Limosilactobacillus mucosae FZJTZ26M3 prevents NAFLD in mice through modulation of lipid metabolism and gut microbiota dysbiosis
Danting Dang, Bowen Li, Mengfan Ding, R. Paul Ross,Catherine Stanton,e, Jianxin Zhao, Bo Yang,c,, Wei Chen,f
a State Key Laboratory of Food Science and Resources, Jiangnan University, Wuxi 214122, China
b School of Food Science and Technology, Jiangnan University, Wuxi 214122, China
c International Joint Research Center for Probiotics & Gut Health, Jiangnan University, Wuxi 214122, China
d APC Microbiome Ireland, University College Cork, Cork T12 YT20, Ireland
e Teagasc Food Research Centre, Moorepark, Fermoy, Cork T12 YT20, Ireland
f National Engineering Research Center for Functional Food, Jiangnan University, Wuxi 214122, China
Keywords: Limosilactobacillus mucosae Nonalcoholic fatty liver disease (NAFLD)Probiotic Lipid metabolism Gut microbiota
ABSTRACT Lactobacillus are considered promising therapeutic methods for nonalcoholic fatty liver disease (NAFLD).The effects of two strains of Limosilactobacillus mucosae on NAFLD were investigated in this study. Fourweek-old male C57BL/6J mice were divided into 4 groups (n = 8 per group, Control, Model, FZJTZ26M3,FGSYC17L3). L. mucosae FZJTZ26M3 reduced the mice’s body weight, liver weight, and adipose tissue weight after 12 weeks of therapy. According to serum analysis, total cholesterol, triacylglycerol, and low-density lipoprotein cholesterol signif icantly decreased after L. mucosae FZJTZ26M3 intervention. Liver pathology showed that L. mucosae FZJTZ26M3 was effective to ameliorate lipid deposition in NAFLD mice. Additionally, the expression of the gene related to lipid metabolism in the liver and adipose tissue was analyzed, and the results indicated that L. mucosae FZJTZ26M3 could alleviate NAFLD by regulating lipid metabolism. Furthermore, the results of 16S rRNA gene sequencing revealed a drop in the relative abundance of Ruminococcaceae, which is linked to inflammation, but the relative abundance of a potential probiotic Akkermansia significantly increased after L. mucosae FZJTZ26M3 intervention. Generally, L. mucosae FZJTZ26M3 could be a candidate to prevent NAFLD.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is rapidly becoming the most prevalent type of chronic liver disease around the world[1-2].Previous research suggested that the prevalence of NAFLD was greater than 25% globally[3]. Obesity, dyslipidemia, hypertension,and diabetes are frequently associated with NAFLD[4-5]. There are currently several traditional medicines for NAFLD that are effective,however, most of them have adverse effects or have had inconsistent results[6]. Moreover, lifestyle adjustments such as healthy diets and moderate exercise are also accessible[7], but the effectiveness of these treatments is dependent on the compliance of patients. Therefore, safer and more effective therapeutic approaches are essential to explore.
Recently, numerous studies have shown that gut microbiota played crucial physiological roles in the digestion and metabolism of the host[8-9]. In NAFLD patients, the gut microbiota was distinct from that of healthy persons[10]. Short-chain fatty acids (SCFAs),lipopolysaccharide (LPS), vitamins, and other microbial metabolites can all be transported directly to the liver through the gut-liver axis since the intestines and liver are linked by the portal tract[11-12].Furthermore, microbiota-assisted therapy has grown in popularity in recent years[9], and gut microbiota modulation by probiotics is considered as a promising therapeutic approach for NAFLD.
Limosilactobacillusmucosaewere initially isolated from the intestine of piglets through amub-derived gene probe (mub, for mucus binding) and were also considered the source ofmub[13]. Consequently,L.mucosaeis a possible probiotic due to its strong mucin-adhesion capability[14]. Four strains ofL.mucosaewere reported to be able to regulate lipid metabolism[15-18]including lowering total cholesterol(TC) in serum, easing liver steatosis via activating production of IL-10 and attenuating the lipid accumulation in the aortic sinus.But the research on the effects ofL.mucosaeon the gut-liver axis including intestinal barrier and lipid metabolism need further exploration. The goal of this study was to assess the potential ofL.mucosaeisolated from the feces of healthy humans to treat NAFLD via analyzing lipid deposition and metabolism in the liver,white adipose tissue (WAT), and brown adipose tissue (BAT), the inflammatory state in the liver, and gut microbiota modulation.
2. Materials and methods
2.1 Strains and animals
L.mucosaeFZJTZ26M3 (CCFM1276) and FGSYC17L3 were previously isolated from the feces of healthy subjects from various provinces in China[19]and were deposited at Culture Collections of Food Microorganisms (CCFM), Jiangnan University, Wuxi, China. The bacteria were subcultured three times in an MRS broth, centrifuged at 8 000 ×gfor 15 min, then washed twice with saline, resuspended in 30% (V/V) glycerite, and stored at −20 °C. Each milliliter of the 30%glycerite solution contained about 4 × 1010CFU of bacteria. When utilizing, the bacteria were collected by centrifuging at 6 000 ×gfor 3 min, then resuspended in an equivalent volume of sterile saline.
The Ethics Committee of Experimental Animals at Jiangnan University approved the animal trial protocols (JN. No.20210915c1201225[306]). The Vital River Laboratory Animal Technology Co., Ltd. (Jiaxing, China) supplied 32 male C57BL/6J mice (4 weeks old). The mice were maintained in an SPF-grade laboratory, with water and food available at all times and a typical 12 h/12 h light/dark cycle. After 1-week adaptation period, the mice were randomly separated into 4 groups (n= 8) including the Control group fed with a normal chow diet (NCD, 10% fat, total energy 3.6 kcal/g); the Model group, and twoL.mucosaetreated groups fed with high-fat diet (HFD, 60% fat, total energy 5 kcal/g). Mice in the twoL.mucosaegroups were intragastric administered with 0.1 mL above-mentioned bacteria-containing saline daily, respectively, while the Control and Model groups were intragastric administration with 0.1 mL saline. The detailed feeding scheme is shown in Table S1.After receiving therapy for 12 weeks, the mice were sacrificed for blood, liver, epididymal WAT, perirenal WAT, BAT, and colon. 4%neutral paraformaldehyde solution was used to fixate a portion of the liver and perirenal WAT, and the remaining tissue samples were kept at −80 °C for future analysis.
2.2 Oral glucose tolerance test (OGTT) and insulin tolerance test (ITT)
In the 10thweek of the trial, an OGTT was conducted. Mice were orally gavaged with 40%D-glucose (2 g/kg body weight) following a 12-h fast. A glucose meter (Roche, Mannheim, Germany) was used to test the blood sugar levels at 0, 15, 30, 60, 90, and 120 min.The glucose tolerance values were calculated by plotting the curve according to time and blood glucose level and then computing the area under the curve.
In the 11thweek of the trial, an ITT was conducted. Mice were intraperitoneally administered with 0.75 U/kg of body weight of insulin after a 6-h fast, the subsequent procedure was identical to OGTT.
2.3 Histological analysis
Hematoxylin-eosin (H&E) staining of liver tissue and perirenal WAT, as well as Oil-Red-O staining of liver tissue, were carried out according to a prior study[20]. Images were obtained by a microscopy imaging system (Pannoramic MIDI, 3DHISTECH, Hungary).Furthermore, the measurement of the adipocyte size was performed using Fiji imaging software with the Adiposoft v1.16 plugin[21], and the proportion of lipid area in liver slices was performed with Image Pro Plus.
2.4 Serum biochemistry
The serum was collected by centrifuging (3 000 ×g, 10 min).Using a Mindray biochemical analyzer (Mindray, Shenzhen, China),the following serum biochemical indices were measured: alanine aminotransferase (ALT), aspartate aminotransferase (AST), TC,triacylglycerol (TG), low-density lipoprotein cholesterol (LDL-C),high-density lipoprotein cholesterol (HDL-C).
The levels of interleukin-1β (IL-1β) and tumor necrosis factor-α(TNF-α) in the liver and the concentration of leptin (LEP) and adiponectin (ADP) in serum were measured using enzyme-linked immunosorbent assay (ELISA) kits (Sangon, Shanghai, China)following the instructions. The level of serum LPS was measured using ELISA kits from Senbeijia Biological Technology Co.,Ltd., Nanjing, China. The protein content in liver homogenate was determined using bicinchoninic acid (BCA) protein assay kits(Waltham, MA, USA).
2.5 Gut microbiota analysis
Fecal microbial genomic DNA was extracted with FastDNA SPIN Kit for Feces (MP Biomedicals, CA, USA)following the manufacturer’s protocol, then amplified using primers 341F (5’-CCTAYGGGRBGCASCAG-3’) and 806R(5’-GGACTACNNGGGTATCTAAT-3’), the condition and system of PCR were based on a previous study[22]. Agar gel electrophoresis was loaded with PCR product. The products of electrophoretic gel were then recovered using the QIAquick Gel Extraction Kit (QIAGEN,Hilden, Germany), then quantified and mixed with equal quality.Sequencing data were analyzed using QIIME2. Alpha diversity was indicated as the Chao1, Shannon, and Simpson indexes. Beta diversity was estimated and represented using principal coordinate analysis(PCoA) plots. LEfSe (linear discriminant analysis (LDA) effect size)was performed online (https://huttenhower.sph.harvard.edu/galaxy/)to identify biomarkers. To predict the physiological function of gut microbiota, 16S rRNA sequences were normalized by PICRUSt and then produced a table of functional genes according to PICRUSt and Kyoto Encyclopedia of Genes and Genomes (KEGG), then drew the graph on a Tutools platform (https://www.cloudtutu.com).
2.6 Quantification of SCFAs in feces
Fresh feces were dried in a vacuum freeze drier and SCFAs were extracted using diethyl ether based on previous studies[23-24], then measured by gas chromatography-mass spectrometry (GC-MS), refer to previous research for specific process[24].
2.7 Quantitative real-time polymerase chain reaction (qRT-PCR)
A high-throughput grinder was used to break up the cells after the RNA-easy isolation reagent (R701-01 Vazyme Biotech Co.,Ltd., Nanjing, China) was applied to the tissue (liver, WAT, BAT,and colon), added RNase-free ddH2O and centrifuged (12 000 ×g,15 min) for the upper phase. Then an equivalent volume of isopropyl alcohol was added to the upper phase, and centrifuge at 12 000 ×gfor 10 min. Total RNA was present in the white precipitates at the tube’s bottom, then the cDNA was synthesized with a reverse transcription kit (R333-01 Vazyme Biotech Co., Ltd., Nanjing, China). A qRT-PCR is conducted with a BioRad thermocycler using the qPCR Master Mix (Q711-02 Vazyme Biotech Co., Ltd, Nanjing, China).The primer sequences are listed in Table S2.
2.8 Statistical analysis
Data were shown using mean ± SEM, and a one-way ANOVA with Duncan’s multiple range test or Tamhane’s T2 (if the data was not normally distributed) was performed for significant analysis.Different low case letters above columns indicated statistical differences atP< 0.05.
3. Results
3.1 Effects of L. mucosae on body weight, food intake, organ weight, and pathological section in NAFLD mice
The body weight was shown at only 10 weeks because of the effect of the OGTT and ITT. Compared with the beginning of the animal experiment, the body weight of the Control and Model group increased by 62.81% and 28.78% after 10 weeks of treatment,respectively, whileL.mucosaeFZJTZ26M3 prevented this increase,and the FZJTZ26M3 group had a 6.92% lower body weight than the Model group, but the body weight in the FGSYC17L3 group was almost the same as that of the Model group (Figs. 1A, B). In addition,without significant energy intake difference (Fig. 1C), FZJTZ16M3 decreased the liver weight (Fig. 1D), epididymal WAT weight(Fig. 1E) and perirenal WAT weight significantly (P< 0.05) (Fig. 1F).Vacuoles of different sizes and numbers appeared in the liver according to the H&E staining (Fig. 1G) and the lipid area in the liver of the Model group was significantly greater than the Control group(Figs. 1H, J) (P< 0.05), additionally, the mean area of adipocyte in perirenal WAT was significantly increased in the Model group compared with the Control group (Figs. 1I, K) (P< 0.05), however,FZJTZ26M3 significantly prevented the lipid deposition in liver and WAT (P< 0.05).

Fig. 1 Effects of L. mucosae on body weight, food intake, organ weight, and pathological section. (A) Body weight, (B) Percentage weight gain, (C) Energy intake, (D) Liver weight, (E) Epididymal WAT weight, (F) Perirenal WAT weight, (G) Representative images of liver H&E staining, ×200, (H) liver Oil-Red-O staining, ×200, (I) perirenal WAT H&E staining, ×200, (J) Liver lipid area (% total area) according to liver Oil-Red-O staining, (K) Perirenal white adipocyte mean area. Groups that did share the same letter were significantly (P < 0.05) different from each other. The same below.
3.2 Effects of L. mucosae on insulin resistance and serum lipids in NAFLD mice
The results of the OGTT and ITT showed that the area under the curve (AUC) value in Model group was significantly higher than that in the Control group (P< 0.05).L.mucosaeFZJTZ26M3 and FGSYC17L3 ameliorated glucose resistance significantly compared with the Model group(P< 0.05) (Fig. 2). Serum TC, TG, HDL-C, LDL-C, and LDL-C/HDL-C in the Model group were all significantly higher than in the Control group (P< 0.05), and all lipid levels except HDL-C were significantly lower after FZJTZ26M3 intervention (P< 0.05) (Figs. 3A-E).The levels of ALT and AST in FZJTZ26M3 were also lower than them in the Model group but without significance (Figs. 3F, G).

Fig. 2 Effects of L. mucosae on blood glucose. (A) OGTT, (B) AUC of OGTT, (C) ITT, (D) AUC of ITT.

Fig. 3 Effects of L. mucosae on serum lipids. (A) Serum TC, (B) Serum TG, (C) Serum HDL-C, (D) Serum LDL-C, (E) LDL-C/HDL-C, (F) Serum ALT, (G) Serum AST.
3.3 Effects of L. mucosae on adipocytokines and liver inflammation in NAFLD mice
LEP, ADP, and plasminogen activator inhibitor-1 (PAI-1)were considered to be connected to energy metabolism and lipid storage[25-26]. The serum concentration of LEP in the Model group was significantly increased compared with the Control group (P< 0.05),L.mucosaeFZJTZ26M3 supplement significantly decreased it (P< 0.05)(Fig. 4A), however, the level of ADP was not significantly changed after FZJTZ26M3 supplement (Fig. 4B). Regarding the level of liver inflammation, the concentrations of IL-1β (Fig. 4C) and TNF-α(Fig. 4D) in the Model mice were significantly higher than that in the Control group (P< 0.05). Compared to the model group, IL-1β level significantly dropped inL.mucosaeFZJTZ26M3 treated mice(P< 0.05), and TNF-α level also decreased. Additionally, compared with the Control group, the level of serum LPS significantly increased in the Model group (P< 0.05), andL.mucosaeFZJTZ26M3 intervention decreased it significantly (P< 0.05)(Fig. 4E).

Fig. 4 Effects of L. mucosae on adipocytokines and inflammatory factor. (A) Serum LEP, (B) Serum ADP, (C) Liver IL-1β, (D) Liver TNF-α, (E) Serum LPS.
3.4 Effects of L. mucosae on gut microbiota in NAFLD mice
Including the Shannon, Chao1, and Simpson indexes,α-diversity indicated the evenness and richness of gut microbiota.L.mucosaeFZJTZ26M3 and FGSYC17L3 intervention significantly increased the Chao1 index (P< 0.05) but the Shannon and Simpson indexes was not influenced (Figs. 5A-C).β-Diversity was calculated using PCoA,and the results showed the microbiota composition in Control, Model,FZJTZ26M3, and FGSYC17L3 were different (Fig. 5D).

Fig. 5 Effects of L. mucosae on α-diversity, β-diversity, and distribution at the phylum level of gut microbiota. (A) Chao1 index, (B) Shannon index,(C) Simpson index, (D) PCoA, (E) Microbial distribution at the phylum level, (F) The ratio of Firmicutes/Bacteroidetes.
Moreover, in comparison to the Control group, the Firmicutes/Bacteroidetes ratio increased in the Model group, correspondingly,L.mucosaeFZJTZ26M3 intervention decreased the ratio, however,L.mucosaeFGSYC17L3 intervention increased the ratio significantly(P< 0.05) (Figs. 5E, F).L.mucosaeintervention altered the composition of the gut microbiota (Figs. 6A, B). In the top 10 families(Fig. 6C), the compositions of Lachnospiraceae, Erysipelotrichaceae,Lactobacillaceae, Ruminococcaceae, Muribaculaceae, Rikenellaceae,Peptostreptococcaceae, Tannerellaceae, Bifidobacteriaceae, and Family XIII were different among the groups (Control (7.89%,18.56%, 30.86%, 3.82%, 14.35%, 7.81%, 0.08%, 2.78%, 9.60%,and 0.50%), Model (46.46%, 6.34%, 0.90%, 16.29%, 7.23%, 4.78%,7.36%, 2.73%, 0, and 1.68%), FZJTZ26M3 (34.97%, 10.14%,2.96%, 11.48%, 8.25%, 5.95%, 7.13%, 6.57%, 0.69%, and 3.74%),FGSYC17L3 (36.95%, 12.69%, 12.13%, 14.82%, 1.87%, 5.61%,4.10%, 1.57%, 0, and 1.26%)). In the top 10 genera (Fig. 6D), the composition ofLactobacillus,Faecalibaculum, Lachnospiraceae,Muribaculaceae,Alistipes,Blautia,Ruminiclostridium9,Eubacterium fissicatenagroup,Parabacteroides, and Lachnospiraceae UCG 006 were different among the groups (Control (30.86%, 18.47%, 2.22%,14.35%, 7.81%, 1.15%, 0.27%, 0.42%, 2.78%, and 1.97%), Model(0.90%, 4.86%, 15.95%, 7.23%, 4.78%, 9.87%, 7.27%, 3.79%, 2.73%,and 3.78%), FZJTZ26M3 (2.96%, 8.37%, 8.86%, 8.25%, 5.95%, 5.36%,4.18%, 6.30%, 6.57%, and 3.06%), FGSYC17L3 (12.13%, 11.96%,9.22%, 1.86%, 5.61%, 7.63%, 7.73%, 3.70%, 1.57%, and 3.29%)).

Fig. 6 Effects of L. mucosae on microbial distribution at the family and genus level. (A) Microbial distribution at the family level, (B) Microbial distribution at the genus level, (C) Relative abundance of the top 10 different families, (D) Relative abundance of the top 10 different genera.
According to the results of LEfSe analysis, genera with potential differences were found (Fig. 7A). Compared with the Control group, the relative abundance ofAkkermansiawas significantly increased in the FZJTZ26M3 group (P< 0.05) (Fig. 7B). In addition, compared with the Control group, the relative abundance ofBlautia,Enterococcus,Lachnoclostridium,Ruminiclostridium9,and Ruminococcaceae UCG 009 was significantly increased in the Model group (P< 0.05), andL.mucosaeFZJTZ26M3 intervention significantly decreased the relative abundance ofLachnoclostridium,Ruminiclostridium9, and Ruminococcaceae UCG 009 compared with the Model group (P< 0.05) (Figs. 7C-G).

Fig. 7 Analysis of the difference in the gut microbiota. (A) Gut microbiota biomarker based on LEfSe analysis, the LDA score indicates the level of differentiation among the 4 groups. Relative abundance of (B) Akkermansia, (C) Blautia, (D) Enterococcus, (E) Lachoclostridium, (F) Ruminiclostridium 9,(G) Ruminococcaceae UCG 009.
The gut microbiota undertakes considerable physiological functions in the host. According to the results of the PICRUSt analysis, compared with the results in the Control group, HFD exposure significantly influenced the metabolism of mice in the Model group including metabolism of terpenoids and polyketides, nucleotide metabolism, glycan biosynthesis and metabolism, metabolism of other amino acids, lipid and carbohydrate metabolism (P< 0.05).L.mucosaeFZJTZ26M3 intervention significantly corrected the metabolism of terpenoids and polyketides (P< 0.05), additionally,the metabolism of cofactors and vitamins and energy metabolism were also significantly upregulated afterL.mucosaeFZJTZ26M3 intervention (P< 0.05). Moreover,L.mucosaeFGSYC17L3 intervention significantly downregulated amino acid metabolism and biosynthesis of other secondary metabolites (P< 0.05) (Fig. 8).

Fig. 8 Analysis of the potential function of the gut microbiota. (A) PICRUSt analysis between Control and Model, (B) PICRUSt analysis between FZJTZ26M3 and Model, (C) PICRUSt analysis between FGSYC17L3 and Model.

Fig. 9 Effects of L. mucosae on the relative expression of tight junction protein at transcription level in the colon. (A) Occludin, (B) ZO-2, (C) Claudin-1, (D) Claudin-2.
3.5 Effects of L. mucosae on SCFAs in NAFLD mice
There were statistically significant differences in the level of acetic, propionic, butyric, and isobutyric acid in the feces of mice between the Control and Model groups (P< 0.05). On the other hand,compared with both the Control and Model groups, the concentration of these acids in the mouse feces of the FZJTZ26M3 and FGSYC17L3 groups did not differ statistically significantly. (Figs. S1A-E).
3.6 Effects of L. mucosae on intestinal barrier in NAFLD mice
The tight junction protein revealed the permeability of the intestinal barrier. There was no significant difference between the Control and Model group in the expression of Occludin and ZO-2 (Figs. 9A, B). The expression of Claudin-1 and Claudin-2 in colon was significantly downregulated in the Model group compared with that in the Control group (P< 0.05), whereasL.mucosaeFZJTZ26M3 supplementation significantly upregulated the expression of these two genes compared with the Model group(P< 0.05) (Figs. 9C, D).
3.7 Effects of L. mucosae on lipid metabolism gene expression in liver and adipose tissue in NAFLD mice
Previous research revealed a connection between lipolysis and the expression of some genes, including lipoprotein lipase (LPL) adipose triglyceride lipase (ATGL), monoacylglycerol lipase (MGL), and hormone-sensitive lipase (HSL)[27]. In this study, compared with the Model group, theL.mucosaeFZJTZ26M3 supplement significantly upregulated the expression ofATGL(Fig. 10B),MGL(Fig. 10C), andHSL(Fig. 10D) (P< 0.05) in the liver, besides, compared with the Model group, theL.mucosaeFZJTZ26M3 supplement upregulated the expression of FAS but without significance (Fig. 10A). However,there was no significant difference between the Control and Model group in the expression of FAS (Fig. 10E). Furthermore, compared with the Model group, the expression of LEP receptor in the liver was also significantly upregulated after theL.mucosaeFZJTZ26M3 supplement (P< 0.05) (Fig. 10H). In addition, theL.mucosaeFZJTZ26M3 supplement upregulated the expression of carnitine palmitoyl transferase-1 (CPT-1) (Fig. 10F), apolipoprotein A (ApoA)(Fig. 10G), peroxisome proliferator-activated receptor-α (PPAR-α)(Fig. 10K), and CCAAT/enhancer-binding protein-α (C/ebp-α)(Fig. 10M), as well as downregulated the expression of adenosine 5’-monophosphate-activated protein kinase (AMPK) (Fig. 10I) and peroxisome proliferator-activated receptor-γ (PPAR-γ) (Fig. 10L).
Adipose tissue including WAT and BAT was also indicated as a passive participant in lipid metabolism[28]. Compared to the Model group, theL.mucosaeFZJTZ26M3 supplement downregulated the expression ofLEPin WAT (Fig. 11A) but had no obvious effect on the expression ofADPandPAI-1(Figs. 11B, C). In addition, the expression ofLPL(Fig. 11D) andPPAR-γ(Fig. 11J) in WAT was significantly upregulated by theL.mucosaeFZJTZ26M3 supplement(P< 0.05) compared with the Model group, whileHSL(Fig. 11F) in WAT was significantly downregulated (P< 0.05), but the expression ofATGL,MGL,C/ebp-αandPPAR-αin WAT was not significantly regulated after FZJTZ26M3 intervention (Figs. 11E, G-I). In BAT, the expression ofPPAR-α(Fig. 11K),PPAR-γ(Fig. 11L),andC/ebp-α(Fig. 11M) was upregulated significantly after theL.mucosaeFZJTZ26M3 supplement compared with the Model group(P< 0.05). Moreover,L.mucosaeFZJTZ26M3 supplementation did not observably affect the expression of uncoupling protein 1 (UCP-1)(Fig. 11O) and peroxisome proliferator-activated receptor-gamma coactivator 1-α (PGC1-α) (Fig. 11N) genes in BAT.
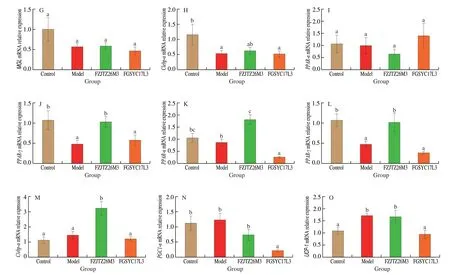
Fig. 11 (Continued)

Fig. 11 Effects of L. mucosae on the relative expression of lipid metabolism genes in adipose tissue. Relative expression in WAT: (A) LEP, (B) ADP, (C) PAI,(D) LPL, (E) ATGL, (F) HSL, (G) MGL, (H) C/ebp-α, (I) PPAR-α, (J) PPAR-γ; Relative expression in brown adipose tissue: (K) PPAR-α, (L) PPAR-γ, (M) C/ebp-α,(N) PGC1-α, (O) UCP-1.
4. Discussion
Two strains ofL.mucosaewere evaluated in this study for their potential to ameliorate NAFLD of two strains, in whichL.mucosaeFZJTZ26M3 treatment was deemed more effective due to its ability to reduce weight gain, decreased serum lipid, regulate lipid metabolism in the liver, WAT, and BAT, and adjust gut microbiota, whereasL.mucosaeFGSYC17L3 was ineffective and even worsened the development of NAFLD in HFD-fed mice.
Gut microbiota was considered an “organ” as its strong influence on health, dysbiosis of gut microbiota will lead to metabolic imbalance and various diseases including NAFLD[29],L.mucosaecould regulate NAFLD-related gut microbiota dysbiosis. Previous studies revealed that the Firmicutes and Bacteroidetes made up the majority of the gut microbiota in mice, and NAFLD caused an increase in the ratio of Firmicutes/Bacteroidetes[7,30], andL.mucosaeFZJTZ26M3 corrected the ratio almost equal to the Control group. In addition, previous studies showed that, compared with the healthy, patients with NAFLD had a higher relative abundance of Ruminococcaceae andBlautia[31-33].Our results verified them, furthermore, theL.mucosaeFZJTZ26M3 supplement restored these changes. In addition,Akkermansiaexhibited substantial correlations with NAFLD[34], treatment withL.mucosaeFZJTZ26M3 led to an increase in the relative abundance ofAkkermansia.Notably, mice in the FZJZT26M3 group had a lower relative abundance ofLactobacillusthan mice in the Control group,asL.mucosaewere proved to have the ability to inhibit the otherLactobacillusspecies[35-36].
Previous studies indicated that NAFLD-related dysbiosis of gut microbiota causes intestinal barrier dysfunction[37], resulting in the migration of gut microbial metabolites such as LPS from the intestine to the liver through blood circulation[38]. LPS in blood circulation and the liver will activate the TLR4 signaling pathway, leading to systemic inflammation and liver injury[7,39]. In our study, by increasing the expression of tight junction protein, theL.mucosaeFZJTZ26M3 supplement modified the intestinal barrier, therefore, the concentration of LPS in the blood was significantly decreased afterL.mucosaeFZJTZ26M3 treatment, thus reducing the accumulation of LPS in the liver. Corresponding to the LPS level, the inflammatory cytokines including IL-1β and TNF-α were downregulated, indicating thatL.mucosaeFZJTZ26M3 could reduce liver inflammation by decreasing serum LPS concentration. This result coincided with previous studies, which provedLimosilactobacillusfermentumCECT5716[40]andLactobacilluskefiranofaciensZW3[41]could ameliorate HFD-induced liver inflammation, by reducing the concentration of LPS in serum and regulate TRL4 related pathways.
LEP was secreted by adipocytes, related to appetite and energy metabolism, it was reported that LEP had the function to limit the storage of triglycerides in non-adipose tissues including the liver[42]. The concentration of LEP increased in patients with obesity and other metabolic diseases since LEP resistance occurred[43-44].L.mucosaeFZJTZ26M3 regulated the production of LEP at the level of both transcription and translation. Moreover, the expression of LEP receptor mRNA in the liver was also upregulated byL.mucosaeFZJTZ26M3. This manifested thatL.mucosaeFZJTZ26M3 could ameliorate NAFLD by alleviating LEP resistance.
Numerous probiotics were indicated to alleviate NAFLD by regulating the expression of genes related to lipid metabolism[20,27,40,45],L.mucosaeFZJTZ26M3 was also proved to have this function in our study. Intrahepatic lipolysis dysfunction happened in NAFLD patients[46],L.mucosaeFZJTZ26M3 treatment upregulated the expression ofLPL,ATGL,MGL, andHSLin the liver, reduced triglycerides in the liver. Additionally, the expression ofHSLmRNA in WAT was downregulated afterL.mucosaeFZJTZ26M3 treatment,preventing triglycerides from entering the blood and enriching in the liver. HFD-induced NAFLD resulted in a decline in LPL activity in WAT, further leading to an increase of chylomicron remnants in blood circulation[4], chylomicron would increase the burden of lipid metabolism in the liver if passing through the circulation to the liver. In our study, LPL activity in WAT was upregulated afterL.mucosaeFZJTZ26M3 intervention, preventing chylomicron remnants in circulation. PPAR-α and PPAR-γ played important roles in NAFLD, fatty acid oxidation[47], lipid storage[48], lipolysis[49], and adipogenesis[28]were influenced by them. The increase of expression of CPT-1 promoted fatty acid oxidation in the liver[50], furthermore,the expression of CPT-1 was stimulated by PPAR-α. In this study,the expression ofPPAR-αin the liver was upregulated, corresponding to that, the expression ofCPT-1in the liver was upregulated, too. It indicated thatL.mucosaeFZJTZ26M3 could accelerate fatty acid oxidation in the liver by regulating the expression ofPPAR-αandCPT-1. The expression ofPPAR-αin WAT was downregulated in this study, preventing lipolysis in WAT. In addition, the expression ofPPAR-γwas downregulated in the liver and upregulated in both WAT and BAT, promoting lipid storage in adipose tissue rather than the liver to prevent mice from NAFLD.
BAT was considered to restrain obesity by inducing thermogenesis[25]. The expression ofUCP-1in BAT facilitated nonshivering thermogenesis. In our study,UCP-1was not regulated byL.mucosaeFZJTZ26M3 but was inhibited afterL.mucosaeFGSYC17L3 intervention. This indicated that the obesity of the mice in the FGSYC17L3 group may result from the dysfunction of BAT. The expression ofPPAR-γandC/ebp-α master the adipocyte differentiation, both the differentiation of adipocytes and the browning of white adipocytes were regulated by them[51]. The expression ofPPAR-γandC/ebp-αwas regulated in our study indicating thatL.mucosaeFZJTZ26M3 could influence adipocyte differentiation in NAFLD mice.
5. Conclusion
In conclusion,L.mucosaeFZJTZ26M3 could downregulate serum lipids, decrease lipid deposition in the liver through modifying the gut microbiota, regulating lipid metabolism in the liver and adipose tissue, and influence adipocyte differentiation to ameliorate NAFLD in mice. It is possible in the future that consumingL.mucosaeFZJTZ26M3 for an extended period will be proved to be an effective microbiota-assist therapy for the treatment of NAFLD and the metabolic illnesses to which it is linked.
Competing interests
Wei Chen is an editorial board member forFood Science and Human Wellnessand was not involved in the editorial review or the decision to publish this article. The authors declare no conflict of interest.
Acknowledgment
This research was supported by the National Natural Science Foundation of China (32021005, 31820103010), 111 project(BP0719028), and the Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.26599/FSHW.2022.9250134.
- 食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango

