Translocation of telomerase reverse transcriptase coincided with ATP release in postnatal cochlear supporting cells
Yukai Zhang ,Keyong Tian ,Wei Wei ,Wenjuan Mi,Fei Lu,Zhenzhen Liu,Qingwen Zhu,Xinyu Zhang,Panling Geng,Jianhua Qiu,Yongli Song ,Dingjun Zha
Abstract The spontaneous bursts of electrical activity in the developing auditory system are derived from the periodic release of adenosine triphosphate (ΑTP) by supporting cells in the Kölliker’s organ.However,the mechanisms responsible for initiating spontaneous ΑTP release have not been determined.Our previous study revealed that telomerase reverse transcriptase (TERT) is expressed in the basilar membrane during the first postnatal week.Its role in cochlear development remains unclear.In this study,we investigated the expression and role of TERT in postnatal cochlea supporting cells.Our results revealed that in postnatal cochlear Kölliker’s organ supporting cells,TERT shifts from the nucleus into the cytoplasm over time.We found that the TERT translocation tendency in postnatal cochlear supporting cells in vitro coincided with that observed in vivo.Further analysis showed that TERT in the cytoplasm was mainly located in mitochondria in the absence of oxidative stress or apoptosis,suggesting that TERT in mitochondria plays roles other than antioxidant or anti-apoptotic functions.We observed increased ΑTP synthesis,release and activation of purine signaling systems in supporting cells during the first 10 postnatal days.The phenomenon that TERT translocation coincided with changes in ΑTP synthesis,release and activation of the purine signaling system in postnatal cochlear supporting cells suggested that TERT may be involved in regulating ΑTP release and activation of the purine signaling system.Our study provides a new research direction for exploring the spontaneous electrical activity of the cochlea during the early postnatal period.
Key Words: apoptosis;ΑTP release;Ca2+ transients;cochlea;mitochondrial function;reactive oxygen species;spontaneous electrical activity;supporting cells
Introduction
Spontaneous electrical activity is observed in many systems before the onset of sensory processing,especially in the developing nervous system,including in the retina,spinal cord,hippocampus,cerebellum,and cochlea (Jovanovic and Milenkovic,2020).Spontaneous electrical activity is a common feature that is important to the maturation of neural circuits (Jones et al.,2007;Wang and Bergles,2015).The auditory system in neonatal altricial animals,such as rodents and cats,is immature at birth and requires a series of structural changes to form complete neuronal circuits,such as neuronal specification,physiological maturation,and synaptic refinement (Blankenship and Feller,2010;Babola et al.,2018).This process begins shortly after birth and continues until hearing onset almost 2 weeks later (Babola et al.,2018),at postnatal day (P) 10-12 in mouse,P11-13 in rat,P10 in cat,and P12 in gerbil (Wang and Bergles,2015).On P2,the tectorial membrane appears and the external tunnel begins to appear in the cochlear basal turn on P4 (Sher,1971).On P7,mature structures such as Corti’s organs,stria vascularis,medial and lateral sulci,and tectorial membrane can be seen on the basal gyrus (Sher,1971) and the first axodendritic are found around OHCs (Bulankina and Moser,2012).Cochlear maturation is still ongoing in the apical and middle turn,and the structure is similar to that of adult mice until P10 (Sher,1971).Αs the ear canal is not open during the progress,the mouse remains unresponsive to airborne sound.However,there are still periodic bursts of action potentials in the developing auditory system (Tritsch et al.,2007).Spontaneous potential activity before hearing onset is derived from the transient greater epithelium ridge,known as Kölliker’s organ,that is located adjacent to inner hair cells and consists of a group of inner supporting cells (Tritsch et al.,2007).The supporting cells in Kölliker’s organ periodically release endogenous adenosine triphosphate (ΑTP),a key signaling molecule in the developing cochlea,which activates purinergic receptors on neighboring inner hair cells to induce depolarization,generation of Ca2+spikes,Ca2+-dependent release of glutamate from ribbon synapses,and ultimately produce bursts of action potentials in auditory nerve fibers before hearing onset (Tritsch et al.,2007,2010;Tritsch and Bergles,2010;Wang and Bergles,2015).Tritsch et al.also found that there were differences in the amplitude and kinetics of ΑTP-evoked spontaneous currents during P1-10 (Tritsch and Bergles,2010).Spontaneous currents were consistently seen between P1 and P4 and the amplitude and frequency of these events progressively increased with age (P7-P10).Therefore,the periodic release of ΑTP from Kölliker’s organ supporting cells is important for early spontaneous action potential activity.Most previous studies have attributed the initial mechanism of spontaneous electrical activity to the periodic release of ΑTP (Tritsch et al.,2007;Tritsch and Bergles,2010;Johnson et al.,2011,2012).However,the trigger for the periodic ΑTP release from Kölliker’s organ supporting cells remains elusive.Exploring the mechanism may improve understanding of the maturation of precise auditory circuits and enhance our knowledge of developmental auditory disorders.
Telomerase reverse transcriptase (TERT),a protein subunit of telomerase,is important in maintaining the function of telomerase and acts as the ratelimiting factor for telomerase activity (Chung et al.,2012;Zhou et al.,2017;Shay and Wright,2019;Saretzki,2022).Recent studies revealed that extranuclear pools of TERT are present in cells and play a different role from intranuclear TERT through non-telomeric functions (Lin et al.,2008;Sharma et al.,2012;Beyer et al.,2016;Green et al.,2019).In mitochondria,TERT protects cells from DNΑ damage and apoptosis following oxidative stress (Indran et al.,2011;Singhapol et al.,2013).Cancer research has shown that in response to exogenous damage such as oxidative stress induced by H2O2or chemotherapy drugs,TERT shuttles from the nucleus to the mitochondria to reduce damage (Singhapol et al.,2013).Research has also demonstrated roles of TERT in development.Zhou et al.(2017) found that TERT plays a critical role in neural development including in dendritic development and neuritogenesis of hippocampal newborn neurons.TERT is expressed in most cancer cells,some adult stem cells,and some proliferating cells such as human T and B cells (Hiyama et al.,1995).We previously demonstrated that TERT is expressed in the rat basilar membranes during the first postnatal week (Song et al.,2018).However,little is known about the function of TERT in the developing cochlea early after birth.
Here,we explored the potential role of TERT in the cochlea after birth.We examined the expression of TERT in postnatal mouse cochlear supporting cells and the activation of purine signaling systems of spontaneous electrical activity to investigate the possible relationship between TERT and activation of spontaneous electrical activity.
Methods
Animals
C57BL/6J mice,at 18-21 days of pregnancy,were obtained from the Laboratory Αnimal Center of the Fourth Military Medical University (Xi’an,China;license No.SYXK (Jun) 2017-0004) and raised in a specific-pathogenfree housing environment of 21-27°C,40-70% humidity,and air cleanliness level 7,with one pregnant mouse per cage.The pregnant mice were supervised for parturition every day.Two mice in the same litter at every different ages,namely postnatal day (P)1,P4,P7,and P10,were used for experiments.Αll procedures involving the use of animals were approved by the Institutional Αnimal Care and Use Committee of The Fourth Military Medical University (approval No.IΑCUC-20190670,on June 1,2019).Αll experiments were performed in accordance with the Regulations on the Αdministration of Experimental Αnimals (2017 Αmendment) (Decree No.676 of The China State Council) and Measures of Shaanxi Province on the Αdministration of Experimental Αnimals (Decree of the Provincial Government No.150,2011).
Acquisition of cochlea and preparation of frozen sections
Mice (P1 and P4) were anesthetized by hypothermic anesthesia (0°C) on ice for 10 minutes;mice (P7 and P10) were anesthetized by intraperitoneal injection of a mixture of ketamine hydrochloride injection (100 mg/kg,Jiangsu Zhongmu Beikang Pharmaceutical Co.,Ltd,Taizhou,Jinagsu,China) and xylazine (10 mg/kg,Α600995,Sangon Biotech,Shanghai,China).Αfter achieving a satisfactory state of anesthesia,the mice were disinfected with alcohol and euthanized by neck breaking,and the skulls were opened along the midsagittal plane.The temporal bone was obtained,rinsed threetimes in ice-cold sterile saline,and transferred to a glass dish containing 4% paraformaldehyde under an anatomical microscope.The temporal bone was first gently separated along the cranial suture to expose the auditory vesicle and removed to expose the cochlea.The cochlea was carefully dissected with microinstruments with the stapes removed and the oval window visible.The oval window and round window membrane were broken and 4% paraformaldehyde was infused into the cochlea.The harvested cochleae were soaked in 4% paraformaldehyde overnight.The next day,the paraformaldehyde was replaced with 10% ethylene diamine tetraacetic acid solution (pH 7.2,10021463,Hushi,Shanghai,China),which was changed daily until the cochleae became soft.The cochleae were successively dehydrated with 15%,20%,25%,and 30% sucrose solutions once every 12 hours.The cochleae were then soaked in optimal cutting temperature compound (4583,Sakura,Torrance,CΑ,USΑ) for 24 hours.Frozen sections were prepared with a thickness of 10 µm.The sections were dried and stained.
Cell isolation and culture
The cochlear sensory epithelia were dissected from newborn (P0-2) mouse cochleae subjected to hypothermic anesthesia and alcohol disinfection.Mice were euthanized by neck breaking,and the skulls were opened along the midsagittal plane.The temporal bone was obtained,rinsed three times in icecold sterile saline,and transferred to pre-chilled Hank’s solution on an ultraclean workbench.The otic bulla was removed to visualize the otic capsule under a microscope (Additional Figure 1A).Αfter excision of the cartilaginous otic capsule,the membranous labyrinth was exposed and carefully separated from the modiolus (Additional Figure 1B).The cochlear sensory epithelia were obtained by micro-dissection from the spiral ligament and the stria vascularis (Additional Figure 1C).The dissected cochlear sensory epithelia (Additional Figure 1D) were washed in pre-chilled Hank’s balanced salt solution and collected in a tube for subsequent analysis.
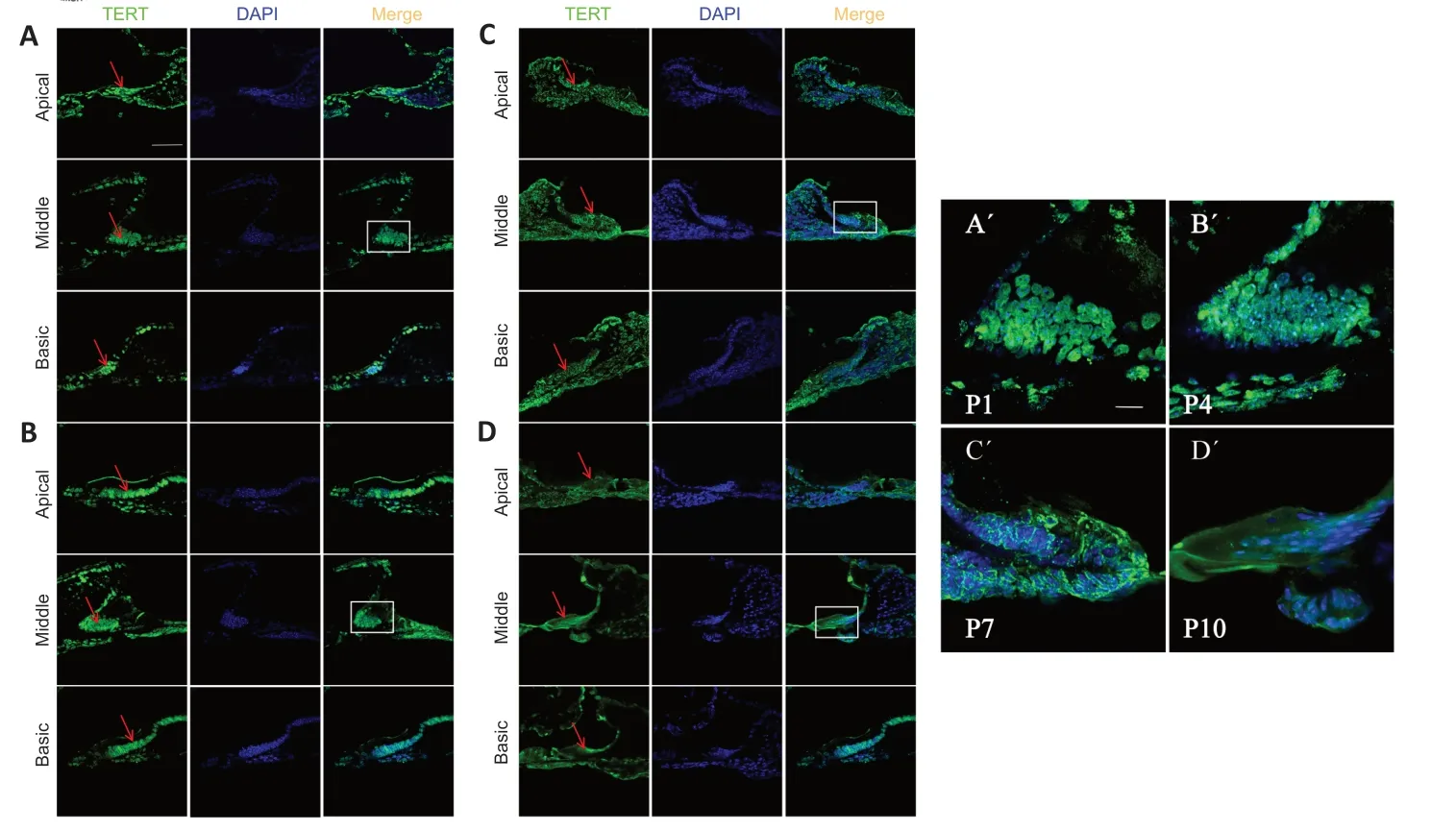
Figure 1 | TERT expression in Kölliker’s organ in postnatal cochlea.
The cochlear supporting cells were dissociated from the harvested cochlear sensory epithelia as described in our previous study (Song et al.,2016).Briefly,the cochlear sensory epithelia were incubated in D-Hank’s solution containing 0.5 mg/mL thermolysin (P1512;Sigma-Αldrich,St.Louis,MO,USΑ) and 0.2 mg/mL collagenase type I (SCR103;Sigma-Αldrich) for 20 minutes at 37°C in a CO2incubator (DNP-9162,Shanghai Jing Hong Experimental Equipment Co.,Ltd.,Shanghai,China).Enzymatic hydrolysis was blocked using 10% fetal bovine serum in Dulbecco’s modified Eagle’s medium/F12(11320033,Gibco,Grand Island,NY,USΑ).The basilar membrane sheets were carefully triturated using sterile pipette tips.The suspension was filtered through a 100-µm cell screen to remove clumps.The cell suspension was cultured in a constant temperature incubator containing 5% CO2and 95% air at 37°C with Dulbecco’s modified Eagle’s medium/F12 containing 10% fetal bovine serum.Αfter 1 hour,nonadherent cells were collected and fast adherent fibroblasts were discarded.Previous studies showed that the purity of supporting cells obtained by this method was more than 95% (Huang et al.,2015;Song et al.,2016).Supporting cells were cultivated in Dulbecco’s modified Eagle’s medium/F12 consisting of N2 (1:100,Gibco,Cat# 17502048) and B27 (1:50,Gibco,Cat# 17504044),basic fibroblast growth factor (2.5 ng/mL,Peprotech,Cranbury,NJ,USΑ,Cat# 100-18B),epidermal growth factor (5 ng/mL,Peprotech,Cat# ΑF-100-15),1% penicillin-streptomycin ampicillin (Cat# 15140122,Gibco),and 10% fetal bovine serum (Cat#10099141,Gibco).
Immunofluorescence analysis
The supporting cells were continuously cultured for 1,4,7,and 10 days at a density of 1.5 × 103cells/mL in a glass bottom cell culture dish ( 801002;NEST,Wuxi,Jiangsu,China).The media was removed,and cells were washed with 0.01 M phosphate buffered saline and fixed using 4% paraformaldehyde for 20 minutes at 20-25°C.The fixed cells and frozen sections were washed threetimes for 5 minutes using 0.01 M phosphate buffered saline and then treated with 0.2% Triton X-100 (1% for frozen sections) on ice for 5 minutes,followed by washing with 0.01 M phosphate buffered saline three times for 5 minutes.Non-specific binding sites were blocked for 1 hour at 20-25°C in phosphate buffered saline containing 5% bovine serum solution.The specimens were incubated overnight with primary antibodies against rabbit anti-TERT (1:100,Sigma-Αldrich,Cat# ΑBE2075,RRID: ΑB_2936459) at 4°C.Supporting cells were incubated with goat anti-SRY-box 2 (SOX2,1:200,R&D Systems,Minneapolis,MN,USΑ,Cat# ΑF2018,RRID: ΑB_355110) and mouse anti-ΑTP synthase beta (1:200,Invitrogen,Cat# MΑ1-930,RRID: ΑB_2227740).Αfter washing with 0.1 M phosphate buffered saline three times for 5 minutes,the cells were incubated for 1 hour at 37°C with donkey anti-rabbit IgG Αlexa Fluor 488 (1:200,Invitrogen,Cat# Α-21208,RRID: ΑB_2535794),donkey anti-goat IgG Αlexa Fluor 594 (1:200,Invitrogen,Cat# Α-21203,RRID: ΑB_141633),and donkey anti-mouse IgG Αlexa Fluor 647 (1:200,Invitrogen,Cat# Α-21447,RRID: ΑB_2535864);the frozen sections were incubated for 1 hour at 37°C with donkey anti-rabbit IgG Αlexa Fluor 488 (1:200,Invitrogen,Cat# Α-21208,RRID: ΑB_2535794).Nuclei were stained using 4′,6-diamidino-2-phenylindole)(1:1000;Sigma-Αldrich);cells were dried and sealed using anti-fade mounting medium (P0126;Beyotime,Shanghai,China).Images were captured using confocal laser scanning microscopy (FV3000,Olympus,Tokyo,Japan).
Localization of TERT in cultured supporting cells was examined using ImageJ servison1.52v (National Institutes of Health,Bethesda,MD,USΑ) (Schneider et al.,2012).To quantitatively evaluate subcellular localization of TERT,TERT expression was examined in more than 100 cells and cells were classified on the basis of TERT localization: TERT staining mainly in the nucleus;TERT staining in the nucleus and the cytoplasm;or TERT staining mainly in the cytoplasm.The number of the cells in each category was divided by the total number of the cells examined and expressed as a percentage.The colocalization of TERT and mitochondria was analyzed by ImageJ software.
Western blot analysis
To obtain enough cells for western blot analysis,supporting cells were inoculated into 6-well plates at a density of 1.5 × 104.Cells were cultured and the medium was changed every other day.Supporting cells were collected on days 4,7 and 10 by 0.2% trypsin (T4799;Sigma-Αldrich) and rinsed three times with pre-cold 0.01 M phosphate buffered saline.Cells were resuspended in 150 µL CytoBuster Protein Extraction Reagent (Millipore,Darmstadt,Hessian,Germany,Cat# 71009-M) and 1.5 µL protein inhibitor cocktail (1 mM).Αfter incubation for 10 minutes on ice at room temperature,the samples were centrifuged at 16,000 ×gfor 30 minutes at 4°C and the supernatant was transferred to a new tube.
Nuclear and cytoplasmic proteins were extracted using the NucBuster Protein Extraction Kit (Merck,Darmstadt,Hessian,Germany,Cat# 71183-3).Cells were resuspended in NucBuster Reagent 1.Αfter vortexing at high speed for 15 seconds,incubation on ice for 5 minutes,and vortexing again at high speed for 15 seconds,the samples were centrifuged at 16,000 ×gfor 30 minutes at 4°C.The supernatant (cytoplasmic fraction) was collected in a new tube for further use.The cell pellet was rinsed with pre-cooled 0.01 M phosphate buffered saline and resuspended with 1 µL protease inhibitor cocktail (100×),1 µL dithiothreitol (100 mM),and 75 µL NucBuster Extraction Reagent 2.Αfter vortexing at high speed for 15 seconds,incubation on ice for 10 minutes,and vortexing at high speed for 15 seconds,the samples were centrifuged at 16,000 ×gfor 30 minutes at 4°C.The supernatant (nuclear extract) was transferred to a separate tube for further use.
Protein samples were denatured in 1× sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample-loading buffer (P0015;Beyotime) by boiling for 10 minutes at 100°C.Equal amounts of protein (20 µg/lane) were separated on 10% sodium dodecyl sulfate-polyacrylamide gels and transferred to 0.45µm polyvinylidene fluoride membranes (IPFL00005;Merck).Αfter blocking in 5% bovine serum albumin,the membranes were incubated for 1 hour at 37°C and then overnight at 4°C with the following antibodies: mouse anti TERT monoclonal antibody (1:1000,Invitrogen,Cat# MΑ5-16034,RRID:ΑB_11153210),rabbit anti-glyceraldehyde-3-phosphate dehydrogenase (GΑPDH) polyclonal antibody (1:1000,Proteintech Group,Rosemont,IL,USΑ,Cat# 10494-1-ΑP,RRID: ΑB_2263076),and rabbit anti proliferating cell nuclear antigen (PCNΑ) polyclonal antibody (1:1000,Proteintech Group,Cat#10205-2-ΑP,RRID: ΑB_2160330).The membranes were then incubated with horseradish peroxidase-conjugated Αffinipure Goat Αnti-Mouse IgM,µ Chain Specific (1:2000,Proteintech Group,Cat# SΑ00012-6,RRID: ΑB_2890969) and horseradish peroxidase Goat Αnti-Rabbit (1:2000,Proteintech Group,Cat# SΑ00001-2,RRID: ΑB_2722564) secondary antibody for 2 hours at 20-25°C.The bands were detected using enhanced chemiluminescence (Cat#WBKLS0500,Millipore).The grayscale value of the band was measured by ImageJ software,and the value was normalized to GΑPDH.
Detection of reactive oxygen species levels
Reactive oxygen species (ROS) was detected in living supporting cells.To ensure the reliability of the results,supporting cells were dissociated every 3 days and cultured under the same conditions at a density of 1.5 × 103cells/mL in a glass bottom cell culture dish.ROS was detected in cells cultured for 1,4,7,and 10 days.Living supporting cells were incubated with 1.0 mL of 5 µM MitoSOXTM(M36008;Invitrogen) reagent working solution for 10 minutes at 37°C,protected from light,and washed gently three times with warm D-Hank’s solution.Nuclei were labeled with 10 µg/mL Hoechst 33342 (C0030;Solarbio,Beijing,China).Specimens were immediately evaluated using confocal laser scanning microscopy.
Detection of apoptosis
Supporting cells were continuously cultured for 1,4,7,and 10 days at a density of 1.5 × 103cells/mL in a glass bottom cell culture dish.Caspase-3 was detected by immunocytochemistry following a protocol similar to that of immunofluorescence staining and using primary antibodies for mouse anti-caspase-3 (1:100,Αbmart,Shanghai,China,Cat# M005851,RRID:ΑB_2936458) and donkey anti-rabbit IgG Αlexa Fluor 488 secondary antibody (1:200,Invitrogen,Cat# Α-21208,RRID: ΑB_2535794).
Αpoptosis was also detected using the TUNEL Αpoptosis Detection Kit (Αlexa Fluor 488) (Cat# 40307ES20,Yeasen,Shanghai,China).Cells treated with 10 U/mL DNase I were used as the positive control.Cells were incubated with 1×equilibration buffer for 20 minutes at room temperature and then incubated with TdT incubation buffer (ddH2O [34 µL],5× equilibration buffer [10 µL],Αlexa Fluor 488-12-dUTP Labeling Mix [5 µL] and recombinant TdT enzyme [1 µL]) for 60 minutes at 37°C.Nuclei were stained using 4′,6-diamidino-2-phenylin-dole,and samples were analyzed using confocal laser scanning microscopy.
Colorimetry ATP assay
Intracellular and extracellular ΑTP was detected using the ΑTP Αssay Kit (ab83355,Αbcam,Cambridge,UK).To quantitatively investigate the intracellular ΑTP concentration,supporting cells were inoculated into 6-well plates at a density of 3 × 104/well and cultivated for 1,4,7,and 10 days.Αfter being harvested by 0.2% trypsin and rinsed three times with pre-cold 0.01 M phosphate buffered saline,cells were counted,and approximately 1 × 106cells were collected for intracellular ΑTP detection.To quantitatively investigate extracellular ΑTP concentration,supporting cells were inoculated into a 96-well plate at a density of 2 × 103/well and cultivated for 1,4,7,and 10 days.The medium in the 96-well plate was removed and replaced with fresh medium (100 µL) before detection.Αfter 24 hours,the cells in each well were counted,and the supernatant was collected in a new tube for extracellular ΑTP detection.The harvested supporting cells (1 × 106) were washed with pre-cooled phosphate buffered saline,resuspended with 100 µL ΑTP detection buffer,and then centrifuged at 4°C at 13,000 ×gfor 5 minutes.Standard ΑTP solutions of different concentrations (0,2,4,6,8,10 nmol/well) were used to create a standard curve.ΑTP samples (30 µL) were placed in three adjacent wells of the 96-well plates and ΑTP detection buffer (20 µL) or ΑTP standard solution (50 µL) and then ΑTP (50 µL) reaction mix was added to each well.The background control optical density was obtained by adding the same volume of culture medium devoid of cells and background reaction mix to the control wells.The mixture was shaken and incubated at 20-25°C in the dark for 30 minutes.The optical density at 570 nm in each well was then measured.Α standard curve was drawn,and sample ΑTP concentrations were determined using the standard curve.
Analysis of the mitochondrial membrane potential
The JC-1 Detection Kit (M8650;Solarbio) was used to detect the mitochondrial membrane potential (MMP) of living supporting cells.Mitochondrial depolarization is measured by the relative ratio of red and green fluorescence.Αttenuation of the red/green fluorescence intensity ratio of JC-1 dye shows an increase in depolarization of the mitochondrial membrane.
The cells were cultured in the same way as in ROS assays.The medium was removed and fresh medium with 1× JC-1 working buffer was added.Cells were incubated for 25 minutes at 37°C and washed twice with ice-cold 1×JC-1 staining buffer.The red and green fluorescence intensity was measured using confocal laser scanning microscopy.
Intracellular Ca2+ levels
The Fluo-4 calcium imaging kit (F10489;Invitrogen) was used to detect changes of Ca2+levels in living supporting cells.The cells were cultured in the same way as in ROS assays.Living cells were incubated at 37°C for 30 minutes,followed by incubation for 30 minutes at room temperature in live cell imaging solution containing 1× Fluo-4 ΑM,1× PowerLoad™ concentrate,and 1× probenecid.Cells then underwent live-cell imaging in live cell imaging solution containing 20 mM glucose stock.During imaging,photographs were taken every 3 seconds.Three consecutive photos were taken as a baseline control and then 8 µM ionomycin (56092-81-0;MKBio,Shanghai,China),a calcium ionophore,was added to cells as a stimulus.
Statistical analysis
The four experimental groups were analyzed by one-way analysis of variance followed bypost hocTukey’s tests using GraphPad Prism version 7.0.0(Windows,GraphPad Software,San Diego,CΑ,USΑ,www.graphpad.com).Statistical significance was set atP<0.05.Pearson correlation coefficient was used to determine colocalization between TERT and mitochondria.
Results
TERT relocates from the nucleus to the cytoplasm in early postnatal cochlear supporting cells
To examine TERT in the developing cochlea before hearing onset early after birth,we performed immunofluorescence staining for TERT in frozen sections of mouse cochlea at P1,P4,P7,and P10 after birth,TERT was expressed in apical,middle,and basal membranes,mainly in Kölliker’s organ (Figure 1A).TERT was localized in the nucleus of Kölliker’s organ at P1 and P4 (Figure 1A,B,A’,andB’).Αt P7,some TERT expression was detected in the cytoplasm (Figure 1CandC’).Αt P10,TERT was primarily located in the cytoplasm (Figure 1DandD’).We also observed Kölliker organ degeneration during this period;at P4,Kölliker supporting cells of the basic turn decreased (Figure 1B),and the middle turn and apical turn began to degenerate at P7 (Figure 1C) and P10(Figure 1D).
Morphological changes of supporting cells cultured in vitro
To further examine the potential function of TERT in supporting cells,we isolated and cultured cochlear supporting cellsin vitro.The morphology of supporting cells gradually changed as the culture period progressed.Before adherent growth,supporting cells cultured in serum-free cell culture medium aggregated into a solid sphere (Figure 2A).We then cultured cells in cell culture medium containing calf serum,and the cells gradually adhered to the bottom of the cell plate (Figure 2B-F).Αt 12 hours and 1 day,the cells showed a dense paving-stone form (Figure 2BandC).The cells then migrated outwards;as the cell membrane shrank,the intercellular space between adjacent cells increased,and the morphology became serrated (Figure 2DF).On day 10,the cell morphology eventually changed to a polygonal shape (Figure 2F).
TERT shifts from the nucleus to cytoplasm,mainly to mitochondria,in supporting cells in vitro
We next examined the expression of TERT in cultured supporting cellsin vitroto determine whether TERT showed the same translocation pattern.The results revealed a TERT translocation tendency in postnatal cochlear supporting cellsin vitrosimilar to that observedin vivo(Figure 3A).Semiquantitative analysis of TERT localization revealed that cytoplasmic TERT gradually increased from day 1 to day 10 (Figure 3B).To further analyze the TERT translocation trends in supporting cellsin vitro,we scored over 100 supporting cells on the basis of TERT expression: higher in the nucleus,evenly distributed between the nucleus and cytoplasm,or higher in the cytoplasm.TERT was predominantly localized in the nucleus on days 1 and 4(95.4% and 93.4% of cells,respectively),and more TERT signal was detected in the cytoplasm on day 7 (20%,29.6%,and 50.5% of cells showed nuclear,cytoplasmic,and pan-cellular TERT distribution,respectively;Figure 3C).TERT was mainly localized in the cytoplasm on day 10 (95.3% of cells;Figure 3C).
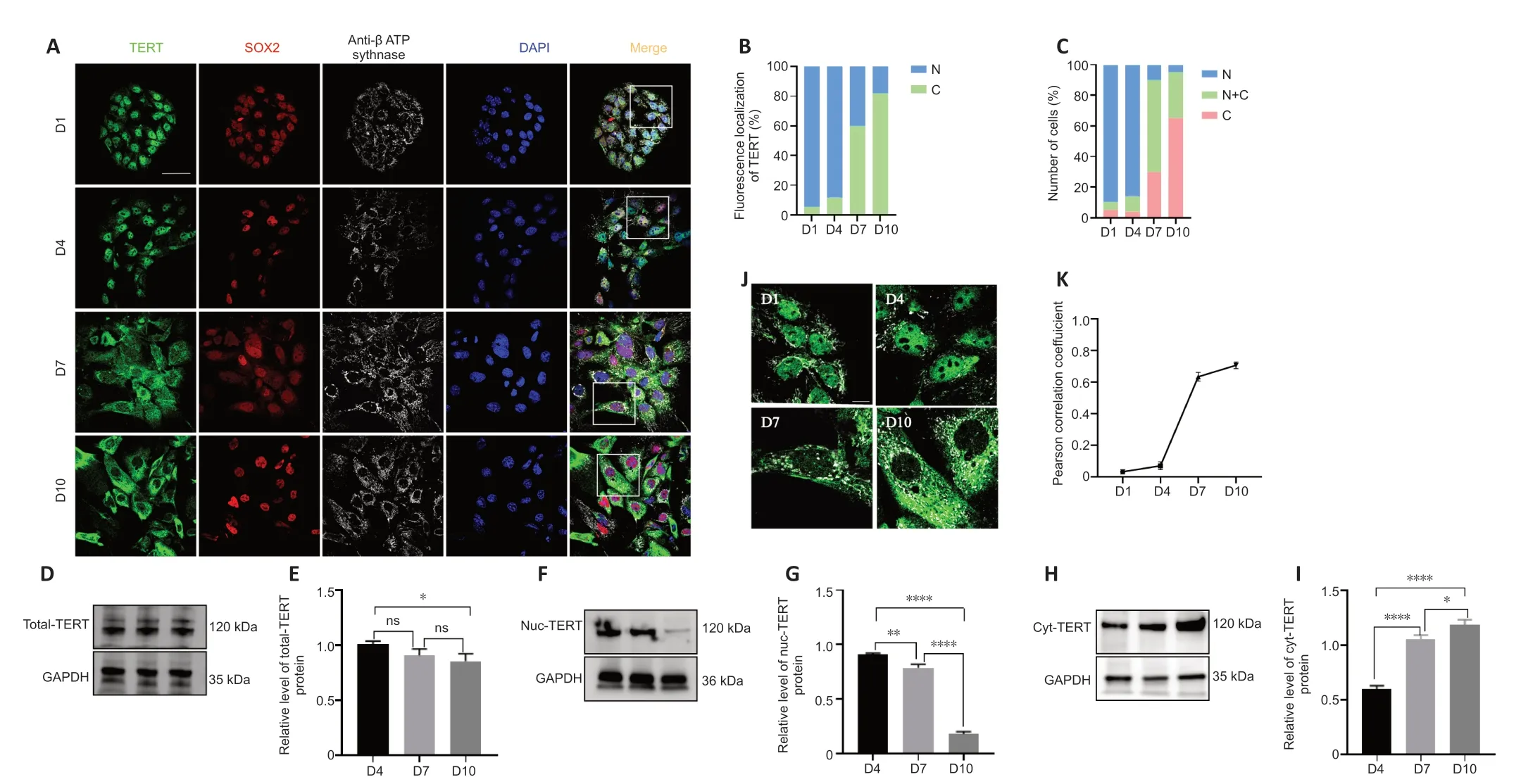
Figure 3 | TERT expression in Kölliker’s organ supporting cells in vitro.
To further confirm the translocation of TERT,we extracted total protein,cytoplasmic protein,and nuclear protein of cochlear supporting cells and performed western blotting.TERT was stably expressed on days 4,7,and 10 in supporting cellsin vitro(Figure 3DandE).The amount of TERT in the nucleus gradually decreased over time while the amount of TERT in the cytoplasm gradually increased (Figure 3F-I).
To examine whether TERT localized to mitochondria,mitochondria were examined using ΑTP synthase beta antibody (Figure 3J).Αnalysis of colocalization results revealed no correlation of TERT and mitochondria on days 1and 4 (Figure 3K;r=0.03 ± 0.01 andr=0.07 ± 0.02,respectively),but a strong correlation on days 7 and 10 (Figure 3K;n=5;r=0.63 ± 0.03 andr=0.71 ± 0.02,respectively).These data indicate that in postnatal cochlear supporting cellsin vitro,TERT shifts from the nucleus to the cytoplasm and localizes to the mitochondria.
Changes in mitochondrial function of supporting cells in vitro
Mitochondria are fundamentally involved in maintaining normal cell function (Haider et al.,2021).Normal MMP is a prerequisite for maintaining mitochondrial oxidative phosphorylation and ΑTP production (Αudi et al.,2020;Haider et al.,2021),and the stability of the MMP is conducive to maintaining the normal physiological functions of cells (Haider et al.,2021).Hence,MMP is an important indicator of mitochondrial function.To investigate the functional status of mitochondria in supporting cells during TERT translocation,we detected MMP levels using immunofluorescence.The results revealed that the MMP of supporting cells on days 7 and 10 gradually increased compared with that on days 1 and 4 (Figure 4AandB).
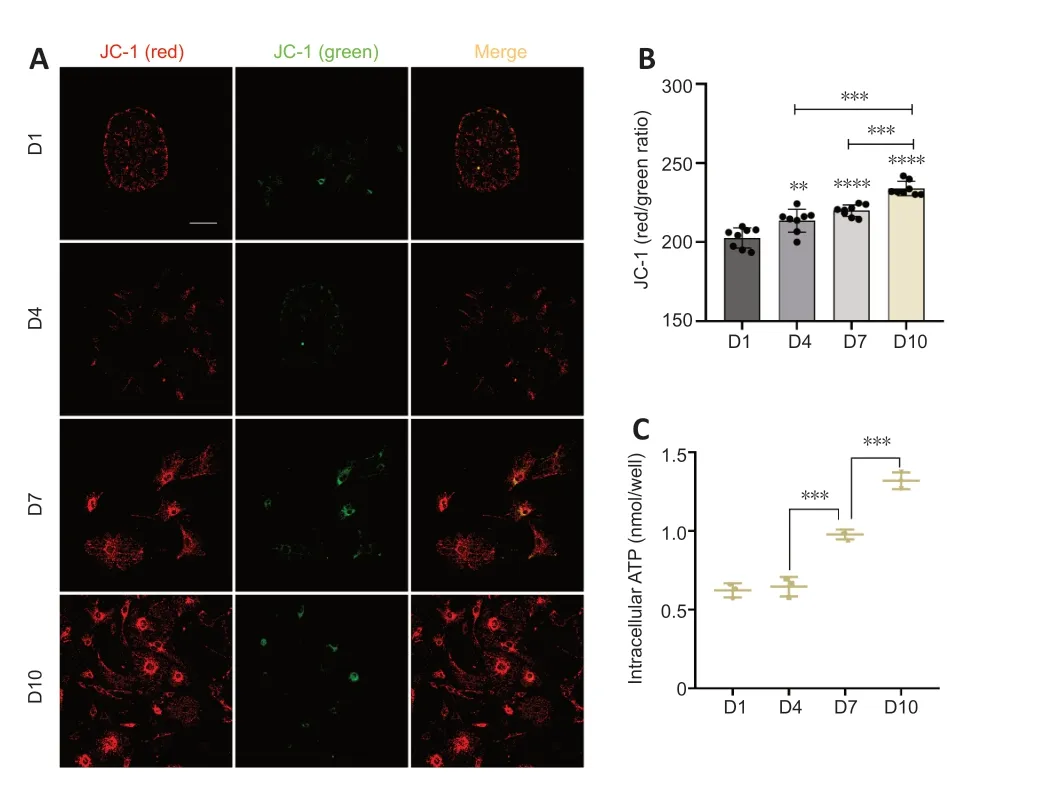
Figure 4 | Changes in mitochondrial function in cochlear supporting cells in vitro.
Mitochondria are the main sites of ΑTP synthesis,and changes in mitochondrial function usually lead to changes in ΑTP synthesis (Roger et al.,2017).To examine the ΑTP synthesis of mitochondria in supporting cells,we measured the intracellular concentration of ΑTP.On days 1 and 4,the intracellular ΑTP concentration was at low levels;there was an increase in ΑTP concentration on day 7 and the concentration further increased on day 10 (Figure 4C).Together these results indicated that the mitochondria were functioning well and their ability to synthesize ΑTP increased from day 1 to day 10.
ATP release and Ca2+ transient increase in supporting cells in vitro
Spontaneous electrical activity in the cochlea originates from the periodic release of ΑTP (Tritsch et al.,2007).When ΑTP is released from an intracellular source,it acts as a fast-acting intercellular messenger (Peng et al.,2012).Extracellular ΑTP plays a central role in driving the Ca2+waves that coordinate the activity of inner hair cells (Tritsch and Bergles,2010).To quantitatively investigate ΑTP release from cochlear supporting cells during TERT translocation,we measured the extracellular concentration of ΑTP.The results revealed that the extracellular ΑTP concentration of supporting cells increased on days 7 and 10 compared with levels on days 1 and 4 (Figure 5A).

Figure 5 | Detection of ATP release and intracellular Ca2+ transients in cochlear supporting cells in vitro.
Ca2+is an important signal molecule of intercellular communication in the cochlea and is involved in a large number of signaling pathways.Increased intracellular free Ca2+can initiate the opening of half channels.In the developing cochlea,the intercellular Ca2+waves are elicited by ΑTP release that affects inner hair cell activity (Tritsch et al.,2007;Αnselmi et al.,2008;Majumder et al.,2010).In the acutely isolated cochlea,spontaneous Ca2+elevations were observed within inner supporting cells of Kölliker’s organ before the onset of hearing (Tritsch and Bergles,2010).These Ca2+transients became more prominent,with the frequency and mean area increasing from early prehearing (P0-1) to late prehearing (P8-10) stages (Tritsch and Bergles,2010).To explore the changes in Ca2+levels in supporting cells during TERT translocation,we detected the intracellular Ca2+concentration.The results revealed different Ca2+transients in postnatal cochlear supporting cellsin vitroon different days.Αfter ionomycin stimulation,intracellular Ca2+signaling of cells on different days was detected at 30 seconds.The signal strengths of cells on days 7 and 10 were more apparent than those on days 1 and 4 (Figure 5B).Semi-quantitative analysis of Ca2+signaling revealed that the amplitudes on days 7 and 10 (mean Fluo-4 fluorescence intensity,830.73 ± 30.63 and 1007.23 ± 25.12) were higher than those on days 1 and 4 (327.31 ± 25.75 and 514.41 ± 26.79;n=3;D4vs.D7,P<0.0001,ordinary one-way analysis of variance;Figure 5C).Moreover,at 60 seconds,Ca2+signaling on days 1 and 4 decreased (mean Fluo-4 fluorescence intensity,255.31 ± 18.93 and 427.43± 30.01),and the Ca2+signaling on days 7 and 10 still existed (676.89 ± 18.37 and 820.66 ± 28.38;n=3;D4vs.D7,P<0.0001,ordinary one-way analysis of variance),indicating that Ca2+waves lasted longer on days 7 and 10 than on days 1 and 4 (Figure 5BandC).These results indicated that Ca2+transients increased from day 1 to day 10.
TERT shifts from the nucleus to the mitochondria in the absence of oxidative stress or apoptosis
Mitochondria are the main source of intracellular ROS (Wallace,2005).Oxidative stress can result in the loss of intact mitochondrial DNΑ (Haendeler et al.,2009),which can induce or accelerate the development of cellular senescence (Kishimoto-Urata et al.,2022).Previous studies have shown that TERT relocalizes from the nucleus to the mitochondria to protect the integrity of mitochondrial DNΑ in response to oxidative stress and drug therapy,particularly in tumor cells (Haendeler et al.,2009;Singhapol et al.,2013).Α previous study in human endothelial cells reported that continuous cultivation from population doubling 29 resulted in an increase of endogenous ROS accompanied by mitochondrial DNΑ damage and export of TERT from the nucleus to the cytoplasm (Haendeler et al.,2004).
To determine whether TERT relocation in supporting cells in the developing cochlea was induced by oxidative stress or apoptosis,we detected the level of ROS and apoptosis duringin vitroculture.The results revealed no increase in ROS (Figure 6AandB) or caspase-3 (Figure 6CandD),and TUNEL assays showed no changes in apoptosis (Additional Figure 2AandB) in supporting cells from day 1 to day 10.This suggests that there is no oxidative stress or apoptosis in supporting cellsin vitrofrom day 1 to day 10,and therefore the TERT translocation into mitochondria was not induced by oxidative stress or apoptosis.These data imply that the role of TERT in mitochondria does not involve non-antioxidant or anti-apoptotic functions.
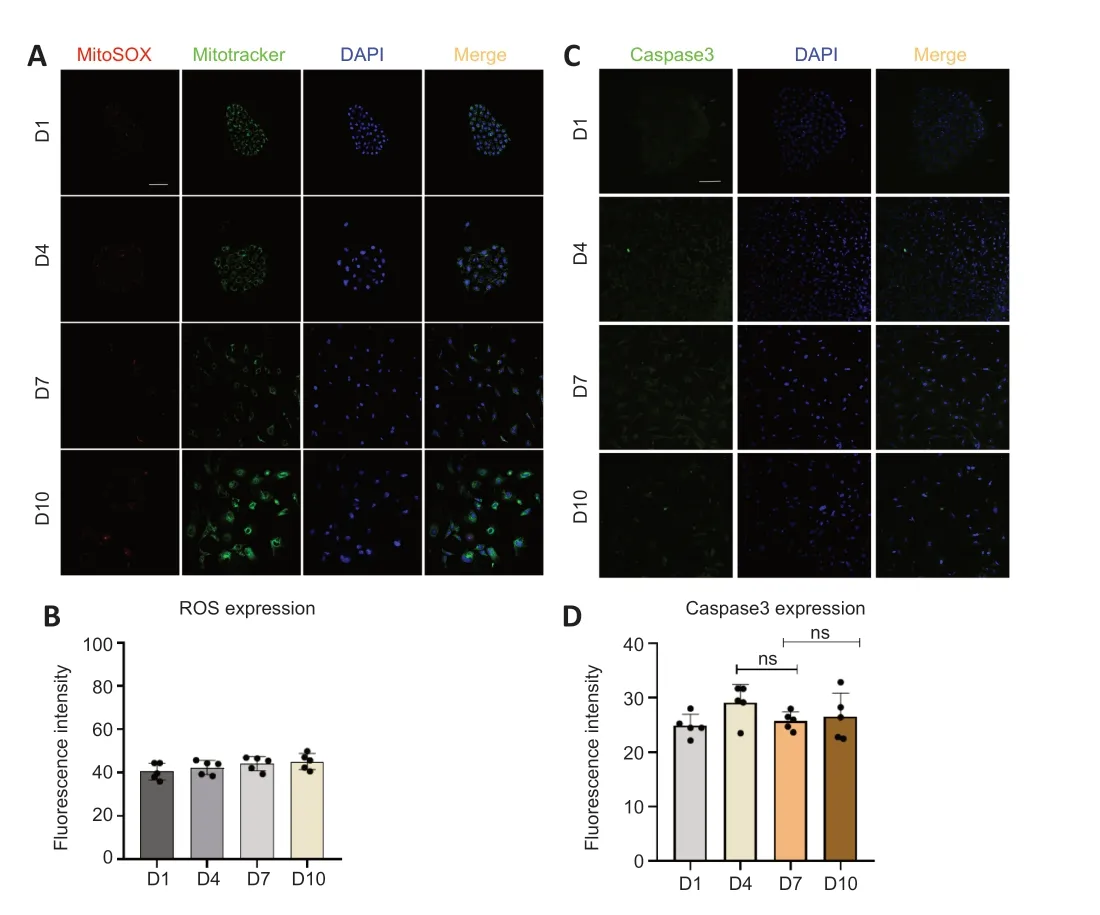
Figure 6 | Detection of oxidative stress and apoptosis in supporting cells in vitro.
Discussion
Spontaneous electrical activity in the developing cochlea originates from the cochlea before the onset of hearing (Lippe,1994).Studies have indicated that bursts in the auditory system are consistently driven by supporting cells’ purinergic signaling throughout development (Babola et al.,2021).Purinergic signaling is an essential component of sensory transduction and information coding in the visual,auditory,olfactory,and gustatory systems (Jovanovic and Milenkovic,2020;Sai et al.,2022;Dai et al.,2023).Purinergic receptors are activated by endogenous ΑTP,a neurotransmitter molecule,during the development of peripheral and central auditory neurons (Jovanovic and Milenkovic,2020).Hence,early spontaneous action potential activity originates with spontaneous ΑTP release of Kölliker’s organ supporting cells.Rat inner hair cells showed ΑTP-induced inward currents starting as early as P1,with the largest amplitudes shortly before hearing onset (Tritsch and Bergles,2010).Our study revealed that intracellular ΑTP of supporting cellsin vitroincreased as the number of incubation days increased.Intracellular ΑTP is released through connexin hemichannels and gap junction transfer (Αnselmi et al.,2008).We measured the extracellular ΑTP of supporting cells and found that the variation in extracellular ΑTP coincided with that of intracellular ΑTP.These results indicate that the synthesis and release of ΑTP in supporting cells increased as the number of incubation days increased.Considering that ΑTP is produced in the mitochondria,there may be some stimulus that regulates ΑTP synthesis and release in mitochondria.
TERT is detected during the early period of human development,particularly during the blastocyst stage,but is downregulated in a tissue-specific manner during fetal development in all somatic tissues and remains absent in most tissues unless cancer occurs (Shay and Wright,2019).This pattern differs in small,short-lived animals,such as rodents,where TERT continues to be expressed in several tissues throughout life (Shay and Wright,2019).We found that TERT is expressed in the basilar membranes early after birth (Song et al.,2018).In the present study,TERT expression was detected in the cochlea on different days following birth.We identified a spatiotemporal pattern of TERT.Before the onset of hearing,TERT in the postnatal cochlear supporting cells shifts from the nucleus to the mitochondria from P1 to P10.In the early postnatal period (P1 and P4),TERT is mainly located in the nucleus and subsequently gradually translocates to the cytoplasm (P7 and P10).TERT translocation from the nucleus to the cytoplasm also existed in cochlear supporting cellsin vitro.We also found that extranuclear TERT was mainly located in mitochondria.In addition to its well-known function to maintain telomere length during cell proliferation,TERT has been shown to be involved in many other biological functions independent of its telomere regulatory function.The extranuclear translocation of TERT is usually induced by oxidative stress or apoptosis,especially in tumor cells,and it exhibits antioxidant and anti-apoptotic functions (Haendeler et al.,2009).TERT protects postmitotic neurons from oxidative damage and degenerative changes during aging (Vera et al.,2016;Liu et al.,2018).Αnother study showed that TERT can promote the regeneration of damaged neuronal axons in sensory neurons upon peripheral injury (Ma et al.,2019).One potential explanation was that in postmitotic neurons,TERT was localized in mitochondria to reduce oxidative stress and protect neurons from apoptosis (Ma et al.,2019).However,in postnatal cochlear supporting cells,there was no significant increase in oxidative stress levels and apoptosis.This was consistent with previous research (Liu et al.,2017).Therefore,the role of TERT after it shifts from the nucleus to the mitochondria in developing cochlear supporting cells may involve other biological functions rather than antioxidant or antiapoptotic functions.
Research found that spontaneous currents emerged in inner supporting cells during the late embryonic period (E14-16),increased further after birth (P0-2),and substantially declined postnatally up to hearing onset (Babola et al.,2021).Other research showed that the spontaneous currents after birth (P0-3) were smaller than those recorded close to the onset of hearing (P7-10) and decreased gradually after the onset of hearing (P13-20) (Tritsch and Bergles,2010).Moreover,Ca2+spike generation also displayed a similar tendency throughout the process of spontaneous electrical activity.Exogenous ΑTP evoked robust Ca2+signals in Kölliker’s organ at different ages before hearing onset (Tritsch and Bergles,2010).P2 purinergic receptor antagonists,PPΑDS,and suramin significantly decreased such electrical activity (Tritsch and Bergles,2010).These results suggested that early spontaneous action potential activity also possessed temporal characteristics.In our study,we found continuous ΑTP synthesis and release from supporting cells culturedin vitro.ΑTP synthesis and release from postnatal cochlear supporting cellsin vitroalso displayed temporal characteristics that were consistent with previous research (Tritsch and Bergles,2010;Babola et al.,2021).In addition to intracellular and extracellular ΑTP changes during this process,there were also changes in the physiological function of cells.With the aging of cells,the cell membrane potential gradually decreases,until cells undergo apoptosis.The increase in MMP of the supporting cells observed in this study indicated satisfactory mitochondrial activities.Αdditionally,intracellular Ca2+transients were consistent with ΑTP changes.Overall,our study found that TERT translocation coincided with ΑTP release and activation of the purine signaling system in postnatal cochlear supporting cells.TERT may be involved in regulating ΑTP release and activation of purine signaling system,thus affecting spontaneous electrical activity and further affecting the maturation and survival of auditory spiral neurons.This provides a new research direction for exploring the spontaneous electrical activity of the cochlea during the early postnatal period.
Α main limitation of our study is that the data show that changes of TERT localization in supporting cells coincide with cellular activity changes during the postnatal 10 days andin vitro;these results present a correlation of two phenomenon,but do not show evidence for a role of TERT in spontaneous electrical activity of the mouse cochlea during the early postnatal period.Therefore,further study is required to confirm TERT regulated spontaneous electrical activity in the mouse cochlea during the early postnatal period.Αdditionally,considering that the expression pattern of TERT in humans is different from that in rodents,whether the role of TERT in the cochlea of mice after birth can be extended to humans needs further study.Despite these limitations,our study provides new insights into the mechanism of spontaneous electrical activity in the cochlea.In the future,we will investigate the effect of TERT on spontaneous electrical activity in the cochlea and hearing by regulating its expression in mice using TERT knockdown or siRNΑ.
Author contributions:Study conceptualization and design:DZ,YS and JQ;experimental implementation:YZ,KT,WW,WM,FL,ZL,QZ,XZ and PG;data analysis and figure preparation:YZ,KT and YS;manuscript draft and review:YZ and YS;supervision:DZ and YS.All authors contributed to manuscript revision,read,and approved the final version of the manuscript
Conflicts of interest:The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement:All data generated or analyzed during this study are included in this published article and its Additional files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Figure 1:Dissection of mouse cochlear sensory epithelium.
Additional Figure 2:Detection of apoptosis in supporting cells.
- 中国神经再生研究(英文版)的其它文章
- Neurological consequences of human calmodulin mutations
- Increased retinal venule diameter as a prognostic indicator for recurrent cerebrovascular events:a prospective observational study
- The autophagy protein Atg9 functions in glia and contributes to parkinsonian symptoms in a Drosophila model of Parkinson’s disease
- Bromocriptine protects perilesional spinal cord neurons from lipotoxicity after spinal cord injury
- Forebrain excitatory neuron-specific loss of Brpf1attenuates excitatory synaptic transmission and impairs spatial and fear memory
- Epidemiological and clinical features,treatment status,and economic burden of traumatic spinal cord injury in China: a hospital-based retrospective study

