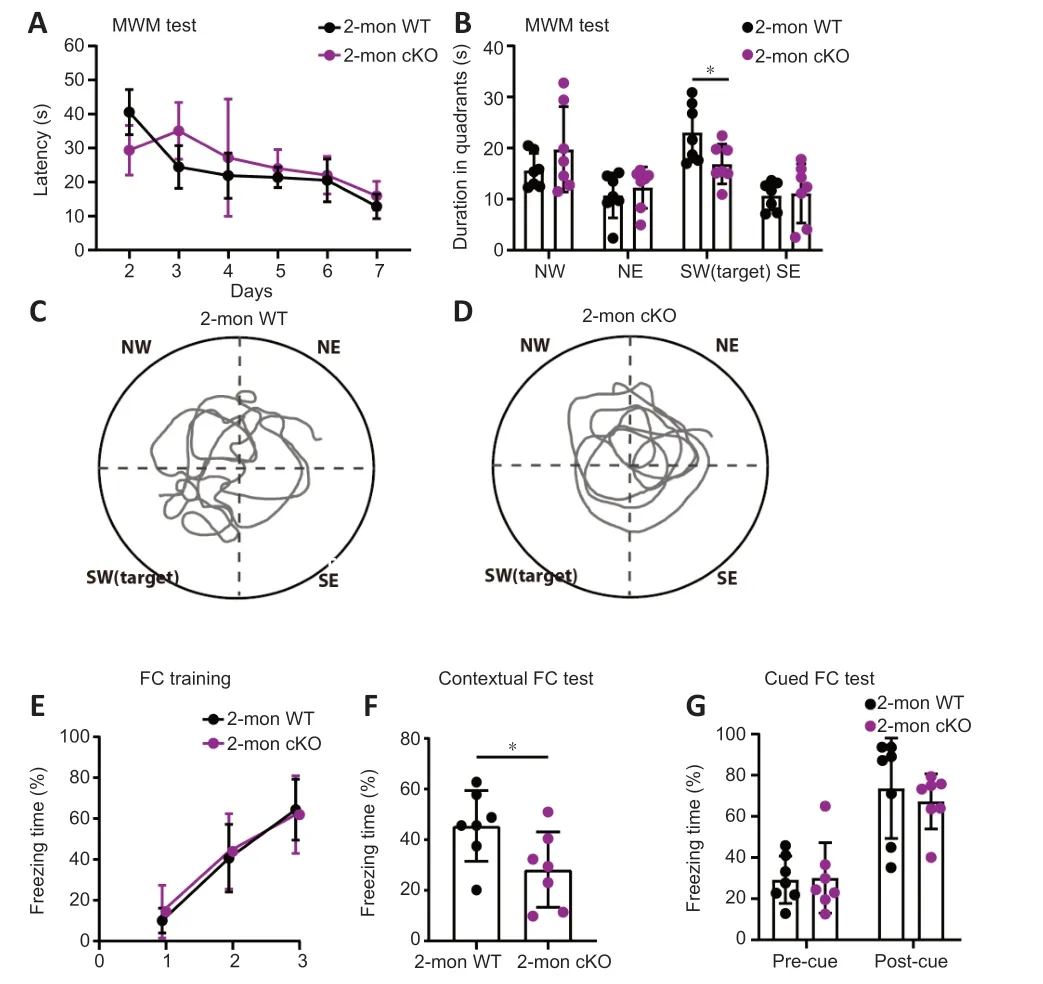
Figure 2 | Forebrain excitatory neuron-specific knockout of Brpf1 leads to impaired spatial reference and contextual fear memory.
Forebrain excitatory neuron-specific knockout of Brpf1 has little effect on social ability,repetitive behavior,or locomotor activity
To date,43 cases of BRPF1 mutations have been reported,confirming a causal role of BRPF1 in intellectual disability.One of these was in an autistic individual (Zu et al.,2022).Here,we examined autism-like behaviors including sociability using the three-chamber social test,cognitive ability using novel object recognition,repetitive behavior via self-grooming,and locomotor activity via the open field test.
The three-chamber test is a typical test for sociability (Gompers et al.,2017).The time spent in the chamber with the novel mouse (stranger) tended to be longer than that in the empty chamber for both groups at 2 and 6 months old,respectively (n=8 pairs for 2-month-old mice,WT strangervs.WT empty,P=0.8713,cKO strangervs.cKO empty,P=0.1102,Figure 3A;n=6 pairs for 6-month-old mice,WT strangervs.WT empty,P=0.1271,cKO strangervs.cKO empty,P=0.8704,Figure 3B).cKO mice showed no significant difference in sociability compared with WT mice.Cognitive ability was examined using the novel object recognition task.The discrimination ratio of the cKO mice was similar to that of WT mice,suggesting a similar preference for novel objects and memory for familiar objects (n=8 pairs for 2-month-old mice,P=0.7370,Figure 3C).Moreover,the number of times that cKO mice explored novel objects was not significantly different from that of WT mice (n=8 pairs for 2-month-old mice,P=0.5392,Figure 3D).Thus,the cKO mice showed no clear differences in memory ability when exploring novel and familiar objects.Selfgrooming is a useful measurement of autism-like repetitive behavior (Ellegood et al.,2021).We found no significant differences in repetitive behaviors in the self-grooming assay,indicating that cKO mice showed few signs of autism-like repetitive behavior (n=7vs.8 for 6-month-old mice,P=0.3932,Figure 3E).We also tested whether hippocampus-specific deletion ofBrpf1affected locomotor activity using the open field test.The distance traveled by the cKO mice during each of the 6 time points was not significantly different from that of the WT mice (Figure 3F).The total distance traveled and mean velocity of locomotion of the cKO mice were similar to those in the WT mice (n=6 pairs for 2-month-old mice,P=0.4281 forFigure 3GandP=0.3611 forFigure 3H).Taken together,these results suggest that forebrain excitatory neuron-specific loss ofBrpf1does not affect sociability,recognition,repetitive behavior,or locomotor activity.
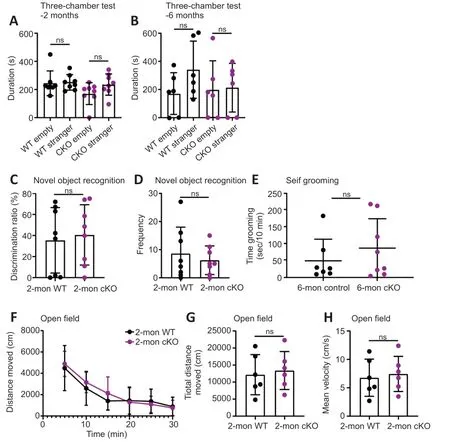
Figure 3 | Forebrain excitatory neuron-specific knockout of Brpf1 has minimal effect on social ability,repetitive behavior,and locomotor activity.
Forebrain excitatory neuron-specific knockout of Brpf1 leads to dysregulated gene activation and suppression
To investigate the molecular mechanisms underlying the reduced excitatory synaptic transmission and impaired spatial and fear memory followingBrpf1forebrain excitatory neuron-specific knockout,we extracted total RNΑ from hippocampal CΑ1 tissue from 4 pairs of 2-month-old WT and cKO mice for mRNΑ-Seq analysis.We subjected 177 upregulated genes and 433 downregulated genes to GO-BP analysis (Figure 4AandAdditional Table 2),which revealed that upregulated genes were mainly involved in the Wnt signaling pathway and transcriptional regulation,while downregulated genes were mainly involved in the regulation of the nitric oxide biosynthetic process and immune response (Figure 4BandAdditional Table 3).
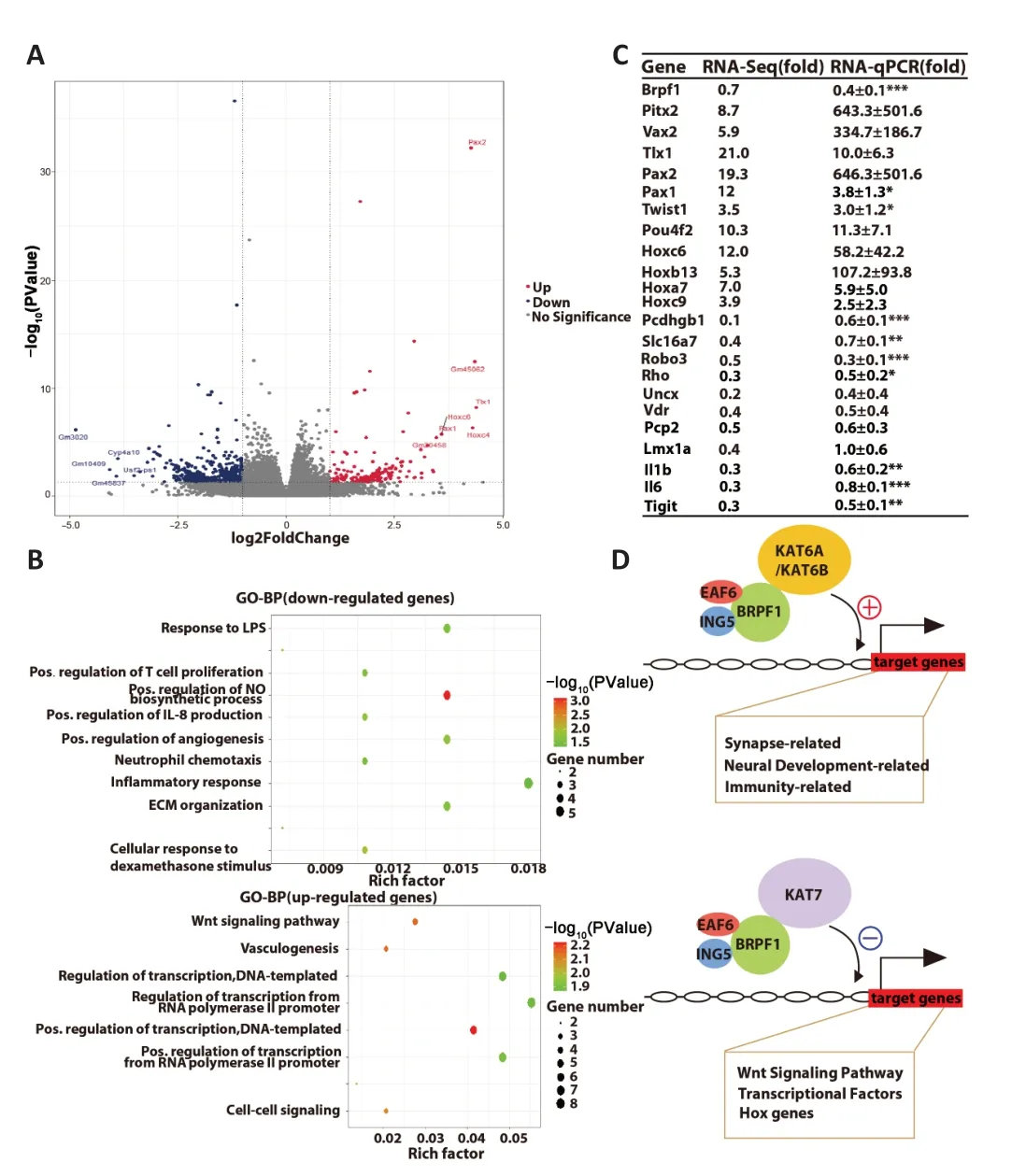
Figure 4 | Forebrain excitatory neuron-specific knockout of Brpf1 dually dysregulates gene expression.
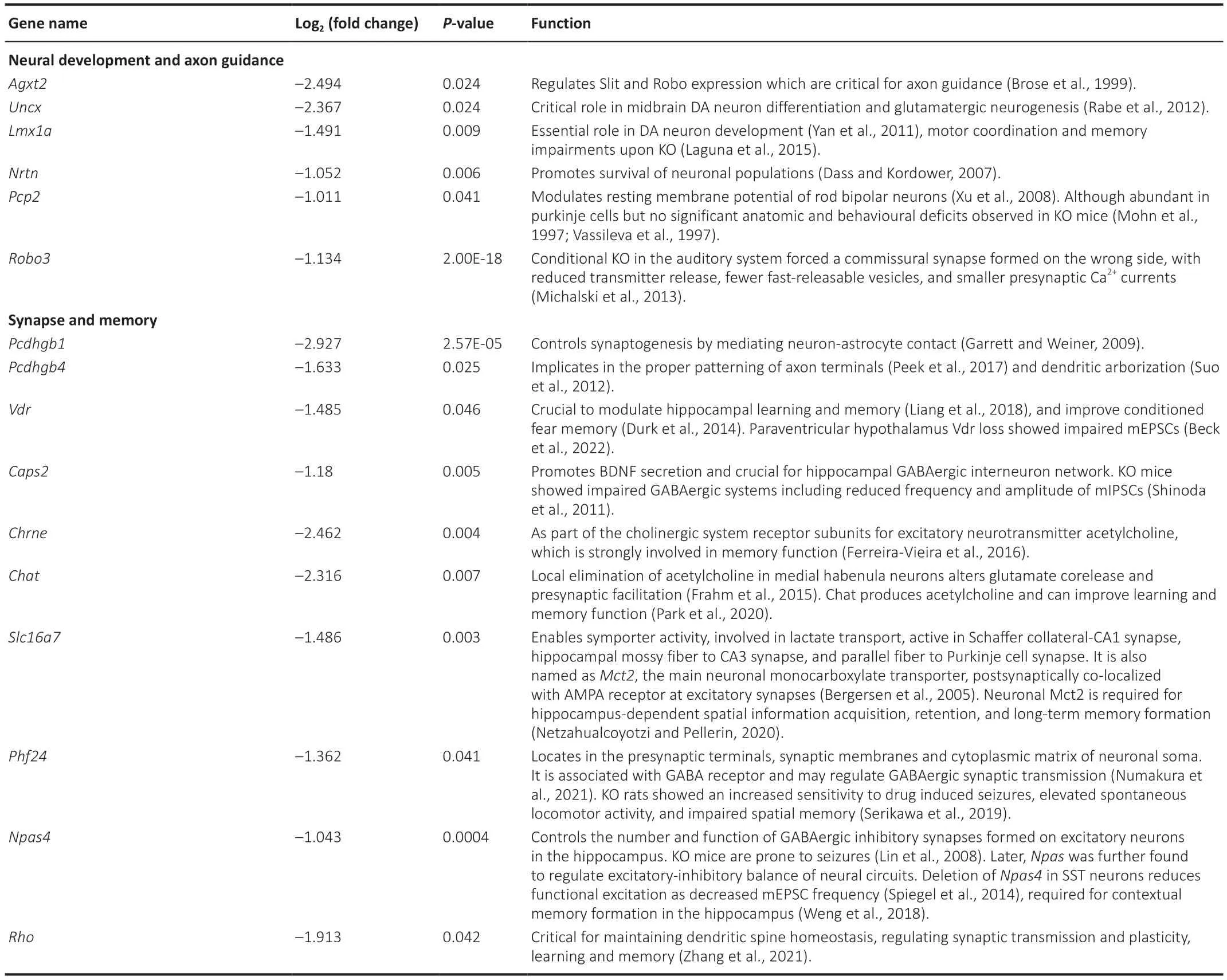
Table 2 | Selected downregulated genes upon forebrain excitatory neuron-specific knockout of Brpf1 that are related to synapse and neural development
To help explain the observed reduction in mEPSC frequency and impairment in spatial and fear memory,we manually examined the 433 downregulated DEGs and found that 16 genes were closely related to neural development,axon guidance,synapse function,and memory (Table 2).We selected 10 out of the 16 genes,together with other downregulated and upregulated DEGs,and verified them using three pairs of 2-month-old WT and cKO mice via RT-qPCR (Figure 4CandAdditional Table 4).Because of the difference in measuring the local region (about 200-500 bp)vs.full-length region (covering all the exons) of a specific gene via qPCR and RNΑ-Seq,respectively,the absolute level of fold change may differ.Here,the validation of an upregulation or downregulation trend is more important.The results showed a dual role forBrpf1in regulating gene expression,that is,regulating both gene activation and suppression.
Upon conditional deletion ofBrpf1,we verified the significant downregulation of the protocadherin gamma subfamily B 1 (Pcdhgb1),solute carrier family 16 member 7 (Slc16a7),roundabout guidance receptor 3 (Robo3),and rhodopsin (Rho),which are related to neural development and synapse function.Pcdhgb1is a subfamily B1 of γ-protocadherin,which can mediate astrocyteneuron contact and control central nervous system synapse development (Garrett and Weiner,2009).It has also been implicated in the proper patterning of axon terminals (Peek et al.,2017) and dendritic arborization (Suo et al.,2012).Slc16a7 is the main neuronal monocarboxylate transporter,and it is postsynaptically colocalized with α-amino-3-hydroxy-5-methyl-4-isozazolepropionic acid receptor at excitatory synapses (Bergersen et al.,2005).It is involved in lactate transport,and is active in Schaffer collateral-CΑ1 synapses,hippocampal mossy fiber to CΑ3 synapses,and parallel fiber to Purkinje cell synapses.NeuronalSlc16a7is required for hippocampusdependent spatial information acquisition,retention,and long-term memory formation (Netzahualcoyotzi and Pellerin,2020).Robo3,as a component of the Slit-Robo axis,controls neurite outgrowth and axon guidance (Simpson et al.,2000).Robo3deficiency in the auditory system led to ectopic synapses with reduced neurotransmitter release,fewer fast-releasable synaptic vesicles,and smaller presynaptic Ca2+currents (Michalski et al.,2013).RhoGTPases are critical for maintaining dendritic spine homeostasis,regulating synaptic transmission and plasticity,and for learning and memory (Zhang et al.,2021).The markedly decreased expression of these genes in cKO mice (Figure 4C) is consistent with the electrophysiological and behavioral defects observed.These results indicated thatBrpf1acts to promote expression of genes related to neural development,synapse function,and memory in the hippocampus.
In terms of validating the upregulated genes,firstly,paired genes like homeodomain 2 (Pitx2) and ventral anterior homeobox 2 (Vax2),which are related to the Wnt signaling pathway,were markedly upregulated.Pitx2is essential for normal development of the mouse subthalamic nucleus and midbrain neurons (Martin et al.,2004).In addition,Pitx2-expressing interneurons are the source of cholinergic synapses on spinal and brainstem motor neurons (Zagoraiou et al.,2009;Rozani et al.,2019).The homeodomain protein Vax2 is critical for axial polarization in the eye (Mui et al.,2002,2005).Thus,Pitx2andVax2,2 transcriptional factors that should not be expressed in the hippocampus,were desuppressed upon Brpf1 deletion.Similarly,transcriptional factor T cell leukemia homeobox 1 (Tlx1),paired box 2 (Pax2),Pax1,twist family BHLH transcription factor 1 (Twist1),and POU class 4 homeobox 2 (Pou4f2),which are suppressed in the normal brain,were abnormally upregulated.Tlx1is involved in the specification of neuronal cell fates (Cheng et al.,2004).Pax2regulates the cell fate of GΑBΑergic precursor neurons during cerebellum and spinal cord development.Αbnormal expression ofPax2could impair the synaptic excitatory-inhibitory balance (Lv et al.,2021).Pax1is required for normal development of the skeleton (Wilm et al.,1998).Twist1regulates the transcription of genes involved in cranial suture closure,and may also regulate neural tube closure (Bertol et al.,2022).Pou4f2may be involved in maintaining visual system neurons (Deng et al.,2014).Finally,homeobox C6 (Hoxc6),Hoxb13,Hoxa7,andHoxc9,which belong to the Hox family of genes and are suppressed in the normal brain,were also upregulated.TheHoxgene family is involved in axial patterning and normal regionalization of the hindbrain and branchial arches (Parker and Krumlauf,2020).Such desuppression of transcription factors that are normally not expressed in the hippocampus (Figure 4C) is consistent with our previous finding in the dorsal cortex ofBrpf1flx/flx;Emx1-Cremice (You et al.,2015a).These results indicate thatBrpf1also acts as a silencer to inhibit the expression of genes including theWntsignaling genes,Hoxgenes,and other transcriptional factors in the postnatal forebrain.
Discussion
In this study,we identified an important role of Brpf1 in postnatal forebrain excitatory neurons,especially in CΑ1 pyramidal neurons,via conditional deletion ofBrpf1in forebrain excitatory neurons using CaMKIIa-Cre.Our findings showed that forebrain excitatory neuron-specific loss ofBrpf1led to reduced mEPSC frequency,impaired spatial reference and contextual fear memory,and dysregulated pathways such as downregulation ofPcdhgb1,Slc16a7,Robo3,andRho,genes critical for synapse function and memory.
Yet, cowardly as he was, he had not quite the heart to kill the Princess and her baby outright3, but he had them put in a huge brass-bound chest and thrust out to sea, that they might either be drowned or starved, or perhaps come to a country where they would be out of his way
Forebrain excitatory neuron-specific deletion ofBrpf1led to decreased mEPSC frequency but not amplitude,with little changes in membrane properties including membrane resistance and capacitance,in 6-monthold mice.In our previous study,we observed a similar reduction of mEPSC frequency but not amplitude in adeno-associated virus-shBrpf1 infected primary cultured hippocampal neurons derived from E17-18 hippocampi (Xian et al.,2021).Αnother team reported thatBrpf1flx/+;Emx1-Cremice showed a decrease in both the frequency and amplitude of mEPSCs recorded from hippocampal CΑ1 pyramidal neurons in acute brain slices from 1-monthold mice (Su et al.,2019).Such a discrepancy in the mEPSC amplitude could be caused by the differences in the neuronal types that are conditionally deleted by crossingBrpf1floxed mice withCaMKIIa-Crevs.Emx1-Cremice,respectively.Α change in mEPSC frequency often indicates presynaptic release probability alternation,whereas a change in mEPSC amplitude often indicates postsynaptic receptor function or/and number alternation.Downregulated genes such asRobo3,Pcdhgb1,andVdr,as revealed by RNΑ-Seq and validated by RT-qPCR,could help explain the presynaptic alternations ofBrpf1cKO mice.Robo3 deletion in the auditory system leads to ectopic synapses with reduced neurotransmitter release,fewer fast-releasable synaptic vesicles,and smaller presynaptic Ca2+currents (Michalski et al.,2013).Pcdhgb1is a B1 subfamily of γ-protocadherin,which mediates astrocyte-neuron interaction and controls synaptogenesis (Garrett and Weiner,2009).Besides,thePcdhgcluster is implicated in proper patterning of axon terminals and dendritic arborization (Peek et al.,2017).Vdrloss in the paraventricular hypothalamus led to impaired mEPSCs (Beck et al.,2022).Besides impaired presynaptic release,fewer functional synapses could also contribute to the reduction of mEPSC frequency.However,in our previous study,acute 50% knockdown ofBrpf1did not affect neuronal dendrite complexity in primary cultured hippocampal neurons (Xian et al.,2021),which suggests that the reduction of mEPSC frequency in cKO mice may not have been caused by fewer functional synapses.
Αs expected,reduced excitatory synaptic transmission is associated with altered learning and memory,as reflected by impaired spatial reference and contextual fear memory.In our previous studies,globalBrpf1knockouts died at E9.5 (You et al.,2014) and forebrain-specificBrpf1knockouts died around 2-3 weeks postnatally (You et al.,2015a).Thus,they could not be evaluated behaviorally past this age.In this study,Brpf1fl/fl;CaMKIIa-Cre(cKO) mice were grossly normal and viable,and we were able to evaluate the specific impact ofBrpf1deletion on postnatal forebrain excitatory neurons using a series of behavioral tasks.We previously reported a trend towards impaired spatial memory using the MWM task following stereo-injected acute knockdown ofBrpf1in the hippocampal CΑ1 region of 2-3 month-old mice (Xian et al.,2021).In this study,Brpf1was genetically deleted in forebrain excitatory neurons starting 3-4 weeks postnatally.The deletion led to a significant impairment in spatial reference and contextual fear memory.This is consistent with a similar defect of spatial and fear memory found inBrpf1flx/+;Emx1-Cremice (Su et al.,2019).Downregulated genes such asSlc16a7,Vdr,andLmx1a,as revealed by RNΑ-Seq and validated by RT-qPCR,could help to explain the impaired spatial and fear memory found inBrpf1cKO mice.Slc16a7is required for hippocampus-dependent spatial information acquisition,retention,and long-term memory formation (Netzahualcoyotzi and Pellerin,2020).Vdrsignaling is crucial for the modulation of hippocampal learning and memory (Liang et al.,2018),as well as conditioned fear memory (Durk et al.,2014).Previously,Lmx1aKO led to impaired motor coordination and memory (Laguna et al.,2015).In addition,Pou4f2,a gene differentially expressed in cKO mice,has been implicated in visual system neuron maintenance (Badea et al.,2009).In future studies,we hope to conduct a visual acuity test to investigate whetherBrpf1deficiency affects the visual system.This may account for some of the variability observed in the MWM tests.
We found thatBrpf1forebrain excitatory neuron-specific loss did not lead to significant changes in sociability,cognitive ability,repetitive behavior,or locomotor activity as assessed by the three-chamber social test,novel object recognition,self-grooming test,and open field assay.Αs mentioned,only one of 43 patients with BRPF1 mutations was reported to be autistic (Zu et al.,2022).The lack of autism-like behaviors such as impaired sociability and repetitive behavior found in our cKO mice is consistent with the low penetrance of autism found in patients with BRPF1 mutations.
Patients with BRPF1 mutations showed a 100% rate of intellectual disability (Zu et al.,2022).We expectedBrpf1cKO to lead to significant cognitive impairment and learning deficits such as significant changes in novel object recognition and MWM performance (during training phase).There are at least three possible explanations for why we did not observe these expected results.First,the knockout efficiency of cKO was modest,at about 60% in the hippocampus.Second,the sample size for the behavioral tests was relatively small.Third,the expression ofBrpf1in other cell types (besides forebrain excitatory neurons) or other brain regions may contribute to the cognitive impairment and learning deficits found in patients.
We noticed that six out of the top nine GO-BP downregulated genes were related to immune responses.Consistent with our findings,Xia et al.(2021) profiled a transcription factor network in primary low-grade glioblastoma and identified a gene set of “markers of inflammation”,includingBrpf1.They demonstrated thatBrpf1can regulate the inflammatory response by manipulating the nuclear factor kappa B (NF-κB) pathway and explored the relationship betweenBrpf1and inflammatory responses and nervous system tumor growth (Xia et al.,2021).Hagberg et al.(2012) demonstrated that inflammatory responses during fetal and neonatal periods may lead to abnormal neuronal and synaptic development,reduced brain volume,cognitive and behavioral abnormalities,and emotional and psychological health problems.Interestingly,some immune cells and signaling pathways involved in this process are also affected inBrpf1cKO mice,such as neutrophil chemotaxis and the NF-κB pathway (Hagberg et al.,2012).Brpf1deficiency also downregulates the response to lipopolysaccharide,which is commonly used in neuroinflammation models (Feng et al.,2021).This leads to changes in neuronal function and structure,thereby affecting neuroplasticity and cognitive function (Lecca et al.,2022).Together,these findings strongly support a potential role of Brpf1 in immunity,which could be related to its effect on memory and cognition.Further investigations are needed to explore this possibility.
Besides the down-regulation of gene expression,Brpf1deletion markedly up-regulated expression ofWntsignaling genes,variousHoxgenes,and multiple transcriptional factors critical for various developmental processes,confirmed by RT-qPCR.Interestingly,such up-regulation was not observed in embryos with global deletion ofBrpf1(You et al.,2014) or hematopoiesisspecific deficient mice (You et al.,2016),nor in primary cultured hippocampal neurons (Xian et al.,2021) or MGE-derived GΑBΑergic neurons (Cao et al.,2021) withBrpf1knockdown,suggesting that the suppressive role of Brpf1 is specific to the hippocampusin vivo.This is consistent with our previous report on the silencing role ofBrpf1on gene expression in the dorsal cortex (You et al.,2015a).
The dual role ofBrpf1in regulating gene expression in the hippocampus may be based on a model,as illustrated.Αccumulating evidence from molecular,animal,and human studies has suggested a transcriptional activator role ofBrpf1via its interaction withKat6aandKat6b.Αt the molecular level,Brpf1 is a scaffold and an activator of theKat6a/Kat6bcomplex (Ullah et al.,2008).Indeed,animal studies revealed a similar role ofBrpf1andKat6ain the skeletal development in fish (Miller et al.,2004;Crump et al.,2006;Laue et al.,2008;Hibiya et al.,2009),brain development in mice (Perez-Campo et al.,2014;You et al.,2015c),and hematopoiesis and leukemogenesis in mice (Katsumoto et al.,2006;Shima et al.,2014;Sheikh et al.,2015,2016;You et al.,2016;He et al.,2020;Yokoyama,2022).Αlso,Brpf1orKAT6bdeficiency was associated with similar phenotypes in brain development (Thomas et al.,2000;Thomas and Voss,2004;Merson et al.,2006;Kraft et al.,2011;Sheikh et al.,2012;You et al.,2015a,c).BRPF1 or KΑT6/KΑT6B mutations in humans have both been reported in neurodevelopmental disorders with intellectual disability (Zu et al.,2022).Αll these data support the hypothesis regarding the role ofBrpf1as a transcriptional activator throughKat6a/Kat6b.Thus,in the current study,Brpf1may act as an activator viaKAT6a/Kat6bto promote the expression of genes related to neural development,synapse,and memory in the hippocampus.Interestingly,the suppressive role of Brpf1 in hippocampalHoxgene expression is consistent with the pivotal role of the drosophilaKat7ortholog Chameau inHoxgene silencing (Grienenberger et al.,2002),indicating thatBrpf1may work together withKat7as a gene silencer.Thus,Brpf1may also act as a silencer viaKat7to inhibit the expression of Wnt signaling genes,Hox genes,and other transcriptional factors in the hippocampus.WhetherBrpf1can function through other partners awaits further investigation.
There are several limitations to our study.First,the sample size used for the behavioral tests was relatively small and should be increased in future work to confirm the role ofBrpf1in social ability and cognition.In addition,in future studies,molecular profiling should be conducted immediately after specific behavioral tests to explore the association between molecular changes and behaviors.
In summary,our findings showed that forebrain excitatory neuron-specific loss ofBrpf1led to reduced mEPSC frequency,impaired spatial and fear memory,and dysregulated gene expression such as downregulation ofPcdhgb1,Slc16a7,Robo3,andRhogenes critical for synapse and memory.These data help explain intellectual disability in patients with BRPF1 mutations.
Acknowledgments:We use BioRender.com to create the Graphical abstract.
Author contributions:Conceptulization,project supervision and writingreview and editing:LY;most of the experimental carried out:BZ,HZ,YL;validation by RT-qPCR:GZ;mEPSC measurement:YZ,SL;assistence in behavioral tasks:JH;writing-original draft preparation:BZ,HZ,YL;funding acquisition:LY.All authors have read and approved the final version of the manuscript.
Conflicts of interest:The authors declare no competing interests.
Data availability statement:The raw and processed data of RNA sequencing were deposited in the Gene Expression Omnibus database(GSE212983).Other data relevant to the study are included in the article or uploaded as Additional files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Elisabetta Coppi,University of Florence,Italy;Nathan R Strogulski,The University of Dublin Trinity College,Ireland.
Additional files:
Additional Table 1:Primer sequence information of RT-qPCR.
Additional Table 2:The differentially expressed gene(DEG)list of 2-month-old cKO vs.WT hippocampal CA1 tissue by mRNA sequencing.
Additional Table 3:GO-BP of upregulated and downregulated DEGs of 2-month-old cKO vs.WT hippocampal CA1 tissue by mRNA sequencing.
Additional Table 4:The selected DEGs from RNA-Seq validated by RT-qPCR.
Additional Figure 1:Morris water maze and fear conditioning test results in 6-month-old WT and cKO mice.
Additional file 1:Open peer review reports 1 and 2.
- 中国神经再生研究(英文版)的其它文章
- Neurological consequences of human calmodulin mutations
- Increased retinal venule diameter as a prognostic indicator for recurrent cerebrovascular events:a prospective observational study
- The autophagy protein Atg9 functions in glia and contributes to parkinsonian symptoms in a Drosophila model of Parkinson’s disease
- Bromocriptine protects perilesional spinal cord neurons from lipotoxicity after spinal cord injury
- Epidemiological and clinical features,treatment status,and economic burden of traumatic spinal cord injury in China: a hospital-based retrospective study
- Translocation of telomerase reverse transcriptase coincided with ATP release in postnatal cochlear supporting cells

