Exploration of cyclooxygenase-2 inhibitory peptides from walnut dreg proteins based on in silico and in vitro analysis
Zishan Hong, Jing Xie, Liang Tao, Jing-Jing Dai, Tingting Li, Li Zhang,Yuying Bai, Xia Hu, Jinlian Chen, Jun Sheng, Yang Tian
a College of Food Science and Technology, Yunnan Agricultural University, Kunming 650201, China
b Yunnan Provincial Key Laboratory of Precision Nutrition and Personalized Food Manufacturing, Yunnan Agricultural University, Kunming 650201, China
c Engineering Research Center of Development and Utilization of Food and Drug Homologous Resources, Ministry of Education, Yunnan Agricultural University, Kunming 650201, China
d School of Tea and Coffee, Pu’er University, Pu’er 665000, China
e Yunnan Provincial Engineering Research Center for Edible and Medicinal Homologous Functional Food, Yunnan Agricultural University, Kunming 650201, China
f Key Laboratory of Pu-er Tea Science, Ministry of Education, Yunnan Agricultural University, Kunming 650201, China
Keywords: Walnut dreg proteins Cyclooxygenase-2 inhibitory peptide Identif ication Virtual screening Molecular docking
ABSTRACT Walnut dreg protein hydrolysates (WDPHs) exhibit a variety of biological activities, however, the cyclooxygenase-2 (COX-2) inhibitory peptide of WDPHs remain unclear. The aim of this study was to rapidly screen for such peptides in WDPHs through a combination of in silico and in vitro analysis. In total, 1 262 peptide sequences were observed by nano liquid chromatography/tandem mass spectrometry (nano LC-MS/MS)and 4 novel COX-2 inhibitory peptides (AGFP, FPGA, LFPD, and VGFP) were identif ied. Enzyme kinetic data indicated that AGFP, FPGA, and LFPD displayed mixed-type COX-2 inhibition, whereas VGFP was a non-competitive inhibitor. This is mainly because the peptides form hydrogen bonds and hydrophobic interactions with residues in the COX-2 active site. These results demonstrate that computer analysis combined with in vitro evaluation allows for rapid screening of COX-2 inhibitory peptides in walnut protein dregs.
1. Introduction
Cyclooxygenase-2 (COX-2) is an important enzyme with a variety of biological roles[1]. In recent years it has attracted attention for its multiple functions and wide range of applications in the food and medical industries. COX-2, a key mediator of inf lammation, has been identified as an important therapeutic target for inflammation,diabetes, and analgesia[2-4]. Although COX-2-inhibiting drugs have been extensively studied, there is considerable research evidence that these drugs have signif icant side effects[5-6]. Therefore, the production of COX-2 inhibitors from natural products is of great importance.
Walnuts(JuglansregiaL.) are one of the world’s most important nuts. According to reports, China’s annual production of walnuts(in shell) is estimated at 2 521 504 metric tons, ranking first in the world[7]. At present, the deep processing of walnuts is mainly based on walnut oil, the production and processing of which generate large quantities of walnut dregs as a byproduct. Walnut dregs are rich in essential amino acids and have high nutritional value with the potential for related product development[7]. However, the utilization of these byproduct resources generated during walnut processing remains insuff icient, and they are generally used as forage or fertilizer.Several studies have demonstrated not only that walnut proteins possess high nutritional value but also that walnut-derived peptides display high levels of biological activity, including anti-inf lammatory and antioxidant effects and the inhibition of dipeptidyl peptidase IV(DPP-IV) and angiotensin-converting enzyme (ACE)[8-10]. Therefore,the proteins present in walnut dregs are a rich source of bioactive peptides. To the best of our knowledge, however, little information is currently available on the production of COX-2-inhibiting peptides from walnut dreg proteins.
Virtual screening is a targeted search for active small molecules from databases[9]. Compared with traditional methods for separating bioactive peptides from proteins (such as ultrafiltration, gel exclusion chromatography, and reverse-phase HPLC)[11], virtual screening and molecular docking techniques are flexible, reliable, fast, and efficient. In addition, a combination of virtual screening andinvitroexperiments can be effective for screening and identifying potential bioactive peptides in proteins[12].
The main objectives of this study were to identify potent COX-2 inhibitory peptides from walnut dreg proteolysates by combining traditional experimental methods withinsilicovirtual screening and to elucidate the interaction mechanisms of these peptides with COX-2.
2. Materials and methods
2.1 Materials and reagents
Walnut dregs were provided by Dali Yunshang Purui Agriculture Co., Ltd. (Dali, China). Alcalase (200 000 U/g), trypsin (25 000 U/g),papain (80 000 U/g), flavourzyme (30 000 U/g), chymotrypsin(100 000 U/g), and neutrase (50 000 U/g) were purchased from Solarbio (Beijing, China). Acetonitrile was purchased from Fisher Chemical (USA). Formic acid, ammonium bicarbonate, dithiothreitol,and iodoacetamide were mass-spectrometry grade and purchased from Sigma-Aldrich (USA). All other chemicals used in this study were of analytical grade.
2.2 Preparation of walnut dreg proteins
Proteins were extracted from walnut dregs as previously described[13]. The walnut dregs were ground into flour and degreased with petroleum ether at a ratio of 1:10 (m/V) for 4 h. After degreasing,the petroleum ether was removed by suction filtration, and the flour was dried and ground into a fine powder. The defatted flour was then dispersed in 1 mol/L NaOH solution at a ratio of 1:15 (m/V), the pH was adjusted to 9.0, and extraction was performed with stirring at 45 °C for 1 h. After adjusting the pH of the supernatant to 4.5 with 1.0 mol/L HCl, the precipitate was obtained through centrifugation at 4 000 r/min for 20 min. Finally, the precipitate was washed with distilled water to pH 7.0, frozen, and stored at −20 °C.
2.3 Preparation of walnut dreg protein hydrolysates (WDPHs)
WDPHs were prepared as described in previous reports[9,14]with some modifications using alcalase, trypsin, papain, flavourzyme,chymotrypsin, and neutrase. The specific enzymatic hydrolysis conditions are listed in Table S1. A protein solution (5%) was prepared, placed in a 90 °C water bath for 15 min to denature the protein, and the pH was adjusted to the optimum range of the corresponding protease. An appropriate amount of the protease was then added, and the reaction solution was placed in a constant-temperature water bath to maintain the optimum temperature of the protease. After 4 h of the enzymatic hydrolysis reactions,samples were taken and the enzymes were inactivated in a boiling water bath for 10 min. After cooling, the solutions were adjusted to neutral pH and centrifuged at 4 000 r/min for 20 min, and the supernatants were lyophilized overnight.
2.4 Ultrafiltration
The digests were resuspended in distilled water (50 mg/mL)and purified using ultrafiltration membranes with molecular weight cutoffs of 10, 5, 3, and 1 kDa (Moso Technology Co., Ltd., Shanghai,China). Four fractions (< 1, 1–3, 3–5, and 5–10 kDa) were collected and lyophilized separately for subsequent use.
2.5 Nano liquid chromatography/tandem mass spectrometry(nano LC-MS/MS) analysis
The samples were characterized using a capillary highperformance liquid chromatography system (Easy-nLC 1200, Thermo Fisher Scientific, USA) connected to an electrospray-ion trap Orbitrap mass spectrometer (Q Exactive Hybrid Quadrupole-Orbitrap Mass Spectrometer, Thermo Fisher Scientific, USA). Five microliters of the sample was injected onto a 150 μm × 15 cm in-house made column packed with reverse-phase ReproSil-Pur C18-AQ resin (1.9 μm,100 Å, Dr. Maisch GmbH, Germany). Mobile phase A was 0.1%formic acid in water, and mobile phase B consisted of 20% of 0.1% formic acid in water and 80% acetonitrile. The samples were separated using the following gradient: from 6% to 9% B over 5 min,from 9% to 14% B over 15 min, from 14% to 30% B over 30 min,from 30% to 40% B over 8 min, and from 40% to 95% B over 2 min.The spray voltage was 2.2 kV, the capillary temperature was 270 °C,the MS resolution was 70 000 atm/z400, and the MS precursor range wasm/z300.0–1 400.0. The raw MS files were analyzed and searched against a protein database based on the species of the samples using Byonic.
2.6 Virtual screening and molecular docking
Peptides with activity scores of > 0.5 were selected using PeptideRanker (http://distilldeep.ucd.ie/PeptideRanker/). The antiinflammatory potential was estimated using AIPpred (http://www.thegleelab.org/AIPred/), and the potential anti-inflammatory peptides were screened.
Peptide structures were constructed using ChemBioDraw Ultra 14.0 and imported into ChemBio3D Ultra 14.0 for energy minimization. The structure of COX-2 was downloaded from the PDB database (PDB ID: 3LN1). PyMOL 2.3.0 was then used to remove the protein crystal water and original ligands, and the protein structure was imported into AutoDockTools 1.5.6 for processing and was saved saved in the “pdbqt” format. Next, AutoDock Vina 1.1.2 was used for docking, and the 3LN1-related parameters were set as follows:center_x= 30.179, center_y= −23.19, center_z= −14.527; search space:size_x= 27.75, size_y= 27.75, size_z= 28.5 (the spacing of each grid point was 0.375 Å), and exhaustiveness = 10. The other parameters were kept at the default settings. Interaction pattern analysis was performed on the docking results using PyMOL 2.3.0 and LIGPLOT 2.2.4.
2.7 Peptide synthesis
Peptides were synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd. (Shanghai, China) using the Fmoc solid-phase synthesis method, and the purity of the synthesized peptides was > 98%.
2.8 Analysis of the physicochemical properties of the peptides
All of the peptides were checked for toxicity using the ToxinPred server (http://crdd.osdd.net/raghava/toxinpred/)[15]. PepDraw (http://pepdraw.com/) was used to estimate the physicochemical properties of the peptides. BioPepDB (http://bis.zju.edu.cn/biopepdbr/index.php)[16]and EROP-Moscow (http://erop.inbi. ras.ru/index.html)[17]were used to check the novelty of the peptides, the GRAVY calculator(http://www.gravy-calculator.de/index.php) was used to assess the hydrophobicity of the peptides, and PeptideCutter (https://web.expasy.org/peptide_cutter/) was used to predict the potential cleavage sites and cleaved peptide sequences of the polypeptides under the action of pepsin (pH > 2) and trypsin.
2.9 Cyclooxygenase-2 inhibitory activity and inhibition mode
The samples were evaluated forinvitroinhibition using a COX-2 inhibitor screening kit (Beyotime, Shanghai, China) based on previous methods with minor modifications[18]. Briefly, the COX-2 cofactor working solution and COX-2 working solution were added to the COX-2 assay buffer, and then the sample solutions of various concentrations were added. After incubation at 37 °C for 10 min, the COX-2 probe and COX-2 substrate were added. After incubation at 37 °C for an additional 5 min in the dark, the fluorescence was measured at an excitation wavelength of 560 nm and an emission wavelength of 590 nm. Each sample was tested in triplicate, the average fluorescence value was calculated, and the inhibition rate was calculated as follows:
whereRFU100%activity,RFUsample, andRFUcontroldenote the fluorescence values for the 100% enzyme control group, test sample,and blank control, respectively. The sample solution of the 100%enzyme activity control group was replaced with equal volume dimethyl sulfoxide (DMSO).
The type of inhibition of COX-2 by the peptides and corresponding inhibition constants were determined using Lineweaver–Burk plots. The assay method was the same as that used for the inhibitory activity, and the peptides were assayed at IC50and 1/2 IC50with different substrate concentrations (0.25, 0.5, and 1.0 COX-2 substrate).
2.10 Statistical analysis
All experiments were conducted in triplicate and the data are presented as the mean ± standard error of the mean (SEM). Statistical analysis was performed using one-way analysis of variance (ANOVA)(P< 0.05) followed by Tukey’s multiple comparison test with GraphPad Prism 6.01 (GraphPad Software, San Diego, USA).
3. Results and discussion
3.1 Degree of hydrolysis (DH) and COX-2 inhibitory activity of WDPHs
Because different proteases possess distinct activation centers and specificities, the biological activities of the resulting hydrolysates can also vary[19]. Therefore, to screen for the WDPH with the highest COX-2 inhibitory activity, six proteases were used to hydrolyze the walnut dreg proteins, and the resulting DH values are plotted in Fig. 1A. We found that the alcalase hydrolysate (Al-WDPH)and neutrase hydrolysate (Ne-WDPH) exhibited the highest DH values of 26.8% and 26.4%, respectively, which is consistent with the results of previous studies[9]. The main reason for this is that alcalase and neutrase are endonucleases with broad specificity and efficient hydrolysis, thus affording higher DH values for walnut proteins compared with other enzymes[20-21]. Furthermore, although flavourzyme contains endopeptidase and exopeptidase activities, this enzyme preparation failed to produce a high DH value compared with alcalase and neutrase owing to its low endopeptidase activity and lack of cleavage sites[22]. We note that the papain hydrolysate(Pa-WDPH) displayed the lowest DH of 6.00%. Although this finding is inconsistent with the results of Jin et al.[23], it is in accordance with those of Rawdkuen et al.[24]. This discrepancy may be attributable to the different sources of protein substrates, with different protein conformations resulting in different numbers of peptide bonds being accessible to the protease, thus altering the hydrolysis rate[24-25].
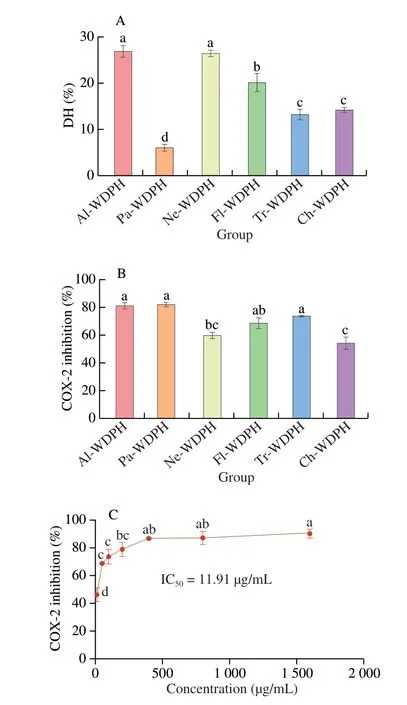
Fig. 1 (A) DH values of the WDPHs obtained using alcalase (Al-WDPH),trypsin (Tr-WDPH), papain (Pa-WDPH), flavourzyme (Fl-WDPH),chymotrypsin (Ch-WDPH), and neutrase (Ne-WDPH). (B) COX-2 inhibitory activities of the six enzymatic hydrolysates. (C) Dose-dependent COX-2 inhibitory activity of Al-WDPH. Different letters (a–d) in the plots indicate significant differences (P < 0.05).
Previous studies have demonstrated that WDPHs can alleviate lipopolysaccharide-induced memory impairment by mitigating brain inflammation and oxidative stress[26]. However, the COX-2 inhibitory activities of WDPHs remain unclear. Therefore, in the present study, we evaluated the COX-2 inhibitory activities of six protease hydrolysates. We found that the different WDPHs inhibited COX-2 to varying degrees, with Al-WDPH, Pa-WDPH, and trypsin hydrolysate(Tr-WDPH) displaying the highest degrees of COX-2 inhibition(Fig. 1B). It has been reported that peptide sequences containing aromatic and hydrophobic amino acids tend to exhibit COX-2 inhibitory activity[27]as well as anti-inflammatory[28]and antioxidant activity[29]. Notably, alcalase is highly specific for hydrolyzing peptide bonds to the right of hydrophobic (leucine, methionine) and aromatic(tyrosine) amino acids[30]. Moreover, papain shows high specificity for amino acids with hydrophobic or aromatic side chains such as valine, phenylalanine, and tyrosine[31]. Therefore, alcalase and papain can produce large amounts of peptides containing hydrophobic and aromatic amino acids when hydrolyzing proteins, thus affording high levels of COX-2 inhibitory activity. Furthermore, according to Rajasekaran et al.[32], arginine- and lysine-containing antimicrobial peptides have strong anti-inflammatory activity. Therefore,because trypsin is an endoprotease that specifically cleaves after lysine and arginine[33], Tr-WDPH likewise exhibited high COX-2 inhibitory activity.
It has been reported that the biological activity of enzyme hydrolysates depends on the source protein, hydrolysis conditions,DH, molecular mass, amino acid composition, and amino acid sequence[34]. The peptides produced through alcalase hydrolysis are known to exert various biological effects, such as hypoglycemic,antioxidant, and anti-inflammatory activities[30]. In the present study, Al-WDPH displayed the highest DH and the strongest COX-2 inhibitory activity, and it was further found to significantly inhibit COX-2 activity in a dose-dependent manner with an IC50of 11.91 μg/mL (Fig. 1C). The IC50value of the hydrolysate obtained from walnut proteins using alcalase in this study indicated superior activity compared with those obtained from other proteins in previous reports. For example, the hydrolysate (concentration 1.75 mg/mL) of lupine proteins obtained using alcalase showed an inhibitory activity of 90% against COX-2[35]. In addition, the IC50value for COX-2 inhibition by Al-WDPH was close to those reported for other active compounds. For example, the IC50values for physalisitins A, B,and C, three new sesquiterpenoids isolated from the medical plantPhysalisalkekengiL. var.franchetii, were (3.22 ± 0.25), (6.35 ± 0.84),and (11.13 ± 1.47) μmol/L, respectively[36]. Furthermore,Nelumbo nuciferaleaf extract was reported to show COX-2 inhibitory activity with an IC50of (8.69 ± 0.76) μg/mL[18]. Therefore, we concluded that Al-WDPH had strong research potential among the six WDPHs and represented an excellent source of bioactive peptides. Notably,although Pa-WDPH exhibited slightly higher COX-2 inhibition,its DH was markedly lower, suggesting that the COX-2 inhibitory activity of Pa-WDPH was not directly related to its DH.
3.2 Purification of Al-WDPH to obtain COX-2 inhibitory peptides
To further screen the molecular weight fractions with COX-2 inhibitory activity, we subjected Al-WDPH to membrane ultrafiltration. We thus divided Al-WDPH into 4 fractions with apparent molecular weights of < 1 kDa (M1), 1–3 kDa (M2),3–5 kDa (M3), and 5–10 kDa (M4) and assayed their COX-2 inhibitory activities. It was found that the lower the molecular weight of the ultrafiltration fraction, the stronger the COX-2 inhibitory activity (Fig. 2A). In addition, the < 1 kDa fraction significantly inhibited COX-2 activity in a dose-dependent manner with an IC50value of 131.6 μg/mL (Fig. 2B). It has been reported that components with molecular weights of < 1 kDa displayed higher activity for both ACE-inhibiting peptides derived fromCucumariafrondosaandα-glucosidase-inhibiting peptides obtained from wheat germ[37].In addition, peptides with lower molecular weights are more easily absorbed and have better physiological activities[38]. This is in agreement with our study, and we therefore selected the < 1 kDa fraction for subsequent characterization by nano LC-MS/MS.
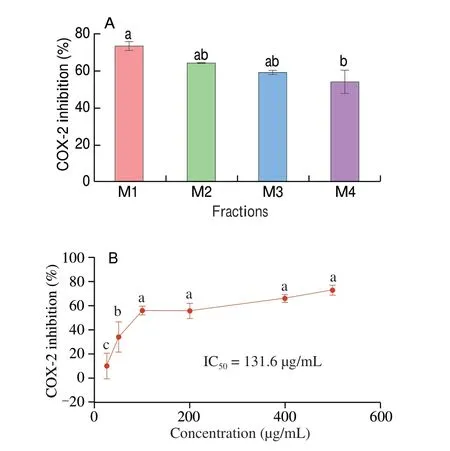
Fig. 2 (A) COX-2 inhibitory activities of the ultrafiltration fractions of Al-WDPH (M1–M4). (B) Dose-dependent COX-2 inhibitory activity of the ultrafiltration fraction M1 (< 1 kDa). Different letters (a–c) in the plots indicate significant differences (P < 0.05).
3.3 Identification and virtual screening of potential bioactive peptides
A total of 1 262 unique peptides were identified from the < 1 kDa fraction of Al-WDPH using nano LC-MS/MS. Previous studies have shown that bioactive peptides with 2–20 amino acid residues can exert significant biological effects[39]. In our study, the peptides from the < 1 kDa fraction of Al-WDPH contained 3–10 amino acid residues. Of these, peptides with 4 and 5 amino acid residues were more abundant at 30.61% and 34.66%, respectively (Fig. S1A),suggesting that peptides derived from walnut dregs also have potential bioactivity for the development of functional foods.
Furthermore, hydrophobic amino acids accounted for 65.90% and 40.13% of the C- and N-terminal residues, respectively. In particular,the hydrophobic amino acid leucine and the aromatic hydrophobic amino acid phenylalanine accounted for 17.37% and 12.45% of the C-terminal residues, respectively (Fig. S1B). It has been shown that peptide sequences containing both aromatic and hydrophobic amino acids can inhibit COX-2[27]. This suggests that peptides derived from walnut dreg proteins could be used to design COX-2 inhibitory peptides. In addition, peptides containing hydrophobic amino acids at the penultimate N-terminal position and at the N-terminus are known to display better biological activity[40]. Similarly, in our results, the amino acid residues at the penultimate N- and C-terminal positions were mainly the hydrophobic amino acids Leu, Phe, and Val(Fig. S1B). Therefore, we speculated that these peptide sequences may display COX-2 inhibitory activity.
Next, to screen for potentially active peptides among the 1 262 identified peptides, their activities were predicted using PeptideRanker,resulting in 468 potentially active peptides with activity scores of > 0.5. Then, peptides with potential anti-inflammatory activity were screened using AIPpred to obtain 363 potential anti-inflammatory peptides.
To further focus on the screening of COX-2 inhibitory peptides,molecular docking was used for virtual screening. The binding capacities of the potential bioactive peptides to COX-2 were evaluated on the basis of their binding energies. In general, a lower score (binding energy value) indicates a more rational and stable interaction between the ligand and protein[41]. Our results indicated that all peptides show different degrees of binding to the active site of the receptor protein,with binding energies ranging from 1.84 kcal/mol to −9.2 kcal/mol.Notably, AGFP, FPGA, LFPD, and VGFP exhibited the best binding affinities with binding energies of −9.1, −8.1, −8.4, and −9.2 kcal/mol,respectively (Table 1), which indicated that the combinations of these 4 peptides with COX-2 were more stable and the stability decreased in the order of VGFP > AGFP > LFPD > FPGA; The secondary mass spectrum of the peptides are shown in Fig. S2. Moreover, these binding energies of the peptides were comparable to those of other known COX-2 inhibitors, such as celecoxib with a binding energy of −8.05 kcal/mol[42].Therefore, we speculated that the peptides AGFP, FPGA, LFPD, and VGFP could interact well with COX-2.
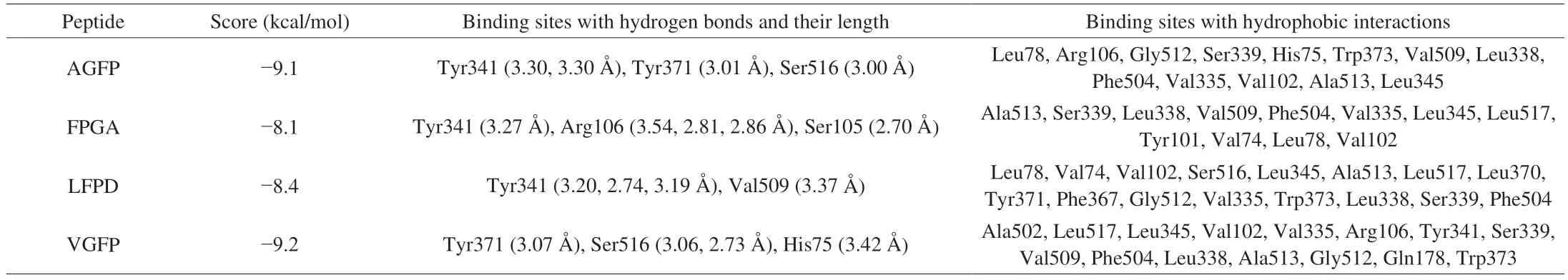
Table 1 COX-2 binding sites of the peptides determined by virtual screening.
3.4 Interaction of four potential bioactive peptides with COX-2
The exact binding sites and interactions between LFPD, FPGA,AGFP, and VGFP and COX-2 (PDB ID: 3LN1) were next examined(Fig. 3). The 4 peptides were found to be deeply docked in the active site pocket of COX-2, in a manner similar to selective COX-2 inhibitors, and deeply immersed in the main hydrophobic pocket[43].The COX-2 active site mainly contains hydrophobic amino acid residues (Val335, Leu338, Ile503, Val509, and Ala513), aromatic amino acids (Tyr341, Tyr371, Trp373, and Phe504), polar uncharged amino acids (Ser339 and Ser516), and basic amino acids (His75 and Arg499)[44]. In our study, the predominant interactions observed between the 4 peptides and residues of COX-2 were hydrogen bonding and hydrophobic interactions (Table 1). Similar findings were also reported by Sharma et al.[27]. FPGA was found to form one hydrogen bond with the active-site Tyr341 (3.27 Å) in the COX-2 pocket, four hydrogen bonds with Arg106 (3.54, 2.81, and 2.86 Å)and Ser105 (2.70 Å), and 12 hydrophobic interactions (Fig. 3B and Table 1). LFPD was found to form four hydrogen bonds with Tyr341(3.20, 2.74, and 3.19 Å) and Val509 (3.37 Å) of the active site within the COX-2 pocket, an electrostatic interaction with Arg106, and 16 hydrophobic interactions (Fig. 3C and Table 1). However, AGFP and VGFP were better aligned to the COX-2 binding site. AGFP formed four hydrogen bonds with the active-site Tyr341 (3.30 and 3.30 Å),Tyr371 (3.01 Å), and Ser516 (3.00 Å) within the COX-2 pocket and 13 hydrophobic interactions (Fig. 3A and Table 1). VGFP formed four hydrogen bonds with the active-site Tyr371 (3.07 Å), Ser516(3.06 and 2.73 Å), and His75 (3.42 Å) and 15 hydrophobic interactions (Fig. 3D and Table 1). This may be the reason for the superior docking scores for AGFP and VGFP.

Fig. 3 3D structural diagrams and 2D interaction diagrams depicting the molecular docking of COX-2 and (A) AGFP, (B) FPGA, (C) LFPD, and (D) VGFP.
Furthermore, Sumaryada et al.[45]demonstrated that Val335,Leu338, Ser339, Phe504, Val509, Ala513, and other amino acids play important roles in the interaction of COX-2 with its ligands and predicted the mechanisms of COX-2 inhibition. In our study, the 4 peptides were found to engage in hydrophobic interactions with these amino acid residues of COX-2, suggesting that the peptides could be good inhibitors of COX-2 activity. Studies have shown that amino acids containing cyclic structures or aromatic amino acids such as phenylalanine can be used to design structures that mimic COX-2 inhibitors[46]. In addition, the presence of hydrophobic amino acid residues such as proline can increase the anti-inflammatory specificity of a peptide[47]. Indeed, it has been demonstrated that the walnut-derived peptide LPF (leucine-proline-phenylalanine) has antiinflammatory activityinvivo[8]. Moreover, there is additional evidence that the active site of COX-2 mainly contains hydrophobic amino acid residues, which can facilitate the binding of hydrophobic peptides to COX-2[47]. In our study, the peptides AGFP, FPGA, LFPD, and VGFP contain hydrophobic and aromatic amino acid residues that can bind tightly to COX-2 and exert anti-inflammatory activity, which fully supports our speculation. However, because molecular docking is a virtual screening method, the screened and predicted potential COX-2 inhibitory peptides also had to be confirmed through biological activity evaluation.
3.5 Analysis of the properties of COX-2 inhibitory peptides
To examine the novelty of the identified peptides, searches were performed against the BioPepDB and EROP-Moscow databases.We found that these are novel peptides that have not been reported previously. In addition, online tools were used to predict the physicochemical properties of the COX-2 inhibitory peptides, as shown in Table S2. The COX-2 inhibitory peptides were non-toxic.In addition, the hydrophilic indices estimated using the GRAVY calculator indicated that the COX-2 inhibitory peptides were all hydrophobic, which was ascribed to the presence of hydrophobic amino acids, such as Ala, Phe, Pro, Leu, and Val, in the tetrapeptides.To predict the stability of the COX-2 inhibitory peptides, mock cleavage was performed using the PeptideCutter tool. The results showed that these peptides contain potential cleavage sites for pepsin (pH > 2) and trypsin, which were predicted to afford peptide fragments of various sizes and free amino acids. However,insilicotools have certain limitations[48]and evaluation of theinvitrostability andinvivotoxicity and bioactivity would require future experimental validation.
3.6 Inhibitory activity and inhibition kinetics of COX-2 inhibitory peptides
Although molecular docking is a valuable tool for screening active peptides, including DPP-IV[9],α-glucosidase[37], and COX-2 inhibitory peptides, it does not accurately determine whether a given peptide is a substrate or an inhibitor. Therefore, to avoid false positives and verify our speculation that LFPD, FPGA, AGFP, and VGFP possess COX-2 inhibitory activity, these peptides were chemically synthesized (Fig. S3) and tested for inhibitory activity toward COX-2. As shown in Fig. 4, FPGA and LFPD displayed the most potent COX-2 enzyme inhibitory activity with IC50values of 0.495 and 0.455 mmol/L, respectively, although their Vina scores were not the lowest. In addition, the IC50values of AGFP and VGFP were 2.308 and 2.777 mmol/L, respectively. It was noted that LFPD and FPGA formed hydrogen bonds or involved in hydrophobic contact with residues Arg106 and Ser105 outside of the COX-2 active pocket. We speculate that COX-2 may contain at least two inhibitor-binding sites,such that LFPD and FPGA displayed higher inhibitory activities.
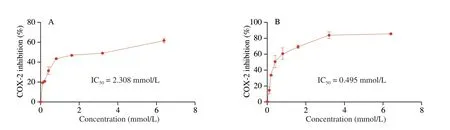
Fig. 4 COX-2 inhibitory activities of (A) AGFP, (B) FPGA, (C) LFPD, and (D) VGFP. The error bars represent the standard deviation of triplicate samples.
Furthermore, Nongonierma et al.[49]showed that N-terminal hydrophobic amino acid residues can improve the DPP-IV inhibitory activity. Similarly, in our study, all 4 peptides contained hydrophobic residues at the N-terminus, suggesting that these may be related to their high levels of COX-2 inhibition. Interestingly, we also observed that LFPD and FPGA were stronger inhibitors than AGFP and VGFP,which may suggest that a hydrophobic amino acid at the penultimate N-terminal position also plays an important role in inhibiting COX-2.
To further analyze the interactions between the 4 peptides and COX-2, the exact type of enzyme inhibition was evaluated using Lineweaver–Burk plots. The rate constants (KMandVmax) were determined from the Lineweaver–Burk plots using the GraphPad Prism software (Table S3). As shown in Figs. 5A-C, the doublereciprocal plots for the peptides LFPD, FPGA, and AGFP revealed that the fitted curves for different inhibitor concentrations intersected in the second quadrant, and the value of the kinetic parameterVmaxdecreased with increasing inhibitor concentration, which indicates that the inhibition mode of COX-2 by LFPD, FPGA, and AGFP was mixed inhibition. By contrast, in the case of VGFP, the fitted curves for different inhibitor concentrations intersected on thexaxis, the value of the kinetic parameterKMwas almost unchanged, andVmaxdecreased with increasing inhibitor concentration, indicating that the inhibition mode of COX-2 by VGFP was noncompetitive inhibition(Fig. 5D). The study by Wang et al. showed that as the best selective COX-2 inhibitor, compound 4 bound in the cavity of COX-2 through hydrogen bonding, resulting in noncompetitive inhibition[50]. This is similar to our finding that VGFP binds in the COX-2 cavity through hydrogen-bonding interactions with the key residues Tyr371, Ser516,and His75. Notably, LFPD, FPGA, and AGFP were found to form one or more stable hydrogen bonds with Tyr341 within the COX-2 active site. By contrast, VGFP formed a hydrophobic interaction with Tyr341, which may be the reason for the different inhibition mode of VGFP compared with the other three peptides.
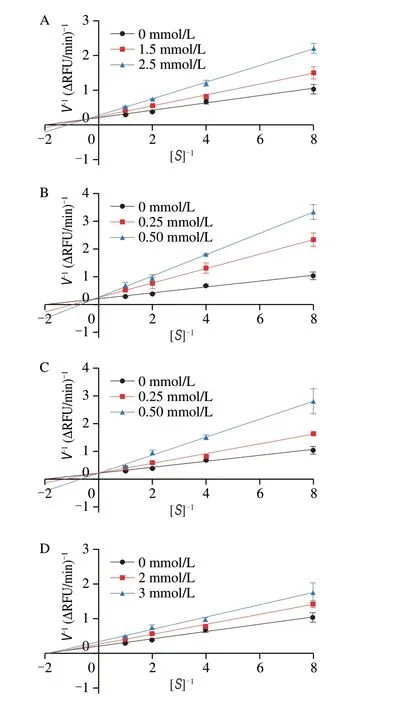
Fig. 5 Lineweaver–Burk double-reciprocal plots for (A) AGFP, (B) FPGA,(C) LFPD, and (D) VGFP.
4. Conclusions
In summary, Al-WDPH exhibited good COX-2 inhibitory activity(IC50= 11.91 μg/mL) among the 6 walnut dreg proteolysates. Further analysis revealed that the < 1 kDa fraction of Al-WDPH with strong COX-2 inhibitory activity contained 1 262 peptides, 4 of which(LFPD, FPGA, AGFP, and VGFP) displayed strong interactions by forming hydrogen bonds and hydrophobic interactions with residues inside the COX-2 active site. In addition, LFPD, FPGA, and AGFP exhibited mixed inhibition of COX-2, whereas VGFP displayed noncompetitive inhibition. These results suggest that the tetrapeptides LFPD, FPGA, AGFP, and VGFP derived from walnut dregs are excellent candidates for COX-2 inhibitors. However, the present study was limited toinvitroexperiments, and furtherin vivoevaluation is required to confirm the bioavailability and bioactivity of the COX-2 inhibitory peptides.
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by the Major Project of Science and Technology Department of Yunnan Province (202002AA100005 and 202102AE090027-2), the Project of Yunnan Province Food and Drug Homologous Resources Functional Food Innovation Team (A3032023057), the YEFICRC project of Yunnan provincial key programs (2019ZG009), Yunnan Province Ten Thousand Plan Industrial Technology Talents project (YNWR-CYJS-2020-010),and the Yunnan Provincial Department of Science and Technology Agricultural Joint Special Project (202101BD070001-120).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.26599/FSHW.2022.9250143.
- 食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango

