Bio-screening and quantif ication of methyl paraben in vinegar and coconut juice separated by HPTLC
Yisheng Chen, Xingjun Xi
a College of Food Science and Engineering, Shanxi Agricultural University, Taigu 030801, China
b Insitute of Food Nutrition and Safety, Shanxi Agricultural University, Taiyuan 030031, China
c Institute of Agricultural Food Standardization, China National Institute of Standardization, Beijing 100191, China
Keywords: High performance thin-layer chromatography Bio-screening Bioluminescence Image analysis Methyl paraben
ABSTRACT As a widely used food preservative, methyl paraben was experimentally evidenced with serious hormonelike adverse effects. Herein, a high performance thin-layer chromatography platformed bioluminescent bioautography and image analysis for the selective quantif ication and conf irmation of methyl paraben was proposed and validated in vinegar and coconut juice. First, the detectability of the bioautography to the analyte on different layer materials was estimated, revealing that normal silica gel was the best choice. After that,the liquid of sample extract and working solution were separated to overcome the background noises due to co-extracted matrices. The separation result was then coupled to the optimized bioautography, enabling instant and straightforward screening of the targeted compound. For accurate quantif ication, bioluminescent inhibition pattern caused by the analyte was processed by image analysis, giving useful sensitivity (LOD > 16 mg/kg),precision (RSD < 10.1%) and accuracy (spike-recovery rate 76.9%–112.2%). Finally, the suspected result was conf irmed by determining its MS f ingerprint, further strengthening the reliability of screening.
1. Introduction
Methyl paraben (MP) is a broad-spectrum anti-microbial/fungal food additive, widely used in a large array of food and beverage. As a food preservative, MP displayed two featuring advantages, compared to conventional edible antimicrobials like sorbic acid and benzoic acid. First, MP is conventionally considered harmless because of its relatively low toxicity. Apart from that, the usability and eff icacy of MP is much more robust, showing favorably high stability to various conditions and therefore a wider inhibition spectrum to different spoilage microorganisms. As such, MP is regarded as a promising alternative to conventional preservatives and therefore increasingly used in the food industry.
Nevertheless, the endocrine-disrupting effect of MP observed in bothinvitroandinvivoassays attracted growing public attention[1].As a matter of fact, increasing experimental evidences links MP exposure with potential health risks like breast cancer in women,decreasing semen quality and testosterone levels in males[2]. As for infants and fetuses, chronic exposure to MP may lead to growth problems, including congenital malformations of the genital organs,testicular cancer, reduced production of sperm, immaturity and precocious puberty. Therefore, restrictions on the usage of MP have been world-widely stipulated. For example, the allowable level of MP in vinegar and fruit juice are 250 mg/kg in China. So far,analysis based on high performance liquid chromatography were the “gold method” for paraben quantification. But the analytical procedure was time-consuming and sophisticated sample cleanup was demanded[3-4], therefore may not able to fulf ill the requirement of fast and reliable screening.
High performance thin-layer chromatography (HPTLC)platformed detections as a hyphenation technology enabled a new chance for analytical chemistry[5-7], because it provides an excellent tool for screening the analyte in samples that were difficult for traditional methods based on column chromatography[8-11]. First, this hyphenation technology inherits the essential advantages of HPTLC like high throughput and matrix-tolerance[8,12-14]. A single separation of many samples can be performed simultaneously. Besides, as a flexible and versatile analysis platform, HPTLC is compatible with many state-of-the-art detections not working with conventional column chromatography, such as densitometry[15], effect-direct assay[16-18],surface enhanced Raman[19-20], nuclear Magnetic Resonance[21-22]and mass spectrometry[23-24]. More importantly, HPTLC could be used as an image-giving platform enabling the visualization of the results straightforwardly for eye-inspection[25-27]. This point would substantially elevate the simplicity and cost-efficiency of screening tasks, therefore particularly suitable for controlling laboratories dealing with high number of samples.
Bioluminescent bacteria is one of the most widely used microorganism coupled to HPTLC. In the last decades, bioluminescent bacteria based bioautography with HPTLC had been applied in many fields[28-30]. It displays outstandingly broader detection spectrum compared to those of the other bioautography based on enzyme reactions. More specifically, any chemicals causing the up/downregulation of cell bioluminescence could be documented[31]. Since they are mostly isolated from the sea/lake water, bioluminescent bacteria will not be associated with safety and availability problems compared to those genetically modified microorganism[18,32-33]. When jointly used with HPTLC, bioluminescent bacteria have been successfully applied for detection multi-class veterinary drugs and pesticides, displaying satisfactory usability[31,34]. More importantly, the instant change of bioluminescence emission when exposed to bioactive compounds staying on the HPTLC platform shortens the visualization to minutes,further increasing the applicability of this combination.
The objective of this study is to develop cost-efficient analysis for the simple and reliable screening of MP in different vinegar and coconut juice samples. To do so, the HPTLC separation is directly linked to a bioautography based on the instant inhibition of bioluminescence, which enables direct and noise-free indication of the presence of the targeted or non-targeted compound. In addition,accurate quantification is readily accomplished by extracting the digital information of the inhibition pattern of bioluminescent image.As a confirmative tool, MS measurement was performed to the elution of positive bands.
2. Materials and methods
2.1 Materials
Standards of methyl paraben (CAS 7128-64-5, > 99% purity)were purchased from Aladdin (Shanghai, China). Organic solvents and chemicals of analytical grade were from Sigma Aldrich (Shanghai,China). Ultra-pure water was prepared by a synergy system (Millipore,Germany). Three blank vinegar and three blank coconut milk samples were purchased in the local supermarket.
Glass backed silica gel G60 plates with or without F254indicator(layer thickness 0.2 mm, 10 cm × 20 cm) were from Merck(Darmstadt, Germany); NH2-silica gel plates with or without F254indicator (layer thickness 0.2 mm, 10 cm × 20 cm) from Macherey-Nagel (Düren, Germany); neutral cellulose and microcrystalline cellulose plates (layer thickness 0.2 mm, 10 cm × 10 cm) were from Qingdao Haiyang (Qingdao, China). Prior to using, all plates were pre-washed by developing with methanol to the top, then dried at 100 °C by TLC heater III (CAMAG, Muttenz, Switzerland) for 20 min, and stored in a airproof bag. The bioluminescent bacteriaPhotobacterium phosphoreum(ATCC 11040) was provided by Institute of Soil Science, Chinese Academy of Sciences.
2.2 Preparation of bioluminescent suspension
The preparation of bacteria suspension with brilliant bioluminescence was principally based on ISO 11348-1, Section 5(2007) and with some improvement[31]. Briefly, 1 L liquid medium containing 30 g NaCl, 6.1 g NaH2PO4·H2O, 2.75 g KH2PO4, 0.204 g MgSO4·7 H2O, 0.5 g (NH4) H2PO4, 3 mL glycerol, 5 g peptone, and 0.5 g yeast extract was neutralized and autoclaved at 121 °C for 20 min. To a 500 mL triangle flask filled with 100 mL such liquid medium, a bacterial colony from an agar plate was seeded. The triangle flask was then covered with a piece of aluminum foil and shaked at 100 r/min. After 12 h growth, the matured bacteria suspension was diluted with another 100 mL fresh medium.
2.3 Sample preparation
Stock solutions (10 mg/mL) of MP were prepared in pure water and stored in a refrigerator (4 °C). The working solutions for spiking into real samples and establishing calibration curves was prepared by further diluting stock solutions to 0.1 and 0.01 mg/mL.
To precipitate the protein, 10 mL sample liquid was mixed with 10 mL acetonitrile, which was shaked on a vortex oscillator for 1 min. If necessary, 60, 100 or 200 μL stock solution was added into the mixture, resulting 60, 100 or 200 mg/kg spiking. The mixture was then centrifugated at 5 000 r/min for 5 min. 5 mL supernatant was carefully collected and freezed at -10 °C for 5 min to remove the lipid constituent. After that, the sample extract was filtered through a disposable syringe filter of 0.45 μm pore size, in case of needle blockage. If the MP concentration exceeded the linear range, the sample extract was further diluted.
2.4 HPTLC
With a semiautomatic TLC sampler Limonat 5 (CAMAG,Muttenz, Switzerland), 1–10 μL working solution of MP and 5 μL sample extracts were carried by a 0.5 MPa nitrogen gas stream sprayed by onto the plate surface as 6 mm bands with automatically calculated intervals, initially 15 mm from the left side, and 10 mm distance from the bottom. The band area was allowed to dry shortly,in order to evaporate the solvent. Then, plate development was carried out by ADC-2 (CAMAG) filled with 10 mL the mixture of toluene/ethyl acetate (9/1,V/V) to a migration distance of 60 mm from the lower edge.
2.5 HPTLC-bioluminescence assay and documentation
The developed plate was heated at 60 °C for 5 min to completely evaporate the residue of organic solvents. After cooling down to room temperature, the plate was dipped into the mature bacteria suspension with strong bioluminescence at a vertical speed of 2 mm/s, 0 s staying, via a TLC Immersion Device III. Immediately after that, the wetted plate was placed into the detection chamber of Bioluminizer(CAMAG, Muttenz, Switzerland) equipped with a cooled CCD camera. The bioluminescence image was documented every 60 s with exposure time 30 s and sequence display delay 250 ms. Quantitation of the bioluminescence inhibition results was performed by image processing software ImageJ that was tailored to measure and process the gray value of digital pixels.
2.6 HPTLC-MS
Guided by the bioautography, the positive bands were further identified by their MS fingerprints. The targeted bands visualized under 366 nm illumination was scraped off the plate layer, of which was eluted with 0.1 mL methanol. Directly, 30 μL of the eluent was injected into an electrospray ionization source and simultaneously analyzed by a triple quadrupole mass spectrometer (Quattro Premier XE, Waters). Full scan MS data acquisition was carried out in ESImode with following settings: capillary voltages 3.0 kV, ion source temperature 100 °C, desolvation temperature 400 °C, desolvation gas flow rate 400 L/h and cone gas flow rate 50 L/h. The spectra were recorded in the ranges ofm/z50–1 000. The MS data was evaluated with MassLynx version 4.2.
3. Results and discussion
3.1 Layer material optimization
Impact of the material exposure on the performance of the living cell had be previously evidenced, implying that the detectability could be optimized by changing the layer material[19,31,34-36]. Guided by the systematic screening result previously[37], layers consisting of alkyl/cyano/diol bonded silica gel and aluminum oxide were generally not compatible to the bioluminescent cell, causing complete bioluminescence quenching or strong background noises of the image. Concerning this point, 3 types of HPTLC plate working with the sensor cell was comparatively estimated, with the aiming to explore a match that gave the highest detectability of the analyte.As comparatively summarized in Table 1, it was apparent that the bioautography result displayed strict layer-dependence. As for the layer made of cellulose and amino silica gel, the presence of MP did not result in any detectable bioluminescence inhibition, though a noise free background can be observed. Remarkably, clear inhibition spots of the analyte on the silica gel plate became readable instantly after immersion. The observed differences in bioautography might be attributed to the impact from layer interface on the bacteria metabolism[38]. Therefore, the silica gel plate was selected for further optimization.
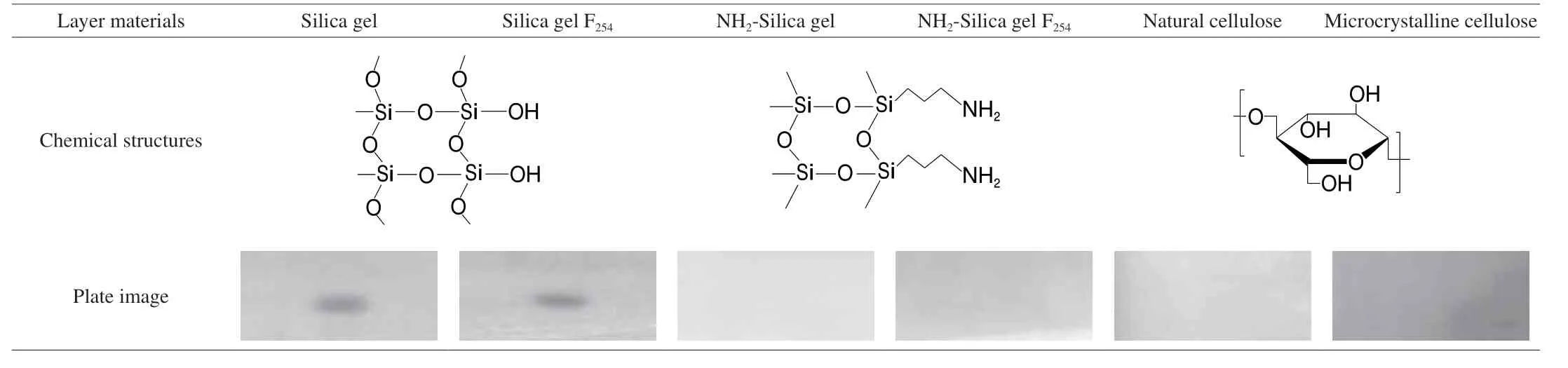
Table 1 Comparison of the layer-effect on the bio-visualization of the analyte spot (100 ng/zone) via cell bioluminescence inhibition.
3.2 Bioautography optimization
In this study, the speed of the biosensor coupled to HPTLC was a factor of crucial importance, determining its real usability.If the exposure time was prolonged, the bands visualized by bioluminescence inhibition would become blur, leading to a poor quantitative capacity of the analysis. Therefore, it really made sense to perform an analysis of the bioluminescence image documented as early as possible. With this regard, the variation of the inhibition spot induced by MP was profiled within the initial 5 min directly after immersion. The graphic results and quantitative data were present in Fig. 1, from which it can be observed that the visualization was a dynamic process in the first 3 min, with fast increasing intensity. After that, the intensity variation of inhibition spot became stable. This evidenced that the optimal exposure time was 3 min, which was fixed for following experiments.
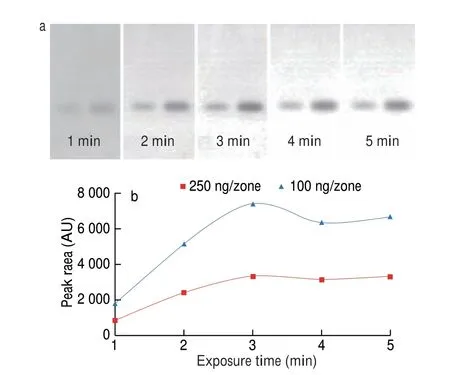
Fig. 1 Variation profile of the bioluminescence inhibition by MP (100 ng/zone-right,250 ng/zone-left) spots on the silica gel plate in the initial 5 min of exposure (a);the time dependent does-signal curve of MP inhibition spots (b).
3.3 HPTLC separation
Background interference due to co-extracted bioactive matrices was the major bottleneck obstacling the application of cells as biosensor for food analysis. With this regard, the function of HPTLC in this method was not to achieve full separation of any single compound in the sample extract, but to realize spatial resolution of the analyte from the background noise. In another word, the HPTLC played a role of cleanup platform which can be then directly combined with the sensor cell. In order to fix an optimal mobile phase,mixtures of toluene and ethyl acetate at different ratio was screened,concerning the relatively hydrophobic property of MP molecules. A comparison of the result revealed that 10 mL methanol+ethyl acetate(1+9 mL) gave the best separation. As showed in Fig. 2, it was clear that the analyte migrated toRf= 0.4 after separation, while the sample matrices causing noises stayed at the lower area. Besides, insignificant variation of the inhibition strength of EP at the presence of sample matrices can be observed as well. Obviously, the optimized HPTLC substantially enhanced the selectivity of the bioautography.

Fig. 2 Bioluminescence inhibition of the HPTLC separation results visualized by the optimized bioautography. Track assignment: 1–3 three blank vinegar samples, 4–6 three vinegar samples spiked with MP at 60 mg/kg,7–8 MP standard at 100 ng/zone, 9–10 MP standard at 250 ng/zone, 11–13 three blank coconut juice samples, 14–16 three coconut juice samples spiked with MP at 60 mg/kg, respectively.
3.4 Image analysis
Quantification based on pixel color information was another featuring advantage of HPTLC, showing remarkably simplicity and cost-efficiency in the screening-oriented task[27,39]. In this study, the dark band of the plate image in black/white mode was further digitalized and processed with ImageJ, in order to quantify the biosensing result. Compared to other software like Videoscan especially designed for HPTLC image analysis, ImageJ displayed superior in availability. It implied that the software can be simply download from the internet and HPTLC Image saved in almost any format can be processed, without paying for expensive software license. Apart from that, ImageJ enabled a straightforward way of image processing, in which chromatogram transformation, baseline correction and peak integration can be easily done. Obviously,these merits were ideally suitable for routine application. Here, the gray scale intensity of the pixel within the separation direction was digitalized with a rectangle (width = 20) covering the middle of the lane. The obtained digital data was plotted in the formation of chromatogram by using the “gel” function. The five chromatograms of both the standard and real samples were exemplarily shown in Fig. 3, further evidencing that the bioautography result was of high selectivity. Then, the base line of the targeted peak can be manually drawn, from which the integration data of the targeted peak was generated by a simple click.
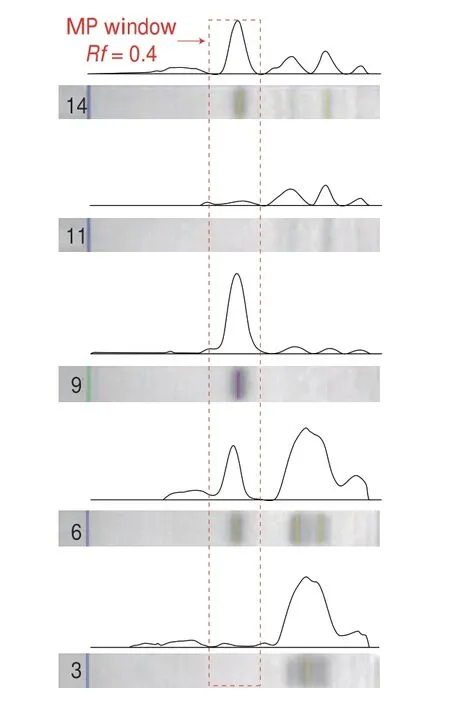
Fig. 3 Detection selectivity assessment: chromatogram was converted from digital images via ImageJ, exemplarily shown with track 3, 6, 9, 11 and 14 in Fig. 2.
3.5 Method validation
After detection optimization, the real performance of the established method was further validated by determining the quantitative performances, including limit of detection (LOD),linearity, precision and accuracy, summarized in Table 2. Different from optical signals like UV-vis and fluorescence, it was found that the bioluminescence inhibition result on the HPTLC plate was not dose-dependent at low concentrations. The result of 5 MP bands with gradient concentrations of 10–50 ng/band revealed that the visual spot suddenly disappeared at 30 ng/band, while clear inhibition was still visible at 40 ng/band. Therefore, a conclusion can be reached that the LOD of this method was lower than 40 ng/band. Taking the sample preparation into consideration, this LOD was equal to 16 mg/kg,far below the tolerance limit (250 mg/kg) stipulated for MP in vinegar and coconut juice, suggesting that this method complied with demands of regulatory authorities. Then, the concentration-signal profile of the calibration curve from 40–400 ng/band was estimated.It was found that the calibration curve obeyed good linearity of 50–300 ng/band withR2= 0.997 2. As for the accuracy of quantification,the recovery rate of MP spiked into real samples at 100 and 200 mg/kg were calculated, respectively. As summarized in Table 3,it was apparent that the obtained recovery rates located within the range of 76.9%–112.2% (RSD < 10.1%), displaying insignificant sample-dependence. As such, it can be concluded that this method was suitable for quantitative screening.
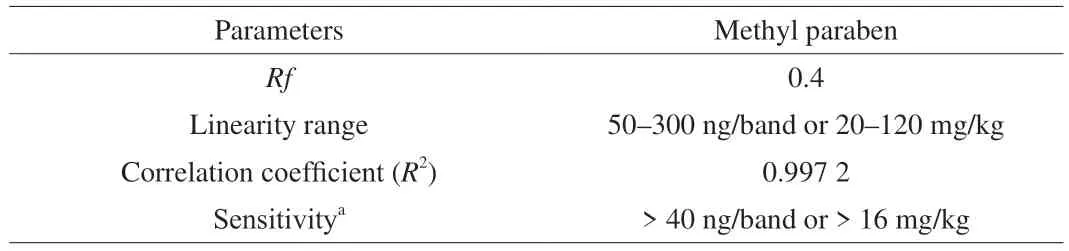
Table 2 Quantitative performance of the HPTLC-bioautography.
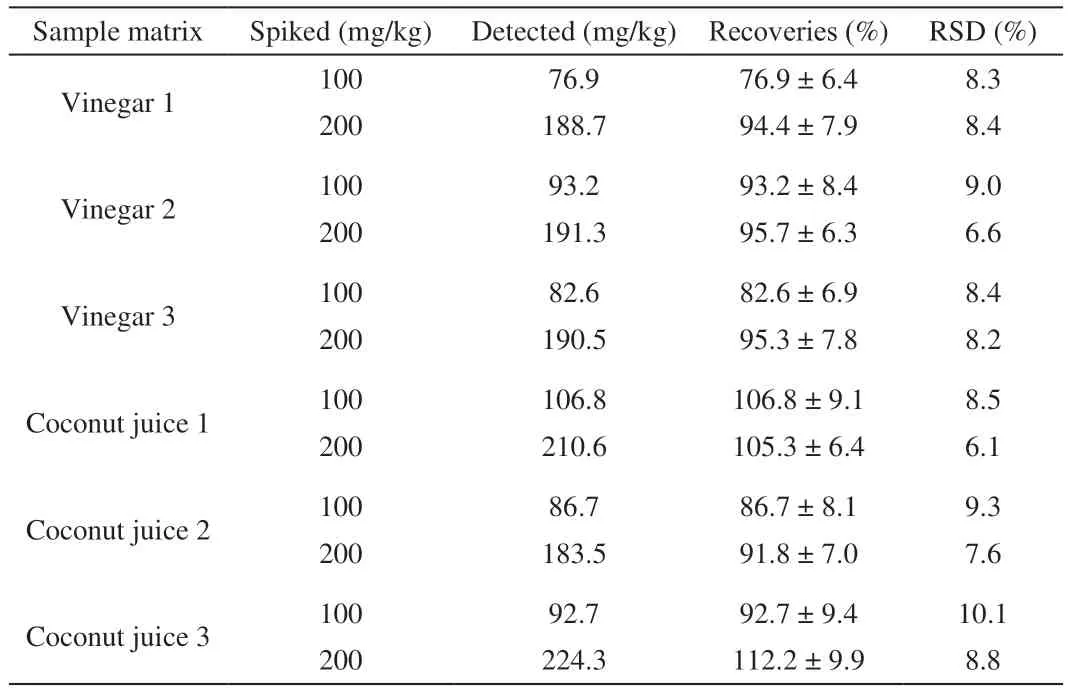
Table 3 Assessment on method accuracy.
3.6 MS confirmation
As a powerful and efficient tool able to eliminate false positive,MS was further jointly employed to ensure the molecular fingerprint of the targeted band visualized by the bioautography. In our previous work, the combination of HPTLC with MS was performed via a instrumentalized TLC-MS device. Apart from that, desorption electrospray ionization was also employed for directly sampling from the HPTLC layer[26]. However, the accessibility of this equipment may be a problem, especially for those laboratories with limited resources.To strengthen the practicality of this method, bands of interest was therefore manually scraped and eluted in this study. From the spectra generated by the mass spectrometry as shown in Fig. 4, three major signals atm/z151, 136 and 92 can be observed. These signals agreed well with the theoretical molecular ions characteristic to MP,which can be attributed to the negatively ionized fragment [M-H]-1,[M-CH3-H]-1and [M-C2H3O2-H]-1, respectively. Besides, analysis of the MP band in the matrix-matched run further evidenced that the MS detection was of enough selectivity with neglectable background noises and sensitivity down to 5 ng/band, able to cover the range of biosensing on HPTLC.

Fig. 4 MS spectra of a MP spot eluted from the HPTLC plate.
4. Conclusions
This study proposed a simplified and reliable screening method for MP in vinegar and coconut juice, based on the link of HPTLC with biosensing quantification and MS confirmation. Analytical performances of this method including sensitivity, linearity, precision and selectivity were validated, while its suitability for screeningoriented tasks was also evidenced. The core advantage of the proposed method relied on that it reached an ideal balance between simplicity, selectivity and compatibility to multi-detectors, which was remarkably valued in screening tasks. Such balance was of crucial importance for food and drug analysis dealing with high number and sophisticated samples.
Conflict of interests
Yisheng Chen is an youth editorial board member forFood Science and Human Wellnessand was not involved in the editorial review or the decision to publish this article. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was financially supported by National Natural Science Foundation of China (21804058), Shanxi Postdoc Reward(SXBYKY2022001), Shanxi Scholarship Council of China (2021-068), Shanxi Agricultural University High-Level Talent Project(2021XG013).
- 食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango

