Effects of Maillard reaction and its product AGEs on aging and age-related diseases
Huan Peng, Yuqi Gao, Chenye Zeng, Rui Hua, Yannan Guo, Yida Wang, Zhao Wang
Protein Science Key Laboratory of the Ministry of Education, School of Pharmaceutical Sciences, Tsinghua University, Beijing 100084, China
Keywords: Maillard reaction Advanced glycation end products Physiologic aging Pathological aging Drug intervention
ABSTRACT Maillard reaction (MR) is a non-enzymatic browning reaction commonly seen in food processing, which occurs between reducing sugars and compounds with amino groups. Despite certain advantages based on Maillard reaction products (MRPs) found in some food for health and storage application have appeared,however, the MR occurring in human physiological environment can produce advanced glycation end products(AGEs) by non-enzymatic modif ication of macromolecules such as proteins, lipids and nucleic acid, which could change the structure and functional activity of the molecules themselves. In this review, we take AGEs as our main object, on the one hand, discuss physiologic aging, that is, age-dependent covalent cross-linking and modif ication of proteins such as collagen that occur in eyes and skin containing connective tissue. On the other hand, pathological aging associated with autoimmune and inflammatory diseases, neurodegenerative diseases, diabetes and diabetic nephropathy, cardiovascular diseases and bone degenerative diseases have been mainly proposed. Based on the series of adverse effects of accelerated aging and disease pathologies caused by MRPs, the possible harm caused by some MR can be slowed down or inhibited by artif icial drug intervention,dietary pattern and lifestyle control. It also stimulates people’s curiosity to continue to explore the potential link between the MR and human aging and health, which should be paid more attention to for the development of life sciences.
1. Maillard reaction
1.1 Discovery
Louis-Camille Maillard (1878–1936), an early French biochemist who studied protein biosynthesis, devoted himself to exploring alternative pathways for peptide synthesis in physiological systems.As early as 1912, during his second doctorate in Nancy, Maillard used glucose as an polyalcohol to heat with amino acids in a short period, and to his extremely surprise, the reaction did not go in the direction he expected. Unlike heating amino acids with glycerol,which promoted the formation of peptides, unexpectedly, peptides were not produced by the reaction of glucose with amino acids[1]. His focus then shifted to observing the changes in the reaction process of other amino acids, peptides, peptones and sugars other than glycine and glucose, thereby noting varying degrees of browning and accompanying carbon dioxide production[2]. Further on the reactivity of individual sugars, he paid particular attention to that saccharose did not react at all, pentoses reacted more rapidly than hexoses, and disaccharides was inferior to hexoses[2]. Besides, Maillard boldly predicted that the cause of carbon dioxide production may be attributed to the decarboxylation of amino acids based on incubation experiments in different gaseous atmosphere (O2, N2, H2), but he did not extract any related substances that the structures were identified from the reactants so that there were no sufficient evidences to test the hypothesis[2]. This serendipitous discovery of that the mixtures of amino acids and sugars turn brown when heated, Laid the original foundation for the Maillard reaction (MR)[3].
The development of a theory always rises in twists and turns,and needs to be supplemented continuously. The above reactions and associated problems raised by Maillard did not involve food,soon other scientists noticed the reactions of amino acids and sugars in kilning, brewing and aroma formation[4-5], and gradually realized that decarboxylation of amino acids in the presence of glucose and some carbonyl compounds results in the production of intermediate aldehydes, named “Strecker degradation” since 1948[6]. Italian chemist Mario Amadori also carried out the verification work of the condensation reaction of aromatic amines and glucose[7], the “stable product” and “labile product” were obtained. Subsequent studies on the basis of the “stable product” were used to distinguish theα-hydroxy aldehyde to which aldoses belongs from the common aldehydes,the rearrangement of labileN-glycosides was thus called “Amadori rearrangement”. Thanks to the discovery by Kurt Heyns et al.[8],the aldose derivatives from amino acids and ketoses were known as “Heyns rearrangement” in an analogous way to the “Amadori rearrangement”[9].
Up to now, we have clearly identified that the MR is a nonenzymatic browning reaction between carbonyl compounds, mainly reducing sugars, and amino acids, amines or proteins and other substances containing free amino groups. Based on these fundamental findings of Maillard and later expanded research by scientists, we are increasingly aware that as starting compounds, amino acids and sugars are widely distributed in nature and can react almost anywhere.Meanwhile, as inseparable basic nutrients and metabolites in the human body, the MR can occur under physiological conditions with sufficient incubation time and even without necessarily heating, it is inextricably linked in the process of human physiology and pathology.Therefore, studying the occurrence, development, pros and cons of the MR in humans is in line with people’s emphasis on life and health today.
1.2 The three main stages
The MR can be roughly divided into 3 main stages, as shown in Fig. 1, the early stage including the formation and degradation ofN-substituted glycosylamines into rearrangement products or fission products. Generally speaking, the carbonyl group from reducing sugars such as glucose forms an addition compound with an unprotonated amino group, then reacts to produce a Schiff base,further was cyclized to obtain anN-substituted glycosylamine,which are very unstable and readily continue the reaction in both directions[10]. 1) Degraded to rearranged products. The acidcatalyzed rearrangement ofN-substituted aldosylamine from aldoses will form 1-amino-1-deoxy-2-ketose or Amadori rearrangement products (ARPs), which may then interact with reducing sugars and reactants with amino groups to yield dideoxyketosamines. While ketose reactions like fructose undergo ketosylamine rearrangement to 2-amino-2-deoxy-1-aldose or Heyns rearrangement products(HRPs), HRPs are easily involved in the next reaction[11]. Amadori rearrangements can be accompanied and replaced by other reactions,in particular, enzymes may also be involved in ARP productionin vivo, and Amadori/Heyns rearrangements are reversible under physiological or room temperature storage conditions, but the half-life of ARPs under biological conditions is 6–8 months[12]. 2) Degraded into fission products.N-Substituted glycosylamines generate reaction intermediates via free radicals formed from glycosylamines,such as highly reactive enaminols, glycolaldehyde alkylamines,the two-carbon fission products of these sugars[13]. Schiff bases were generally considered to be intermediates in the formation of ARPs/HRPs, but other intermediates may also exist in the Amadori/Heyns rearrangement reaction depending on the reaction conditions such as reducing sugars, amino compounds, catalysts,solvents, and temperature[12]. ARPs and HRPs are found in various foods such as cocoa, coffee, barley malt, wheat malt, wheat beer and bell peppers[14].
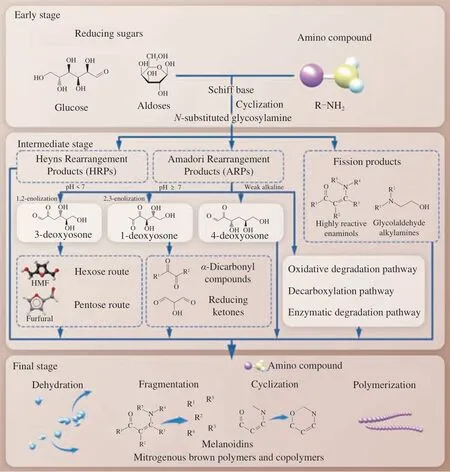
Fig. 1 Schematic representation of the three stages of the Maillard reaction.
The intermediate stage includes the degradation of the rearrangement products and the subsequent secondary reactions. The ARPs and HRPs formed in the initial stage can be degraded into all kinds of carbonyl compounds during heating, mainlyα-dicarbonyl compounds, such as glyoxal, methylglyoxal and diacetyl, leading to sugar fragmentation products and release of the amino group.The acyclic forms of ARPs are even more reactive in subsequent enolization and degradation reactions than the cyclic forms ofα- orβ-pyranose and furanose[15]. The breakdown of ARPs mainly involves 3 different pathways, which depend on the acidity and alkalinity of the reaction environment: 1) Under acidic conditions, ARPs can undergo 1,2-enolization to form 1,2-enaminol, through an irreversible route to generate 3-deoxyosone, whose cyclization and dehydration can bring about the production of 5-hydroxymethylfurfural (HMF)from the hexose route and furfural from the pentose route. 2) In neutral and alkaline environments, ARPs can form 1-deoxyosone through 2,3-enolization, which can be further degraded intoα-dicarbonyl compounds and reducing ketones. 3) In a weak alkaline environment, ARPs can also be decomposed to form 4-deoxyosone,which is a rarer pathway than the above two[16-17]. The above three pathways all have deoxyosones as reactive intermediates, and some of their decomposition products are often involved advanced glycation end products (AGEs). Of course, it has also been reported that ARPs have other degradation pathways, including oxidative degradation pathway, decarboxylation pathway[14], and enzymatic degradation pathway[18]. Notably, a class of substances called pyrrole aldehydes was detected in the reaction of a mixture of HMF and amine, and the further reaction of 3-deoxyosones with ARPs, which may give rise to the occurrence of intermolecular and intramolecular cross-linking of proteinsin vivo[19].
The final stage of the MR makes the amino group continue to participate in various reactions such as dehydration, fragmentation,cyclization and polymerization. Many of the low molecular weight compounds aggregate and react with each other in the later stage to form heterogeneous polymers, which are represented by the formation of unsaturated, nitrogenous brown polymers and copolymers, the molecular weight of this class of materials called melanoidins can be as high as about 100 000 g/mol, and so far there is no extremely clear formation mechanism and chemical structure[20].
1.3 Biological function
1.3.1 Antioxidant activity
Maillard reaction products (MRPs) are widely found in the heat treatment and storage of foods containing reducing sugars and amino compounds. In sugar-amino acid/peptide/protein model systems or food processing, some MRPs, such as amino reductones and ARPs, especially melanoidins, have been detected to exert excellent antioxidant capacity by chelating metal ions, breaking down free radical chains and hydrogen peroxide (H2O2), and scavenging reactive oxygen species (ROS)[21]. The combination of lysozyme (antibacterial polypeptide) and guar gum enhanced 2,2-diphenyl-1-picrylhydrazyl(DPPH) radical scavenging ability, possibly due to the formation of melanoidins in the MR, and the same result was reported for the lysozyme-xanthan gum conjugate[22]. The electron-donating ability of amino acids in the conjugates formed by MR may be the reason for the increase of reducing power[23]. The cross-linking of gelatin and sodium alginate in the aqueous model systems has significant scavenging activity against DPPH radicals, which can be attributed to the fact that MRPs including intermediates or final brown polymers can be used as hydrogen donors, it is beneficial to the anti-radical activity[24]. Melanoidins have anion properties and can chelate metal ions through nitrogen atoms. As well, pyridone or pyranone of melanoidins may provide chelating donors such as hydroxyl and ketone groups[25]. The antioxidant properties of food mainly depend on the production of melanoidins and brown MRPs, and contribute to the flavor, color and stability of the product, which has been confirmed in some food studies, such as coffee[26], bread crust[27],beer, biscuit[28], vinegar[29], honey[30], fruit juices[31], meat[32], pasta[33],bakery[34], potato[35]and dairy products[36].
In vitro, some predecessors have found that brown MRPs generated by reaction have antioxidant protection effect on lipids[37-38],rat microsomes[26], hepatocytes[39-40], HepG2 cells[28]and human lymphocytes[41]. Diets with large amounts of MRP extracted from biscuits increased serum antioxidant activity and decreased lipid oxidation in Wistar rats, and the increase in plasma antioxidant activity was well correlated with the decrease in blood pressure[42]. An intervention trial in humans showed a significant increase in oxidative resistance of low-density lipoprotein (LDL)in vitrofor dark beer,bread crust, and roasted coffee compared with pale beer, bread crumb,and raw coffee, and MRPs-rich thermally processed foods have the ability to reduce oxidative modifications of LDLin vivo[43]. While in a group of male adolescents samplesin vivoassay, consuming a diet rich in MRP does not significantly affect the oxidative damage markers (serum thiobarbituric acid-reactive substances and erythrocyte hydroperoxides) and antioxidant defence parameters(serum antioxidants and enzymatic activities of catalase, superoxide dismutase and glutathione peroxidase)[44].
1.3.2 Antimicrobial activity
The Rufián-Henares team[45]has conducted extensive and intensive research on the antimicrobial activity of melanoidins. He and his colleagues found that the antimicrobial activity of coffee melanoidins against different pathogenic bacteria involved three concentration-dependent mechanisms. Melanoidins achieved antimicrobial properties at low concentrations through iron chelation in the medium. Melanoidins can also chelate siderophore-Fe3+complex to reduce the virulence of pathogenic bacteria, which could produce siderophore. At high concentrations, melanoidins destroyed the permeability of bacterial cell membrane by removing magnesium ions. H2O2produced by MRPs or MRPs-rich foods has also been determined to have antibacterial activity, which is involved in the reaction leading to intrinsic damage related to cell membrane destruction[46]. Melanoidins from aqueous model systems and foods like coffee, beer, and dessert wine were more likely to inhibit Grampositive bacteria than Gram-negative bacteria[47]. The high molecular weight melanoidins from darker coffee and biscuits were more potent againstEscherichia colithan the low molecular weight fractions[48].When catalase was added, the antibacterial activity of MRPs and coffee was almost completely inhibited, this also indicated that the formation of H2O2in coffee and MRPs is a significant contributor to its antibacterial ability[49]. Importantly, the antimicrobial properties of MRPs against different strains of bacteria likeE. coli,Bacillus cereus,Proteus mirabilis,Pseudomonas aeruginosa,Staphylococcus aureus,Listeria monocytogenes,Salmonella typhimurium,Aeromonas hydrophilaorHelicobacter pylorihave been proposed in model experiments[50-53], so MRPs have also been investigated as active ingredients in antimicrobial packaging materials[54].
MRPs, including melanoidins, are widely found in food. They can be directly used as antibacterial agents in food. Under the premise of not harming human health, the concentration of MRPs can be controlled to regulate the bacteriostasis or bactericidal effect, so as to improve the shelflife and safety of food. Chitosan glucose complex is currently being studied for its antimicrobial properties in meat[55],fresh shrimps[56]and noodles[33].
1.3.3 Anti-inflammatory effect
Some reports suggest that MRPs also have anti-inflammatory effects, but research has focused on the cellular level. For example,fructose-tyrosine MRPs were found to exhibit anti-inflammatory activity in astrocytes and BV-2 cells in cell experiments[57].A compound, named BF-4, isolated from the products of the ribose-tryptophan Maillard reaction, reduced the levels of pro-inflammatory factors and inhibited nuclear transcription factor-κB(NF-κB) activation and mitogen-activated protein kinase (MAPK)phosphorylation through suppressing phosphorylation of IκBα,p65, p38 and c-Jun N-terminal kinase (JNK) to achieve its antiinflammatory activity[58]. There are many researchs on the cellular anti-inflammatory capacity of MRPs, and it is not worth describing them all.
Notably, the anti-inflammatory properties of MRPs have been suggested to play an important role in intestinal diseases in recent years. Anti-inflammatory effects of sugar- (glucose, fructose,or ribose) amino acid (lysine or glycine) MRPs have also been reported in intestinal epithelial cell models using Caco-2 cells[59].In the study of chronic recurrent inflammatory bowel disease,glucose-lysine MRPs demonstrated prophylactic and therapeutic potential by inhibiting mRNA levels of inflammatory cytokines and NF-κB in colon tissue in rat models of colitis induced by 5% dextran sulfate sodium[60]. Similarly, the intake of ovalbumin and its MRPs could alleviate the symptoms of colitis and improve the richness and diversity of intestinal flora, which was conducive to the maintenance of intestinal health[61]. The above studies indicate that MRPs can play a protective role in intestinal tract by regulating microbial composition and metabolic characteristics, inhibiting inflammatory response.
1.3.4 Memory improvement
MRPs come from a wide variety of sources, among which MRPs from some processed foods have been shown to have a certain anti-amnesic effect. After oral administration of the MRPs and the phenolic compounds from roasted peanut flour extracts in scopolamineinduced amnesia mice, behavioral tests and the detection of acetylcholine and other parameters showed that they could improve cognitive ability via cholinergic regulation and antioxidant effects[62]. In addition, Anchovy(Coilia mystus) protein hydrolysate and its Maillard reaction product(APH-M) were also found to alleviate the abnormal changes in the hippocampal ultrastructure of mice, and protect PC12 from H2O2-induced oxidative stress, which mainly demonstrated the well-being potential of APH-M for combating memory-impairment in mice by inhibiting acetylcholinesterase (AchE) activity[63]. These studies have shown that some MRPs, when produced in foods and ingested, could also play a role in improving memory. However, how to screen and identify MRPs with this function, and the specific molecular mechanism of improving cognition are not well explained.
2. Accumulation and metabolism of AGEs
2.1 Accumulation
So far, studies on age-related MRPs have mostly focused on AGEs, and the levels of certain AGEs have been taken as biomarkers of aging or related diseases, which means that to explore the relationship between MR and aging, it is impossible to ignore and inevitable to discuss the influence of AGEs on human beings.Therefore, the following part will focus on AGEs to analyze their roles and functions in the body. AGEs are found in the blood and tissues of the human body, together with AGEs found in the diet,more than 20 kinds of AGEs have been identified, which can be divided into fluorescent and non-fluorescent types[64-65]. The commonly mentioned pentosidine and methylglyoxal-lysine dimer (MOLD)belong to fluorescent substances[66-67], whileNε-carboxymethyl-lysine(CML),Nε-carboxyethyl-lysine (CEL) and pyrraline are typical non-fluorescent substances[68-69]. We list the common AGEs including cross-linking and non-cross-linking AGEs in Fig. 2. The general commonality of these so-called AGEs is based on the presence of lysine residues.

Fig. 2 Structures of representative AGEs. GOLD, glyoxal-lysine dimer;MG-H1, Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)ornithine.
In particular, foods with high protein or fat content derived from cooking at high temperatures are rich in dietary AGEs. According to statistics, we consume about 75 mg AGEs in our average diet per day,and about 30%–80% of them are absorbed by the digestive system,but this statistic about human absorption is still controversial[70].Under the context of normal metabolism in the human body, AGEs are catabolized in renal proximal tubular cells and excreted through the kidney, and one-third of the absorbed AGEs can be excreted in the urine within 2 days[71-73]. Cigarette smoke is also one of the sources of exogenous AGEs, which has to be paid attention to. Glycotoxins that enter the body through the lung alveoli are transported and absorbed into blood or lung cells, where they interact with other glycation products to form AGEs[74]. The formation of endogenous AGEs under physiological conditions is a relatively slow process, and the slow deposition mainly occurs in protein molecules with a slow turnover rate such as tissue collagen or lens crystals. Accumulation in tissues is regulated by many factors, including turnover rate of the targeted proteins, metabolic rate, redox equilibrium, sugar levels,detoxification system activity, and underlying small fraction of dietary absorbed AGEs, primarily affecting the function of long-lived proteins such as collagen, laminin, elastin, hemoglobin, lysozyme, and alkaline phosphatase[75]. Protein conformational changes induced by glycation can resist protein degradation and promote the accumulation in cells and organs of proteins that are unable to perform their normal physiological functions.
The key to the formation of AGEs involves the stress of reactive carbonyl compounds. Glyoxalase (GLO) system link to the enzymatic degradation of AGE-precursors, such as glyoxal and methylglyoxal,plays a very important role in inhibiting the production of AGEs[76-77].Nevertheless, the expression of GLO1 and GLO2 presented in the cytosol of all mammalian cells, and glutathione, an important cofactor of these two enzymes, both decreases during aging, which give rise to the accumulation of AGEs and the development of diseases[78-79].Some early detoxifycation proteins present in blood and body fluids,such as defensins, lactoferrin and lysozyme, can bind to AGEs to inhibit their uptake by cells or participate in cross-linking.
2.2 Abnormal degradation
AGEs do not just have a single receptor called RAGE, a member of the immunoglobulin superfamily that have got a lot of attention,other receptors contain the AGE receptor complex (AGE-R1/OST-48,AGE-R2/80k-H, AGE-R3/galectin-3) and scavenger receptor family(SR-A, SR-B, SR-1, SR-E, LOX-1, FEEL-1, FEEL-2 and CD36)[80].AGE-modified proteins usually have characters of denaturation and misfolding, in terms of a cellular level, damaged proteins, through receptor mediated internalization pathway, are detoxified or degraded by lysosomal system (cathepsins D and L) or the 20S proteasome of the ubiquitin-proteasome system (UPS). AGE-modified long-lived and AGE-modified proteins in organelles could also be removed by autophagic degradation. However, the presence of AGEs inhibits the access of damaged proteins to the proteasomal core and prevents the degradation of proteins that have lost normal biological functions.To make matters worse, the decrease of proteasome activity with age further enhances the accumulation of AGE-modified proteins. With the decline of renal function and chronic inflammation during aging,the decreased renal excretion and excessive accumulation of AGEs contribute to the circulatory invasion of aging and renal dysfunction related diseases[71,81].
3. Effects on aging and age-related diseases
MR, as a complex and various intertwined chemical reaction network, is mainly produced in the process of food processing, based on people’s daily nutrient intake has to rely on a variety of foods containing protein and carbohydrates, as well as MR can also occurin vivo, so there is growing evidence linking the MR to human health and even the aging process. The positive side of it has been mentioned above. However, the two sides of MR can be unquestionable, and here we focus on the potential risks related to human health in particular,especially aging.
In biological organisms, the aging process is universal,progressive, irreversible and harmful. Numerous studies have found that the MR, while leading to nutrient loss and production of toxic and harmful compoundsin vivo, and strongly promotes the age-dependent decline of tissue structure and function associated with aging through several deleterious processes, which will be explained in the subsequent section of physiological aging. Of course, apart from physiological aging, which is strongly related to tissue composition,MR is increasingly receiving widespread attention in the direction of pathological aging. At the same time of the inevitable physical aging, MR will trigger and accelerate the occurrence of one or more typical diseases according to the physical state, thus promoting the aging process. Multiple studies have increasingly found that Maillard chemistry has a non-negligible implications on healthin vivo,endogenously formed AGEs may accumulate on proteins, lipids and nucleic acids, both intracellularly and extracellularly, and are also associated with metabolic pathological changes such as oxidative stress, hyperglycemia and hyperlipidemia, and various inflammatory conditions. That is, MR is closely related to age, tissue composition,and pathophysiological development of the related disease (Fig. 3).
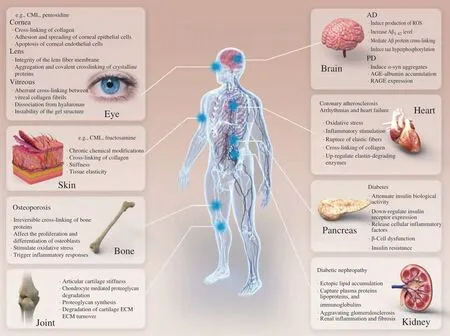
Fig. 3 Effect of MRPs on physiologic and pathologic aging. ECM, cartilage extracellular matrix; Aβ, amyloid β-peptide; α-syn, α-synuclein; AGE, advanced glycation end products; RAGE, receptor of AGE; AMI, acute myocardial infarction.
3.1 Major physiological aging
3.1.1 The skin
Tissues with long-lived proteins, such as the lens, arteries,tendons, lungs, cartilage, or skin, commonly experience a loss of elasticity or an increase in stiffness with age. This phenomenon is not only caused by changes in elastin or collagen content, on the other hand, the MR has also been used to explain the age-related stiffness,protein digestibility and tissue elasticity loss[82]. AGEs formed by nonenzymatic MR between reducing sugars and proteins primary amino groups contribute to increased chemical modification and crosslinking of tissue proteins with age, the chronic chemical modifications impair proteolytic digestibility and alter protein conformation. It is difficult to comprehensively assess the degree of chemical damage to extracellular matrix (ECM) caused by MR, but it is possible to quantitatively evaluate the level of AGEs in human skin. Researchers have found a variety of AGEs in human skin, and more studies have analyzed the content of various glycation products in collagen in the skin of an 85-year-old human. Among which the main cause of damage to arginine residues is ornithine, while glucosepane mainly plays the role of the main cross-linking agent, and the glycoxidation product CML and the glucose-derived ARP, fructosamine, were also the main chemical modifiers of protein glycation.
3.1.2 The eye
The eye relies on tissues such as the lens, uvea, cornea, retina,and ocular blood supply to provide structural integrity, optical clarity,and appropriate nourishment for the highly specialized eye cells to efficiently convert light into neural transmission to generate visual.Based on the collagen in the ECM of connective tissue throughout the body, which has a long biological half-life and metabolic inactivity,AGEs are more likely to accumulate and modify gradually in the collagen-rich cornea and lens with increasing age. Unfortunately,many differentiated cells in the mammalian eye have little or no ability to regenerate. This makes these cellular structures very sensitive to changes in protein structure and metabolic imbalances that occur during aging.
The aging process is accompanied by a decrease in protein stability in the matrix and an increase in immunoreactive AGEs, socalled AGEs that have been partially characterized as pentosidine and CML[83-84]. Notably, AGEs accumulate in the aging cornea as well as in ECM proteins in other tissues[85-87]. A previous epidemiological study showed that glycation-related fluorophore accumulation in the cornea has an association with smoking and glycemic control, and that age-related cross-linking occurs primarily in the collagenous components of the cornea (stroma and lamina)[88-89]. This indicates that AGEs mainly undergo progressive and irreversible crosslinking damage with collagen in cornea to accelerate aging under physiological conditions. To find out mechanism,in vitrostudies have shown that adhesion and spreading of corneal epithelial cells can be significantly weakened by AGE-modified substrates via disrupting integrin/non-integrin receptor-matrix interactions[84,90]. In addition,some researchers found that corneal endothelial cells lead to apoptosis after exposure to AGEs, and also express both RAGE and galectin-33,while the specific regulation of these receptors in the cornea remains unclear[91-92]. With aging, glycosylation will cause changes in the integrity of the lens fiber membrane and the tertiary structure of lens proteins, leading to the aggregation and covalent crosslinking of crystalline proteins, which is easy to cause the occurrence of cataract. Dicarbonyl compounds, such as glyoxal and methylglyoxal,play a prominent role in aging, leading to AGE cross-linking on theα-crystallin, which led to loss of chaperone activity, increased inαβ-crystallin content to form compact aggregates[93-94]. Age-related degeneration of the vitreous, is usually induced by glycation which induced aberrant cross-linking between vitreal collagen fibrils, leading to dissociation from hyaluronan and the resulting instability of the gel structure[95]. It has been reported that AGE has been found at high levels in age-related macular degeneration patients and accumulated in drusen and Bruch’s membranes[96-99]. This is mainly due to the fact that drusen contains lipids, TIMP-3, clusterin, serum albumin,apolipoprotein E, amyloid, and vitronectin, thus it can be speculated that AGE accumulation caused these partial proteins to be easily captured by AGEs during aging[100-101].
3.1.3 The joint
Collagen and proteoglycans in articular cartilage also occurred increased levels of AGEs associated with aging, which has become an important risk factor for the development of osteoarthritis with age[102-103]. Firstly, the gradual accumulation of cross-linked AGEs had a negative regulatory effect on articular cartilage, which was reflected in the increase of articular cartilage stiffness, chondrocyte mediated proteoglycan degradation, decrease of proteoglycan synthesis, and degradation of cartilage ECM[104-105]. Secondly, the accumulation of AGEs in articular cartilage also stimulated excessive oxidative stress and cytokine release to affect ECM turnover[106]. Studies of mice fed high levels of AGEs have also found evidence that could contribute to the development of osteoarthritis, one of the most common chronic diseases in the elderly that is closely related to chronological age.
3.2 Major pathological aging
3.2.1 Autoimmune and inflammatory diseases
Age-related chronic, low-grade and sterile inflammation, was considered to the “pillar of aging”, hints at the inextricable relationship between inflammation and aging[107-109]. AGEs have been identified as elevated in the plasma of diseases associated with autoimmunity and inflammation such as systemic lupus erythematosus[110-111], rheumatoid arthritis[112-113], systemic sclerosis[114-115], adult-onset Still’s disease[116],psoriasis[117-118]and Hashimoto’s thyroiditis[119]. The mechanisms and related signaling pathways of AGEs triggering immune and inflammatory diseases have also been gradually elucidated in a series of studies. For example, albumin, the most abundant protein in plasma, was targeted and modified by dicarbonyl metabolites (glyoxal and methylglyoxal) to produce cross-linking, thus, glycated albumin was granted immunogenicity to trigger inflammation[120].
It has been gradually realized that the up-regulation of RAGE may be an important reason for the failure of the self-tolerance mechanism based on autoimmunity, and increased RAGE expression was found in immune cells including neutrophils, T and B lymphocytes, monocytes, macrophages, and dendritic cells. RAGE,which has been extensively studied, plays a non-negligible role in chronic inflammation and immune dysfunction. As a multi-ligand transmembrane receptor, RAGE can also bind with other ligands besides AGEs including the DNA binding protein high-mobility group box-1 (HMGB-1)/amphoterin, S100 calgranulins, Mac-1 and amyloid-β peptide (Aβ)[121-122]. RAGE may activate autocrine and paracrine signaling pathways after the release of these ligand molecules to amplify tissue inflammation and injury. To dig the AGE-RAGE interaction, there are four transduction signal pathways,JAK2-signal transducer and activator of transcription 1 (STAT1),PI3K-AKT, MAPK-ERK and NADPH oxidase-ROS, respectively.Depending on these pathways, ultimately the phosphorylatedNF-κBenters into the nucleus to transcribe the genes expression of proinflammatory cytokines, growth factors, profibrotic cytokines and oxidative stress[80,121,123-124]. The AGEs and expression of RAGE goes up with age, therefore, binding of AGEs to RAGEs results in a series of adverse consequences, altering innate and adaptive immune responses, producing proinflammatory cytokines, ROS and reactive nitrogen intermediates (RNI) to induce inflammation and fibrotic signals (Fig. 4). We found that inflammation triggered by AGEs, as representative MRPs, is a foundational age-related disorder, that is,other pathologic aging diseases are accompanied by varying degrees of inflammatory damage.
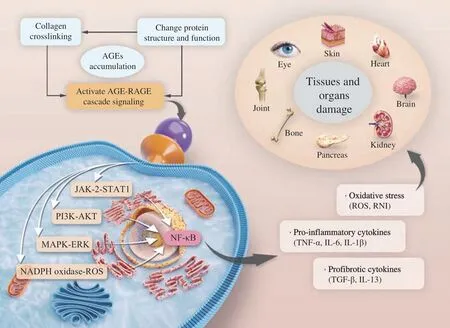
Fig. 4 Mechanism by which AGEs accelerate aging and related diseases. TNF-α, tumor necrosis factor-α; IL-6, interleukin 6; IL-1β, interleukin 1β; TGF-β,transforming growth factor β; IL-13, interleukin 13.
3.2.2 Neurodegenerative diseases
Age-related neurodegenerative diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD) and Huntington’s disease,although their pathological and clinical features vary, are generally associated with abnormal aggregation of proteins, such as Aβ, tau protein,α-synuclein (α-syn) and prions, which can be glycated. The formation of AGEs and oxidative stress in the brain can promote each other, causing the enhancement of pathological damage. RAGE mediates cell-intrinsic and cell-cell network mechanisms and plays a role in the dysfunction of neurons, glial cells and neurovascular cells in the central nervous system[125]. The progressive accumulation of AGEs and RAGE ligands with aging may be associated with endoplasmic reticulum homeostasis, UPS, autophagy flux and neuronal growth factor to cause neuronal damage, which was described in PD patients and in experimental PD models[126-130]. Recent studies have shown high plasma CML levels in patients with AD and PD[131].
As the most common age-related dementing disorder, AD is characterized by the formation of amyloid plaques and neurofibrillary tangles, and the death of neuronal cells. As mentioned above, longlived proteins and other post-translated proteins can be modified to form AGEs, and it is not surprising that AGEs modification and precipitated proteins cross-linking are also present in plaques and tangles. The accumulation of AGEs has been confirmed in senile plaques in different cortical areas, in primitive plaques, coronas of classical plaques and some glial cells of AD brain. Through measuring various AGEs and advanced lipid peroxidation end products in the cerebral cortex of AD patients, the concentrations of CML,Nε-malondialdehyde-lysine,Nε-carboxyethyl-lysine and other protein oxidative adducts increased significantly and heterogeneously.Methylglyoxal is considered to be one of the major carbonyl groups in AD leading to AGE formation[132]. Previous reports showed that AGEs induced the production of ROS, up-regulated the expression of amyloid precursor proteinin vivoandin vitroand promoted the increase of Aβ1-42level[133]. Pretreatment of cells with ROS inhibitorsN-acetyl-L-cysteine blocked the effect of AGEs. However, the further combination of AGEs and aggregated Aβ1-42can enhance ROS and reduce cell viability, which revealed that AGEs was a risk factor and mediated Aβ protein cross-linking to accelerate the formation of senile plaques[134]. In addition, there was evidence that AGEs can induce tau hyperphosphorylation in rats through RAGE-mediated activation of GSK-3, leading to synaptic protein decline and memory deterioration[135].
The main pathological feature of PD is the abnormal accumulation ofα-syn in dopaminergic neurons in the substantia nigra of the midbrain.α-Syn aggregates are considered to be cytotoxic.α-Syn is known to undergo several posttranslational modifications such as oxidation, phosphorylation and glycation. Relatively speaking,α-syn, as a lysine-rich protein containing 15 residues, is a potential target for glycation modification[136].In vitrostudies have highlighted AGEs-inducedα-syn aggregates production that can cause oxidative stress and toxicity in cell[137-138]. AGEs andα-syn were observed to be similarly distributed in very early Lewy bodies in the human brain in cases of incidental Lewy body disease, providing support providing support that AGEs promote protein cross-linking and formation of insoluble, non-degradable aggregates[139]. In addition, there is growing evidence that RAGE expression is also associated with PD in human subjects and animal models. Not only substantia nigra,amygdala and frontal cortex showed increased AGE expression,but also RAGE cell expression in substantia nigra and frontal cortex increased and heterogeneous in cases with early stages of Parkinsonian neuropathology[140]. AGE-albumin was found to be the most abundant AGE in human PD brain. It was synthesized in activated microglia cells and accumulated in extracellular space, upregulated AGE receptors and bring about apoptosis of human primary dopamine neurons[141].
3.2.3 Diabetes and diabetic nephropathy
Data from 103 controls, 200 patients with type 2 diabetes and 200 patients with complications of diabetes showed that glycation adducts were higher in patients with diabetes and higher in patients with nephropathy than in controls. The expression of CML was higher in nephropathy patients, and the level and expression of soluble RAGE(sRAGE) were mainly increased in patients with nephropathy. The expressions of membrane RAGE, NF-κB and inflammatory markers were significantly increased in diabetes patients with nephropathy[142].Plasma glycation adducts, various RAGE isoforms are inextricably related to complications of type 2 diabetes, this has to draw people’s attention to the relationship between AGE and diabetes.
Under normal physiological conditions, the glycation reaction is slow and moderate, but the degree of the reaction is also regulated by the substrate. The aggregation and protein modification of AGEs are more likely to occur in the body with elevated blood glucose level. In particular, there is a dependent association with diabetes,during diabetic hyperglycemia, the cross-linking of proteins greatly reduces the permeability and elasticity of the ECM, in addition, the accelerated formation of AGEs in collagen promotes further effects on vascular elasticity and basement membrane permeability[143]. As far as type 2 diabetes mellitus is concerned, AGEs can induce insulin resistance by directly modifying insulin to attenuate its biological activity[144]. The adverse effects of AGEs on β-cell function also may be attributed to the regulation of RAGE signaling pathway. The regulation of AGEs/RAGE signaling pathway lead to the activation of MAPK, p38 and protein kinase C (PKC). These kinases directly mediated insulin resistance by down-regulating insulin receptor expression, impairing insulin receptor substrate (IRS-1) tyrosine phosphorylation and promoting IRS-1 serine phosphorylation,and leading to defects in insulin receptor signaling[145-147]. The RAGE-induced activation of NF-κB was transferred to the nucleus and promoted the release of a series of cellular inflammatory factors such as interleukin 1β (IL-1β), interleukin 6 (IL-6), tumor necrosis factor α(TNF-α), which eventually led to insulin resistance[145]. Besides,RAGE could also induce sustained activation of transcription factors such as STAT3, hypoxia inducible factor 1α (HIF-1α) and activated protein 1 (AP-1), the cascade increased inflammation and further attenuates insulin sensitivity in target cells[146-149]. On the other hand,β-cell dysfunction is also closely related to AGEs, which can cause β-cell damage and death, resulting in reduced insulin secretion[150-151].Extensive evidence has shown that RAGE selectively binds to toxic islet amyloid polypeptide (IAPP) intermediates, resulting in NADPH oxidase-mediated ROS generation, and induced cellular stress and inflammation are toxic to β-cells, leading to apoptosis and decrease in number[152-153].
A series of clinical and experimental studies have also demonstrated the involvement of AGE in the pathogenesis of diabetic complications, including diabetic nephropathy. Yuan et al.[154]found that CML induces ectopic lipid accumulation in human renal tubular epithelial cells (HK-2) and in the kidney of type 2 diabetic rats, which may lead to diabetic nephropathy by disrupting the intracellular feedback regulation of cholesterol. Mainly, on the one hand, the formation of AGEs is accompanied by inter/intramolecular cross-linking of collagen, thus changing the metabolism of ECM components. Glycated collagen of the basement membrane may predispose platelets to the potential for aggregation[155]. AGEs accumulated in ECM components capture plasma proteins,lipoproteins, and immunoglobulins, affecting homeostasis of normal renal function and aggravating glomerulosclerosis[156]. In the second aspect, the accumulation of AGEs induced renal inflammation and fibrosis, the expression of transforming growth factor β (TGF-β) and other cytokines induced via activation of RAGE can mediate the epithelial-myofibroblast transdifferentiation of renal tubular cells[157].
3.2.4 Cardiovascular disease
Previous studies have suggested that the level of CML in the normal arterial wall increased with rising individual age, mainly in the intimal ECM, and the level of CML in the atherosclerotic vessel was about three times higher than that in the normal person[158], this indicated that AGEs were necessarily related to the occurrence of cardiovascular diseases. The mechanisms associated with AGEs and extensively studied involve the glycation modification of proteins affecting physiological functions, the inflammatory response triggered by AGEs and the collagen cross-linking mediated by AGEs.Obviously, the above don’t happen independently of each other but they cross over.
AGEs levels measured by AGEs-enzyme linked immunosorbent assay (ELISA) were about 2.5-fold higher in aged hearts than in younger ones. In addition, proteins with molecular weight of 50–75 kDa and isoelectric point of 4–7 were significantly modified in aged hearts, which may be evidently related to the concentration of AGEs and the modification of protein[159]. There is substantial evidence that glycated LDL promoted atherosclerosis, not least because AGELDL activated toll-like receptor 4-mediated signaling pathways and induces proinflammatory cytokine production in human coronary artery endothelial cells macrophages[160]. Methylglyoxal modification of high-density lipoprotein (HDL) reduced its stability and plasma half-lifein vivoand may lead to an increased risk of cardiovascular disease[161].
The activated endothelium of intramyocardial blood vessels after acute myocardial infarction (AMI) and myocarditis, showed significantly increased CML deposition. The experimental data suggested that the rising of CML was caused by inflammation rather than ischemia, and CML may be one of the factors triggering AMI[162].CML was also detected in both the myocardium and fat tissue separately in the atria of atrial fibrillation patients, and this result was analyzed to correspond to endothelial activation and inflammatory cell infiltration[163]. The effects of AGEs binding to RAGE can also cause oxidative stress and inflammatory stimulation, resulting in vascular endothelial dysfunction and endothelial cell death. Circulating CML,sRAGE, and endogenous secretory RAGE (esRAGE) levels were also predictably associated with the severity of coronary atherosclerosis and heart failure[164].
Collagen cross-linking, on the other hand, is particularly evident in the aging of the connective tissue ECM throughout the organism and even its relevance to the skin, but collagen cross-linking induced by MR also plays a certain role in the formation of atherosclerosis. AGEs react with amino groups (mainly theε-amines of lysine) on collagen,elastin, and other macromolecules[165], and most of the lysine residues in tropoelastin, the soluble precursor of elastic fibers, are involved in and modified by the cross-linking process during elastogenesis. The rupture of elastic fibers and the cross-linking of collagen fibers limit the turnover and proper assembly of the matrix and result in increased rigidity of the macromolecular structure and gradual hardening of the vessel wall[166]. The same cross-linking reaction process also occurs in the fine collagen network between cardiomyocytes, which may directly give rise to the gradual loss of contractile cells with age, the cross-linking of collagen will increasingly hinder the coordination of the rhythmic contraction of the myocardium and even loss of function.This is the product of the MR that is likely to cause arrhythmias and final heart failure in the body[167].
Of course, there are other factors like elastin-degrading enzymes, researchers have discovered the important role of elastin degradation in atherosclerotic lesions. cellular-level experiments have demonstrated that glycation-products added to human skin fibroblast cell cultures up-regulate elastase-type endopeptidase activity to increase elastin degradation[168]. In a large population study of vascular and cognitive aging in 1 389 men and women aged 59–71 years,ELISA titration of blood samples showed elastin peptides produced by the degradation of elastic fibers in the blood circulation[169]. This may also have something to do with the accumulation of AGEs.
3.2.5 Bone-degenerative diseases
Bone is a slowly metabolized tissue that contains long-lived proteins and is particularly susceptible to AGE modification and accumulation over time. Osteoporosis is common in elderly,postmenopausal women and men over 70 years of age, and patients with diabetes, often accompanied by low levels of chronic inflammation[170]. Bone tissue is characterized by low turnover rate and high content of long-lived proteins, so the accumulation and modification of AGEs are also not impossible. According to a study of 104 bone and plasma samples from non-diabetic patients, plasma pentosidine in cortical bone showed an exponential increase in the process of aging[171], likewise, the serum level of AGEs in menopausal women with osteoporosis or osteopenia was higher than that of healthy women[172], revealing AGEs as a potential factor in bone loss with age, perhaps marking the extent of bone density loss[173].
The deleterious effect of AGEs on bone biomechanical properties is partly caused by the long-lived matrix proteins (such as collagen I,approximately 95% of the entire collagen content of bone), in the extracellular environment, and irreversible cross-linking of bone proteins exposed to AGEs for a long time leads to resistance to degradation, structural and functional changes[171,174-175]. Secondly,AGEs-modified collagen has been shown to affect the proliferation and differentiation of osteoblasts. Pentosidine had a dose-dependent deleterious effect on human osteoblastsin vitro, leading to functional changes in osteoblasts to inhibit the formation of bone nodules[176-177].Moreover, there is considerable evidence that AGEs and RAGE pathways can stimulate oxidative stress and trigger inflammatory responses in vascular wall cells, osteoblasts and osteoclasts, thus participating in vascular calcification and osteoporosis in diabetes mellitus[178-179].
4. Intervention and control in the MR
MRPs damage is not solely involved in aging-related diseases,and acrylamide has been reported to have carcinogenic effects. Upregulation of RAGE expression has been detected in colorectal cancer[180], pancreatic cancer[181], prostate cancer[182], lung cancer[183],and breast cancer[184], suggesting that the toxicity of AGEs may involve activation of RAGE to stimulate mutagenesis and cancer cell proliferation and migration. Excessive and undesirable MR in human organisms accelerate the aging process and the occurrence of diseases to a certain extent, making the body subtly violated. Therefore,research on effective interventions for MR is increasingly emerging to seek to minimize the adverse health consequences of MR products.
4.1 Drugs intervention
MRPs, and AGEs in particular, accelerate both physical and pathological aging, which makes it natural for researchers to look for potential pharmacological interventions, such as the much-discussed AGE inhibitors. As shown in Table 1, we summarize some substances that have been studied that can be used to interfere with the adverse MR,inhibit the pathologies with harmful MRPs or resist the toxicity of MPRs.
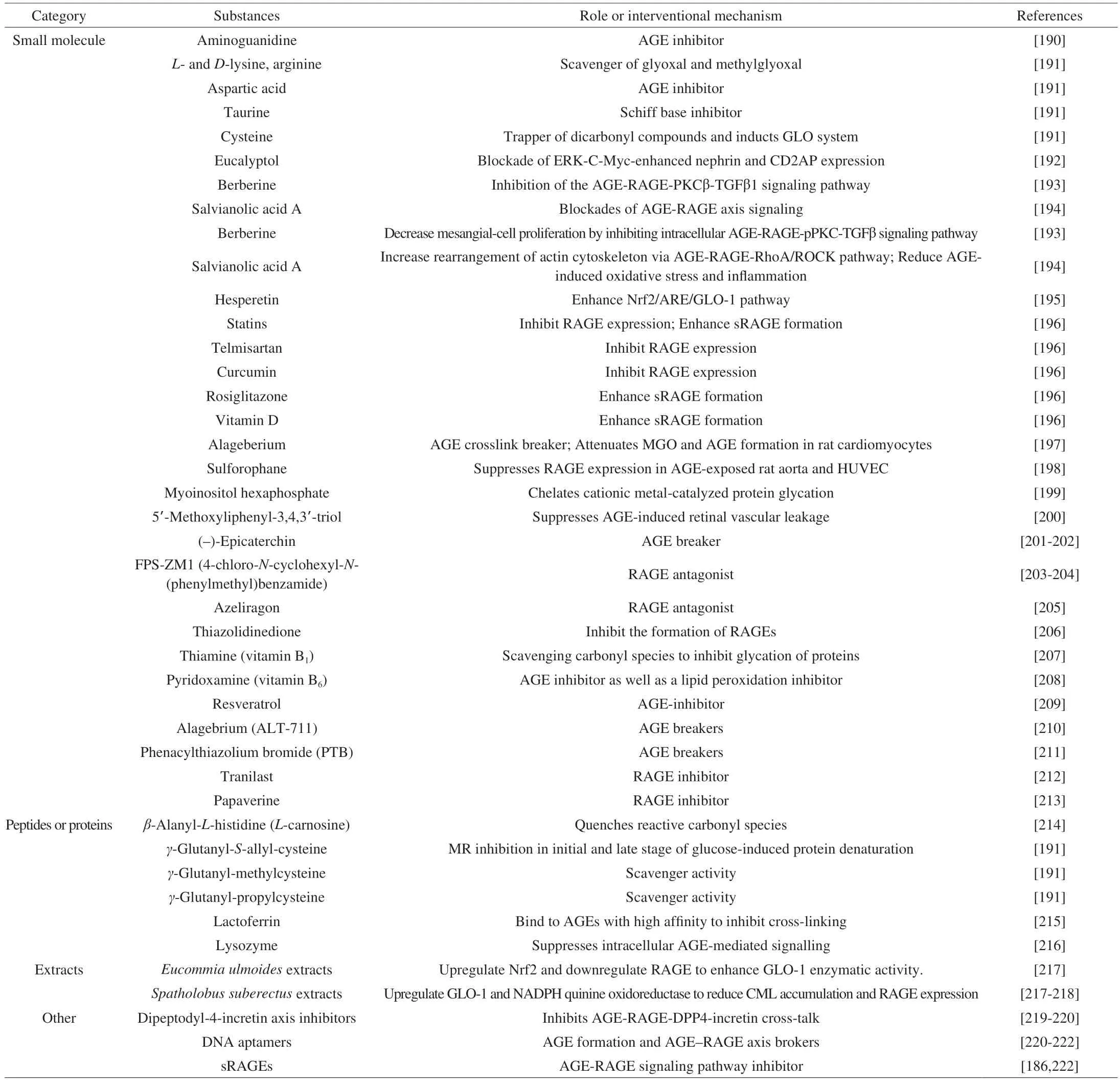
Table 1 Substances applicated to intervene in adverse MR or to inhibit pathologies associated with harmful MRPs.
In terms of considering AGEs, novel therapeutic AGE inhibitors can be divided into 4 types: 1) inhibitors of AGE formation;2) breakers of preformed AGEs; 3) blockades of AGE-RAGE axis signaling; and 4) inducers of intracellular GLO, ubiquitinproteasome, and autophagy pathways[185-188], They set out to slow or eliminate the adverse effects of AGEs on disease through attenuation of glycosylation, resistance to oxidative stress, chelation of metal ions, and removal of active 1,2-dicarbonyl compounds or ROS/RNI[189]. Small molecules, peptides and some natural extracts have been evaluated to inhibit the formation of AGEs and AGEs cross-linking, promote the decomposition of existing AGEs and block the biological response of AGEs. There have been many articles on the drug intervention of AGEs in detail, so it is not necessary to described here. In spite of the development based on the AGE inhibitor or the AGE breaker has made a lot of efforts, the new drug in the animal model shows expected and effective prospects, but many drugs have shown potential toxicity and side effects in preclinical studies and clinical trials, there is still no FDA-approved drugs can be used in the treatment of induced the pathology of the AGEs, people are still looking for relative safety and highly effective therapeutic agent further validation mechanism.
4.2 Control of diet patterns
Studies demonstrated that repeated heating of cooking oil could contribute to an increase in fluorescent AGEs in diet[223]. In a participating trial of 51 participants without type 2 diabetes, a diet high in red, processed meats, and refined grains elevated plasma CEL concentrations compared with an energy-matched diet high in whole grains, dairy products, nuts, and legumes[224]. In fact, dietary AGEs are one of the most significant exogenous AGEs sources that contribute to the body AGEs pool[225]. More and more abundant data confirm that western mixed diet rich in saturated fatty acids is also a plentiful source of exogenous AGEs[226]. There is an inalienable correlation between the intake of AGEs and the concentration of AGEs in serum. To some extent, the diet restricting AGEs is beneficial to reduce the effective total AGEs level which can cause oxidative stress and inflammation. The Mediterranean diet, which is rich in monounsaturated fatty acids and minimally processed natural foods,reduced the serum levels of AGEs in elderly people, thus achieving the goal of strengthening antioxidant defense[227].
Of course, the comprehensive effects of various environmental factors may enhance the formation and accumulation of AGEs, among which attention should be paid to the reasonable control of the daily habits of high-carbohydrate and high-calorie diet, high-temperature cooked food.
4.3 Control of living habits
Long-term smokers have a higher risk of cardiometabolic complications than the general nonsmokers, which has led to speculation that the components of tobacco may form MR, especially AGEs, after burning. Later academic studies have found that active glycosylation products exist in the aqueous extracts and smoke of tobacco in a state of rapid reaction with proteins, although the reaction could be inhibited by aminoguanidine. When glycation products,known as “glycotoxins”, were transferred to the serum proteins of smokers, and the levels of AGE-apolipoprotein B and serum AGE levels in smokers were significantly higher than those in nonsmokers[228]. Accordingly, immunoreactive AGEs were significantly higher in lenses and lenticular extracts and even in coronary artery specimens of smokers than in nonsmokers, irrespective of diabetes[229].Smoking not only affects the health of the individual, but smokingrelated AGEs can also be transferred through breast milk, increasing AGEs in the skin of breastfed infants based on mothers who smoke during pregnancy and lactation[230]. The above evidence proves that the personal habits of smoking will accelerate the generation and exogenous source accumulation of AGEs, and likely to potentially develop chronic obstructive pulmonary diseases as well as skin aging.Sedentary life-style will also cause the formation of AGEs, which requires us to maintain good living habits, and be responsible for the health of ourselves and our relatives.
5. Conclusions and perspectives
With the change of people’s lifestyle and diet structure, MRPs contained in baked foods and hot processed foods are also affecting the physiological process of the human body. Above all, levels of AGEs accumulate over time in the human body, especially in older people with slower metabolic rates. The relationship between AGEs and aging is as follows: 1) The accumulation of AGEs in proteins,which is considered to be one of the causes of aging since this process can modify proteins characterized by aging, such as collagen and elastin, and change their structure and biological function.2) Proteins modified by AGEs may have immunogenicity, stimulate autoimmunity and promote the occurrence of pathology. 3) AGEs can induce oxidative stress and proinflammatory effects by binding with RAGE, and then activate various down-stream signals, resulting in a cascade reaction to accelerate the process of aging. 4) AGEs and aging are not only a simple causal relationship, but complement each other. For our own health, it is necessary to be aware of the toxic risks of AGEs, understand the importance of healthy diet and habits, and minimize the risk of diabetes, cardiovascular complications, and other glycation-related diseases.
MRPs, especially AGEs, are linked to the occurrence and development of diseases. Many mechanisms are still unclear in current studies, and emerging doubts also need to be solved. It is meaningful to conduct more extensive prospective studies on MR.Future research may focus more on the following aspects: 1) Finding MPRs as promising new biomarkers for the diagnosis and treatment of aging and diseases. 2) Developing non-invasive methods for detecting and measuring MRPs. Current methods for measuring MRPs require invasive procedures, which limit their use in clinical settings. Developing non-invasive techniques, such as imaging or biomarker assays, would be highly beneficial for monitoring MRPs in patients and identifying those at risk for developing related diseases.3) To develop safe and effective therapeutic drugs with few side effects to deal with aging and a series of diseases caused by glycation modification. 4) There is increasing evidence that MRPs play a significant role in the development of neurodegenerative diseases such as AD and PD. Future studies can focus on understanding the molecular mechanisms underlying the contribution of MRPs to these diseases and identifying potential therapeutic targets. 5) Investigating the role of dietary interventions in reducing MRPs. Several studies have shown that dietary interventions, such as calorie restriction and the consumption of antioxidants, can reduce the formation of MRPs.Future research can explore the effectiveness of these interventions in preventing or delaying the onset of age-related diseases. 6) Studying the effects of MRPs on stem cell function. Stem cells play a critical role in tissue regeneration and repair. However, their function declines with age, leading to decreased regenerative capacity.Understanding how MRPs affect stem cell function and identifying ways to mitigate their negative effects could have significant implications for regenerative medicine. 7) Since it has been proved that telomere attrition, gene modification and DNA repair defects is associated with biological aging, it may be worth considering whether the accumulation of AGEs in physiological aging is related to these mechanisms.
Declaration of competing interest
Zhao Wang is an associate editor forFood Science and Human Wellnessand was not involved in the editorial review or the decision to publish this article. The authors report no conflict of interest.
Acknowledgment
This review was financially supported by grants from the National Natural Science Foundation of China (82170873; 81871095), the National Natural Science Foundation of China (81974503), and the Tsinghua University Spring Breeze Fund (20211080005).
- 食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango
- Learning about good nutrition with the 5-color front-of-package label“Nutri-Score”: an experimental study

