Effect of wall-disruption on nutrient composition and in vitro digestion of camellia and lotus bee pollens
Yun Yun, Shun Zhong, Zeyun Deng,, Gungyn Li, Jinwu Zhng, Hongyn Li,
a State Key Laboratory of Food Science and Resources, Nanchang University, Nanchang 330047, China
b Nanchang Tongxin Zichao Biological Engineering Co., Ltd., Nanchang 330052, China
Keywords: Bee pollen Nutrients Wall disruption Phenolic compounds In vitro digestion
ABSTRACT The nutrient digestion, absorption and biological activity of bee pollen may be limited due to the complex pollen wall. Here, the effect of superf ine grinding technology on the release of nutrients from bee pollen were investigated, and their antioxidant activities and in vitro digestion were explored in this study. Results showed that the content of nutrients in bee pollen increased after wall disruption. Among them, fat content increased by 22.55%–8.31%, protein content increased by 0.54%–4.91%, starch content increased by 36.31%–48.64%,soluble sugar content increased by 20.57%–29.67%, total phenolic acid content increased by 11.73%–86.98%and total f lavonoids content increased by 14.29%–24.79%. At the same time, the antioxidant activity increased by 14.84%–46.00%. Furthermore, the active components such as phenolic compounds in the wall-disruption bee pollen were more readily to be released during the in vitro digestion, and easier to be absorbed because of their higher bioaccessibility. Antioxidant activities during in vitro digestion were also improved in walldisruption bee pollen. These f indings provide evidence that bee pollen wall disruption was suggested, thus, it is more conducive to exerting the value of bee pollen in functional foods.
1. Introduction
Bee pollen is a substance formed by the addition of nectar and saliva to the pollen collected by honeybees. Bee pollen contains all essential amino acids, fatty acids, free amino acids, vitamins, essential minerals,carotenoids, f lavonoids and many other biologically active substances[1].Therefore, bee pollen has a variety of therapeutic properties, including antioxidant, anticancer, antibacterial activities, and organ protection[1].Nowadays, bee pollen is receiving more and more attention in the f ield of functional foods. In addition, in other applications, bee pollen might be a natural winemaking tool that could improve the sensory quality of wine and could affect the reproductive performance of f ish and the immune response of its offspring as a dietary supplement[2-3].
The mature pollen wall can be clearly divided into two layers. The outer layer, called the exine, is mainly composed of sporopollenin,which is very resistant. The inner layer, also called the inner wall, is mainly made of cellulose and the structure is similar to the plant cell wall[4]. It is necessary to adopt wall-disruption technology to promote the release of nutrients and the biological effects of pollen, and some researchers suggested that cracking pollen walls may be effective in increasing the digestibility of bioactive compounds in bee pollen[5].Other studies, however, claim that whole bee pollen can be digested and absorbed by humans and animals[6]. Thus, it is still necessary to provide direct evidence to prove whether wall disruption can improve the nutrient digestibility of bee pollen.
Wall disruption can be realized mainly by chemical, biological and physical methods[7]. Chemical methods generally use chemical reagents to disrupt the bee pollen wall. However, the chemical reagents will remain in the pollen, which may cause pollution and reduce its nutritional value[4]. The biological method treats bee pollen with various bacteria or enzymes. It is cost-effective, lowefficiency, and may easily contaminate the bee pollen with bacteria[8].Physical methods usually use radiation, swelling, osmosis, ultrasound,microwaves, temperature changes or shear forces to disrupt the bee pollen walls[7]. Compared with the chemical and biological methods,physical methods are more widely used for wall breaking, owing to their simplicity of operation and low cost[8]. The combination of ultrasonic and high-shear technology can effectively break the bee pollen wall and release nutrients[7]. Other research also found that applying ultrasonic treatment to break the wall of rose bee pollen could promote the release of nutritional components such as protein,lipid, water-soluble sugar and flavonoids, but lead to the loss of vitamin B1and vitamin C[9]. Compared with other physical methods,superfine grinding technology is low-cost, simple operation, timesaving, and suitable for large-scale production[10]. In addition,the particle size after superfine grinding is small and uniformly distributed, with an obvious wall-breaking effect[11].
Based on the above, two distinctive and relatively little-studied main categories of bee pollen in China, camellia and lotus, were selected as the research objects. Several studies have reported the chemical composition and function of bee pollen, but there is little information about the effect of superfine grinding technology on the wall of bee pollen. And it is still controversial whether the wallbreaking treatment of bee pollen was needed. Therefore, in this study, superfine grinding technology was used to break the wall of bee pollens, and the nutrient components and antioxidant activities of bee pollens with or without wall disruption were compared.The effects of wall disruption on the release and bioavailability of phenolic compounds in bee pollen duringinvitrodigestion were also studied, and the changes in their composition and antioxidant activity were characterized, so as to provide a theoretical basis for whether bee pollen needed wall disruption or not. These results were helpful to evaluate the effect of wall-breaking treatment on bioactive compounds, and provide evidence for the potential application of bee pollen in functional food processing.
2. Material and methods
2.1 Materials and regents
α-Amylase (527 U/mg), pepsin (≥ 15 000 U/mg), pancreatin(100–350 U/mg), bile salt (> 98%), caffeic acid (> 99%), gallic acid(> 99%), quercetin (> 99%),p-hydroxybenzoic acid (> 99%), benzoic acid (> 99%), glucose (> 98%), gluconic acid (> 98%), sucrose(> 98%) and Trolox (98%) were obtained from Aladdin Reagent Database, Inc. (Shanghai, China). 1,1-Diphenyl-2-picrylhydrazyl(DPPH, > 99%), 2,2’-azino bis(3-ethylbenzthiazoline-6-sulphonate)(ABTS, > 98%,), 1,3,5-tri(2-pyridyl)-2,4,6-triazine) (TPTZ,> 98%), and Folin-Ciocalteu (2 mol/L, 2N) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). KCl, KH2PO4,NaHCO3, NaOH, NaCl, HCl, CaCl2, MgCl2·6H2O and (NH2)4CO3were obtained from XiLong Chemical Co., Ltd. (Guangdong,China). All these reagents were of analytical grade (AR, ≥ 99.7%).Acetonitrile, ethanol, and formic acid (HPLC-grade, ≥ 99.9%) were purchased from Merck (Darmstadt, Germany). Water was purified using a Milli-Q system, Millipore (Bedford, MA, USA).
2.2 Samples
Camellia (Camellia japonicaL.) and lotus (Nelumbo nuciferaGaertn.) bee pollen samples were provided by Jiangxi Nanchang Tongxinzicao Biological Engineering Co. Ltd (Jiangxi, China).The company dried bee pollen samples in a vacuum oven to a final moisture content of < 8%. The species of bee pollen samples were confirmed by scanning electron microscopy (SEM; SU8100, Hitachi Inc., Japan) based on palynology[12].
A high-speed ultrafine pulverizer (DFT-50A, Wenling Linda Machinery Co., Ltd, Zhejiang, China) was used at a speed of 25 000 r/min to break the wall of bee pollen samples. The sample powders were passed through a 60-mesh sieve, and the obtained powder was stored at –18 °C for further analysis.
2.3 Histomorphology analysis
2.3.1 SEM
A small amount of camellia bee pollen before wall disruption(CB), camellia bee pollen after wall disruption (CA), lotus bee pollen before wall disruption (LB) and lotus bee pollen before wall disruption (LA) were fixed on the sample stands with conductive glue,and spattered with gold. The morphology of bee pollens was observed under the appropriate magnification of the SEM (SU8100, Hitachi Inc., Japan) at an acceleration voltage of 5.0 kV and a magnification of 500 ×. All samples were done in triplicate.
2.3.2 Specific surface area
The specific surface area (SSA) analysis was completed according to the pretreatment conditions widely used in Brunauer,Emmett and Teller (BET) analysis. Bee pollen samples were dried in a vacuum oven (DFT-50A, Wenling Linda Machinery Co., Ltd.,Zhejiang, China) before BET determination. Then, samples were put into the automatic SSA analyzer (JW-BK132F, JWGB Sci &Tech Ltd., Beijing, China), and nitrogen was filled in for adsorption.Subsequently, nitrogen adsorption data were obtained and SSA values were calculated[8]. All samples were done in triplicate.
2.4 Chemical composition analysis
Crude fat determination was made through a Soxhlet extractor and petroleum ether (boiling range was 30–60 °C) as the solvent[13].Protein was determined by the Kjeldahl method with a factor of 6.25 for converting the total nitrogen into proteins[14]. The fiber was determined by measuring the loss on ignition of the dried residue remaining after digestion of the sample with 1.25% (m/m) H2SO4and 1.25% (m/V) NaOH solution[15]. The anthrone colorimetric method was used to measure the starch and soluble sugar contents with commercial kits (Solarbio Science and Technology Co., Ltd., Beijing,China). All samples were done in triplicate.
2.5 Fatty acid analysis
The total fatty acids were determined according to previous studies[16]. In brief, the lipid of bee pollen samples was extracted with chloroform/methanol (1:1) and then methylated by 14% BF3in methanol for 1 h at 90 °C. The fatty acid methyl esters (FAMEs)were analyzed by gas chromatography (6890N, Agilent Technologies,USA), equipped with flame ionization detection and a CP-Sil88 column (100 m × 0.25 mm, 0.2 μm, Agilent Technologies, USA). The injector and detector temperatures were both set at 250 °C. N2was used as a carrier gas, H2and air were used as burning gas, and the flow velocity was 1.8 mL/min. The initial temperature of the column was operated at 45 °C for 4 min and programmed at 13 °C/min to 175 °C, then rose to 215 °C at a rate of 4 °C/min and maintained for 15 min. The FAMEs were identified and quantified by comparing the retention times and peak areas with the FAME standard mixture(GLC-463, Nu-Chek Prep Inc., USA), and the results were expressed as a percentage of total FAEMs. All samples were done in triplicate.
2.6 Amino acid analysis
The contents of amino acids were determined by the methods reported by Paramás et al.[17]. In brief, (280 ± 20) mg of defatted pollen was accurately weighed and extracted with 50 mL 80% ethanol for 15 min, and then centrifuged at 4 000 r/min (LD5-2B, Jing Li Centrifuge Co., Ltd., Beijing, China). The residue was reextracted twice, and the supernatants were combined and dried at approximately 40 °C with rotary evaporation (RE-3000, Yalong Biochemical Instrument Factory, Shanghai, China). The residue was dissolved in citric acid buffer solution (pH 4.3), filtered with a 0.22 μm membrane,and analyzed by the amino acid automatic analyzer (L-8900, Hitachi,Japan). All samples were done in triplicate.
2.7 Chemical extraction
Free and bound phenolics were extracted according to Peng et al.[18]with some modifications. Free phenolics were ultrasonic extracted from 1 g of bee pollen for 20 min with 30 mL of 80% (V/V)methanol. After centrifugation, the supernatant was collected, and the extraction was repeated three times. The supernatants were combined and concentrated by rotary evaporation (Buchi, Rotavapor R-210,Shanghai, China) at 40 °C. After extraction of free phenolics, the resulting residue was dried under nitrogen and then digested with 20 mL 2 mol/L NaOH at room temperature for 4 h. The solution was then acidified to pH 2 with 6 mol/L HCl and extracted three times with 20 mL of ethyl acetate. The organic phase was then partially mixed and evaporated to dryness under a vacuum at 35 °C. The bound phenolics were re-dissolved in 1 mL 80% (V/V) methanol for further analysis.
2.8 Total phenolic contents (TPC) and the total flavonoid contents (TFC)
The TPC of bee pollen extracts were determined using the Folin-Ciocalteu method[19]. A total of 25 μL samples or gallic acid standards were mixed with 125 μL of Folin-Ciocalteu reagent (0.2 mol/L)in a 96-well plate and reacted for 6 min at room temperature, then 125 μL 7.5% Na2CO3solution (m/V) was added. The mixture was slowly shaken on an oscillator (SANYO Electric Co., Ltd., Osaka,Japan) and placed at room temperature for 30 min. The absorbance was determined at 765 nm using a Thermo Varioskan Flash Microplate Reader (Thermo Scientific, Waltham, MA, USA). The concentrations of gallic acid standards were 15.625, 31.25, 62.5, 125,250, 500 and 1 000 μg/mL (R2= 0.999 5). The TPC was expressed as milligrams of gallic acid equivalents (GAE) per gram of dry weight(mg GAE/g DW). All samples were done in triplicate.
The TFC of bee pollen samples were measured by the NaNO2-AlCl3method[19]. A total of 25 μL standard solutions of rutin (25,31.25, 50, 62.5, 100, 250 and 500 μg/mL,R2= 0.998 9) or samples were mixed with 110 μL of NaNO2(0.066 mol/L) solution in a 96-well plate at room temperature and the reaction was performed in darkness for 5 min, then 15 μL of 0.75 mol/L AlCl3solution was added to the mixture. Finally, 100 μL NaOH (0.5 mol/L) solution was added 6 min later and the absorbance was measured at 510 nm.The TFC of bee pollen samples was expressed as milligrams of rutin equivalents (RTE) per gram of dry weight (mg RTE/g DW). All samples were done in triplicate.
2.9 UHPLC-ESI-QTOF-MS/MS analysis
A UHPLC30A system (Shimadzu, LC20AD, Kyoto, Japan),coupled with a Trip TOFTM5600 system (AB SCIEX, Foster City, California, USA) was used for analyzing the phytochemical composition. Separation was carried out in an Inertsil ODS-3 C18column (4.6 mm × 250 mm, 5 μm). The mobile phase was composed of 0.1% formic acid in deionized water (A) and acetonitrile (B).The solvent gradient for separation of phytochemical composition in camellia bee pollen was as follows: 0–22 min, 10%–30% B;22–31 min, 30%–49% B; 31–39 min, 49%–60% B; 39–50 min,60%–72% B; 50–54 min, 72%–100% B; 54–56min, 100%–0% B.The solvent gradient in lotus bee pollen was also as follows:0–14 min, 12%–20% B; 14–30 min, 20%–30% B; 30–35 min, 30%–44% B;35–48 min, 44%–60% B; 48–66 min, 60%–74% B; 66–68 min,74%–12% B. The post-running time was 2 min. The column temperature was 35 °C, and the flow rate was 0.6 mL/min. The diode array detector at 280, 320 and 360 nm was used. All standards and samples were dissolved in methanol and the injection volume was 10 μL.
The ESI source was operated in the negative ion mode, and full scan mass spectral data were acquired over a range fromm/z100 to 1 200. The optimum values of the source parameters were as follows:capillary voltage, + 4.0 kV; drying gas flow, 10.0 L/min; drying gas temperature, 350 °C; nebulizing gas pressure, 40 psi. The collision energy was set at 35 eV, and the fragmentor voltage was set at 135 V, using nitrogen as collision gas. The compounds in the extract were identified by methods such as comparison with corresponding standards, literature reports or searching databases.
2.10 In vitro antioxidant activities
The ABTS radical scavenging activity and ferric reducing antioxidant power (FRAP) were determined using our previously published methods[19]. Briefly, 20 μL of the supernatant extract sample was mixed with 200 μL of fresh ABTS cation radical. The mixture was incubated at room temperature for 5 min. The absorbance was recorded at 734 nm using a Thermo Varioskan Flash Microplate Reader (Thermo Scientific). The results were determined using a Trolox calibration curve (R2= 0.997 4) and expressed as Trolox equivalent per gram of dried weight sample (mmol Trolox/g DW).For FRAP analysis, 10 μL the supernatant extract sample was allowed to react with 300 μL of ferric-TPTZ reagent. The mixture samples were kept at 37 °C for 5 min, and they then were measured at 595 nm. Ferrous sulfate was used to prepare the calibration curve(R2= 0.999 3), and the results were expressed as FeSO4equivalent per gram of dried weight sample (mmol FeSO4/g DW). All samples were done in triplicate.
2.11 In vitro digestion
A standardized staticinvitrodigestion method was performed according to the procedures previously described by Minekus et al.[20].In brief, 1 g of each bee pollen powder was thoroughly mixed with 8.5 mL of simulated salivary fluid (SSF) electrolyte stock solution,followed by 0.5 mLα-amylase solution (1 500 U/mL), 25 μL CaCl2solution (0.3 mol/L) and 975 μL of distilled water. The mixture was shaken for 2 min in a 37 °C water bath in the dark. Following oral digestion, 1.6 mL porcine pepsin stock solution (25 000 U/mL),5 μL of 0.3 mol/L CaCl2, 7.5 mL of simulated gastric fluid (SGF)electrolyte stock solution, 0.2 mL of HCl (1 mol/L) to reach pH 3.0 and 695 μL of distilled water were added to the above-digested mixture. The digest was incubated for 2 h in a shaking water bath at 37 °C in the darkness. Finally, the gastric mixture was mixed with 11 mL of simulated intestinal fluid (SIF) electrolyte stock solution,5.0 mL of a pancreatin solution (800 U/mL), 2.5 mL fresh bile salt solution (160 mmol/L) and 40 mL of CaCl2(0.3 mol/L), 0.15 mL of NaOH (1mol/L) to reach pH 7.0 and 1.31 mL of distilled water.Then, unlike the standard process, two separate procedures are performed. In one digestion, the mixture was continued to react at a 37 °C water bath for 2 h. In the other digestion, the intestinal mixture was transferred into a 15-cm cellulose dialysis membrane(Solarbio Biological Technology Co. Ltd., Beijing, China) of 10 kDa molecular weight cut off. The membrane was placed in the phosphate buffer solution (pH 7.4). The dialysis system was performed for 2 h in a water bath at 37 °C. The fraction that came out of the dialysis membrane after dialysis was considered the bioaccessible fraction and it was supposedly available for absorption[21]. After each reaction,phenylmethylsulfonyl fluoride (PMSF) was added to achieve 1 mmol/L in the final digestion mixture to stop the reaction. Then the digestion supernatant at each stage was freeze-dried, and all samples were redissolved with 80% methanol before analysis. All samples were done in triplicate.
The bioaccessibility and dialyzable rate of bee pollen samples were calculated as follows:
Where PC1is the phenolic compound content (mg/g DW) in the soluble fractions afterinvitrogastrointestinal digestion, PC2is the phenolic compound content (mg/g DW) in the samples before digestion, PC3is the phenolic compound content (mg/g DW) in the fractions coming out of the dialysis membrane, PC4is the phenolic content (mg/g DW) in the samples before theinvitrodigestion.
2.12 Statistical analysis
Blank samples were prepared in the same manner as the other samples and were used to correct the measurement signal. The results were expressed as mean ± standard deviations (SD) of triplicate samples. Statistical analyses were carried out using the SPSS 20.0(SPSS Inc, USA), which were carried out using the one-way analysis of variance (ANOVA) followed by Duncan’s multiple range tests.In addition, MassHunter Acquisition B.03.01 was used to collect and analyze the LC-MS data, and the MSndata were analyzed by the MassBank, PubChem, and Human Metabolome Databases.
3. Results and discussion
3.1 Comparisons of the nutritional components in bee pollen before and after wall disruption
In the present study, the superfine grinding technology was applied to disrupt the wall in camellia and lotus bee pollen samples,and the effect on the structure of bee pollen wall was observed by SEM. The micrographs in Fig. 1 showed camellia and louts bee pollen samples with and without wall disruption. It was found that the morphology of bee pollen grains changed greatly after the wall disruption. Before ultrafine pulverizing, the bee pollen wall tightly wrapped the intracellular substances, and a complete pollen grain structure was observed. After ultrafine comminution, the bee pollen grains were broken into fragments, and the cytoplasm was released.The SSA is an important feature of the biological functions of microstructures, and the degree of wall breakage of bee pollen can be characterized by the SSA. Generally, a larger SSA means a greater degree of damage to the structure of bee pollen wall. The SSA of camellia and lotus bee pollen samples after wall disruption were almost twice as large as that of the untreated samples (P< 0.001,Fig. S1), indicating that the intact bee pollen grains were destroyed.
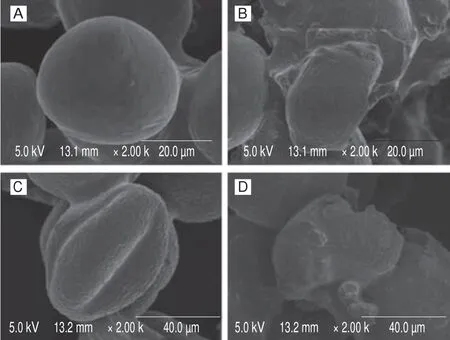
Fig. 1 SEM microphotography of bee pollens before and after wall-disruption.(A, B) camellia bee pollen before and after wall-disruption, respectively;(C, D) lotus bee pollen before and after wall-disruption, respectively.
The results of nutrient components of bee pollen samples before and after wall disruption were shown in Table S1. The contents of crude protein, crude fat, starch and soluble sugars in camellia and lotus bee pollen were increased in varying degrees after superfine grinding. These results showed that wall disruption was beneficial to the release of nutrients. Yan et al.[22]also found that more sugars,including glucose, fructose and maltose, could be detected after the wall disruption of rape bee pollen. However, the crude fiber content of bee pollen ultrafine powders was significantly lower than those of untreated samples (P< 0.01), especially the crude fiber content in lotus bee pollen after wall disruption (LA) was 56% lower than that before (LB,P< 0.001). These were mainly due to the destruction of sporopollenin in the outer wall and the cellulose in the inner wall of pollen cells after superfine grinding, so the contents of crude fiber were obviously reduced. However, it was found that the element content in bee pollen did not change significantly after the wall disruption (Table S2).
Compared with the standard, the fatty acids composition and relative content of bee pollen were determined (Table 1). The content of polyunsaturated fatty acids (PUFAs) in camellia bee pollen was the highest, followed by monounsaturated fatty acids(MUFAs) and saturated fatty acids (SFAs). Furthermore, biologically importantα-linolenic acid (C18:3n-3) was the main fatty acids in camellia bee pollen before the wall disruption (CB) and after the wall disruption (CA), accounting for 45.09% and 42.18% of all fatty acids, respectively. After the wall disruption, the ratio of SFA and MUFA in camellia bee pollen increased significantly (P< 0.05).The most abundant fatty acid in lotus bee pollen was palmitic acid(C16:0), followed byα-linolenic acid. Like camellia bee pollen, which underwent wall-breaking treatment, the ratio of SFA and MUFA was significantly increased in lotus bee pollen (P< 0.01). It was suggested that wall disruption could increase the ratio of SFA and MUFA in total fatty acids from camellia and lotus bee pollen samples, which speculated that the fatty acids inside the bee pollen cell wall were mostly SFAs and MUFAs. MUFAs have favorable anti-inflammatory and cardiovascular benefits. Mechanistically, MUFAs can inhibit the accumulation of lipid ROS at the plasma membrane[23-24]. PUFAs are associated with obesity, metabolic diseases, cardiovascular and immune functions, insulin action, neuronal development and neuropsychiatric diseases[25]. In recent years, PUFA has been proven to be a regulator of lipid metabolism and to have anti-inflammatory and anti-cancer effects[26]. The ratio of PUFAs to total fatty acids in bee pollen decreased after wall breaking, from 56.47% to 53.32% in camellia bee pollen, and from 34.36% to 27.30% in lotus bee pollen.The total fat content in camellia and lotus bee pollen samples after the wall-breaking was significantly increased (P< 0.05, Table S1),especially the fat content of CA was nearly 2 times as much as that of CB. Therefore, the decrease in the ratio of PUFAs did not mean a decrease in PUFAs content of bee pollen. It has been reported that the content of PUFAs in wall-broken bee pollen samples increased,particularlyα-linolenic acid (C18:3n-3) and linoleic acid (C18:2n-6) with important biological activities[7].
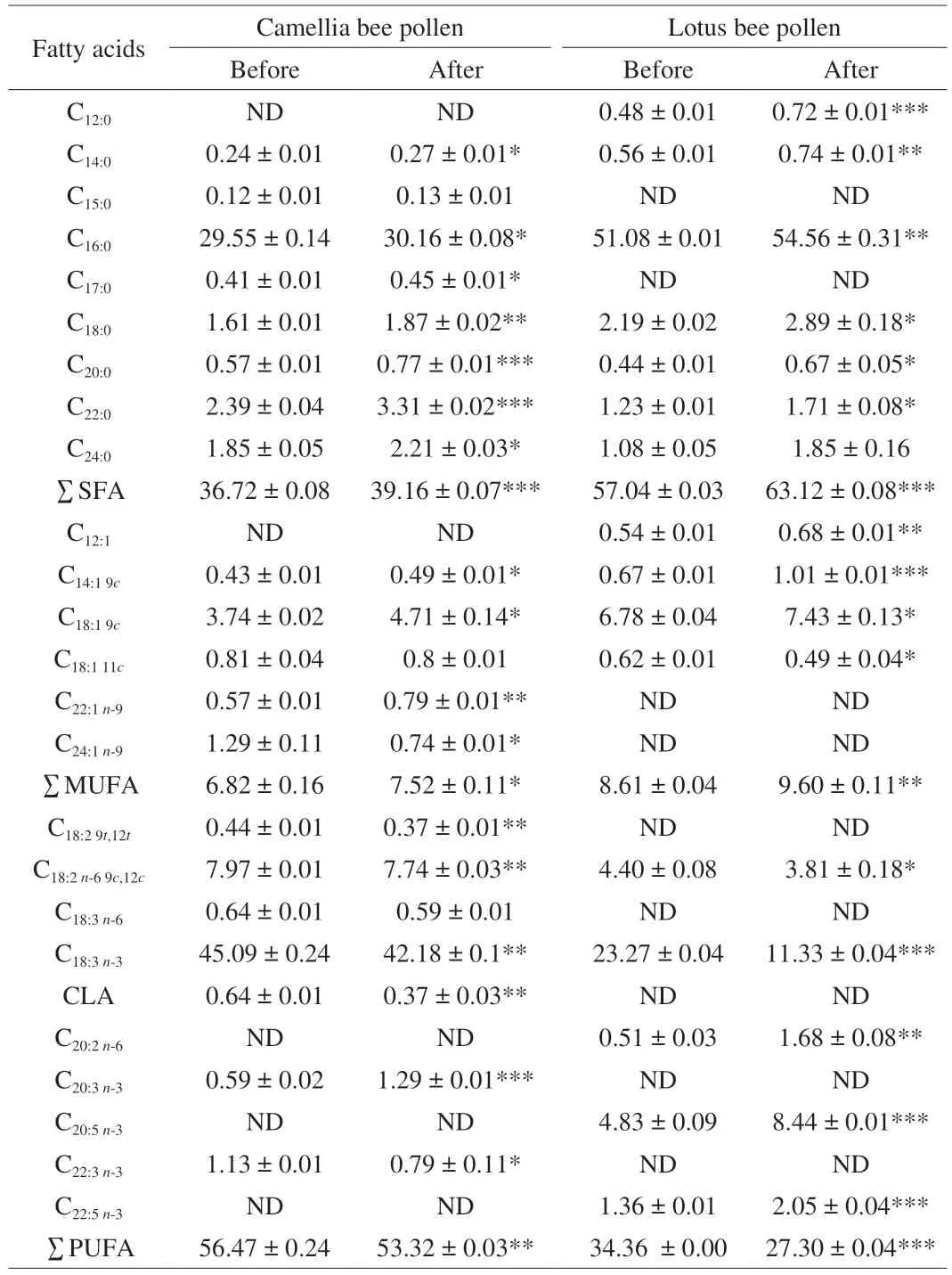
Table 1 Fatty acid profiles of bee pollen samples before and after wall-disruption (%).
According to Table 2, it can be seen that the contents of total essential amino acids (TEAA), total non-essential amino acids(TNEAA) and total amino acids (TAA) in camellia and lotus bee pollens after wall disruption were increased (P< 0.05). Especially the contents of threonine (Thr), lysine (Lys) and aspartic acid (Asp)in camellia bee pollen increased by 375%, 131.58% and 64.31%.Meanwhile, leucine (Leu), lysine (Lys) and arginine (Arg) in lotus bee pollen increased by 32.84%, 31.75% and 21.97%, respectively. In general, the types of amino acids in both two kinds of wall-disruption bee pollen samples increased. CA increased four types of amino acids compared to CB, namely tyrosine (Try), serine (Ser), cysteine (Cys)and arginine (Arg). What’s more, LA increased one type of amino acids, which was the proline (Pro). Combined with the changes in amino acid types and contents, it could be seen that the amino acids contents of camellia and lotus bee pollen samples increased after wall disruption, and Try, Ser, Cys, Arg and Pro may be mainly distributed in the interior of pollen wall.
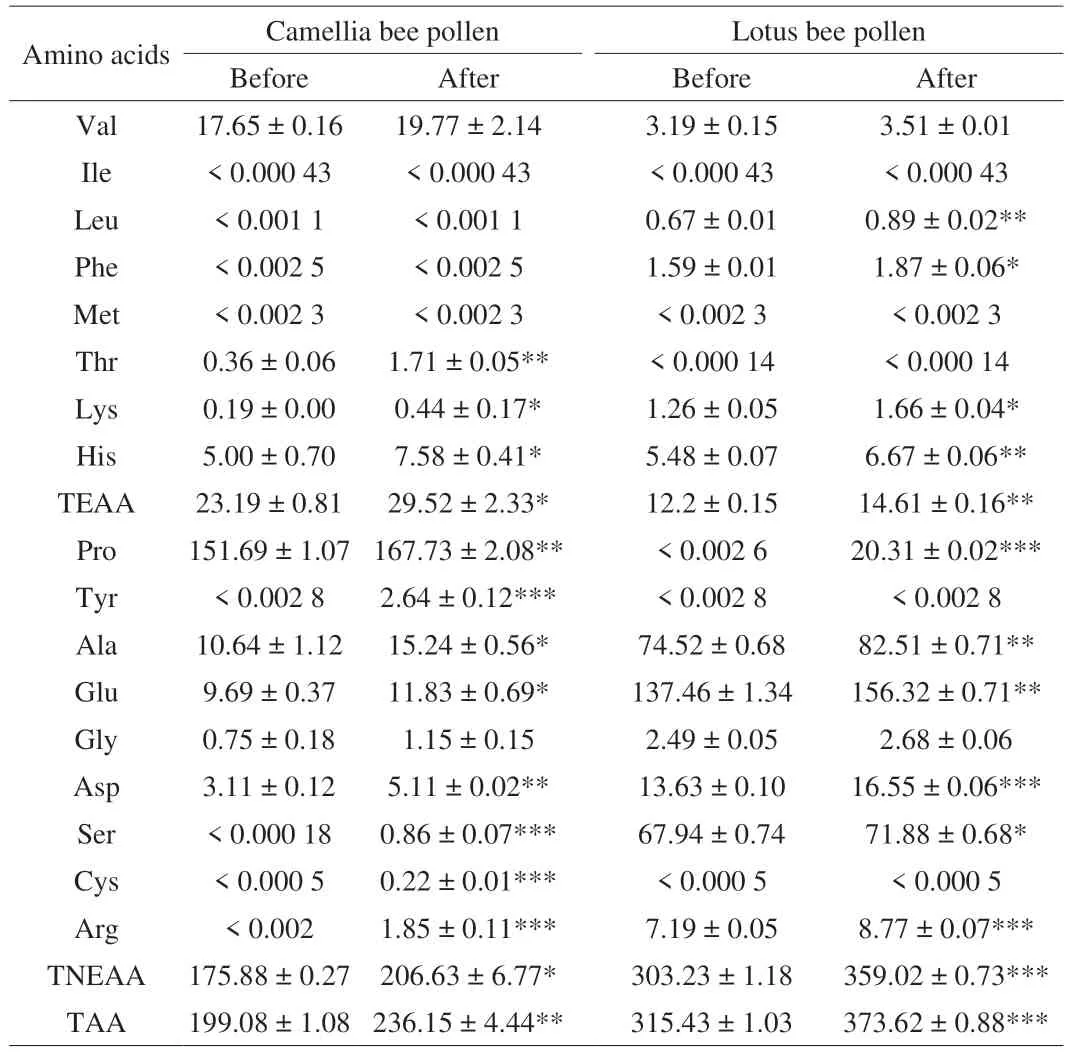
Table 2 Amino acid composition and content of bee pollen samples before and after wall-disruption (mg/100 g DW).
3.2 Identification of phenolics in camellia and lotus bee pollens
Fig. S2 was the base peak chromatogram of free phenolic extracts from camellia bee pollen under the negative ion model. The identified compounds were shown in Table S3. Through retention times, UV spectra and accurate MSndata, it was found that the type of free phenolic compounds contained in CB and CA were the same, and a total of 15 phenolic compounds were identified. Peaks FC1, FC3 and FC6 (tR3.47, 12.2 and 31.6 min,m/z179.056 5, 169.014 4 and 300.999 4) were identified as caffeic acid, gallic acid and quercetin respectively compared to the standards. Based on the MSndata in the literature, peak FC2 was identified as galloyl glucose[27], and Fig. S3A showed its cleavage law. The fragmentation pathway shown by the main fragment ion in peaks FC4, FC12 and FC14 (m/z315) was compatible with isorhamnetin. In compound 4, MS2spectrum indicated a loss of 470 Da corresponding to a rutinoside-glucoside linked together, whereas,in peak FC12, the production was formed by the loss of 308 Da, which was indicative of a rutinoside unit[28]. Peak FC14 had a precursor ion atm/z769.18 [M–H]–, product ion atm/zgenerated by the loss of a rhamnoside group, and isorhamnetin aglycone due to a further loss of a rutinoside group. Peaks FC4, FC12 and FC14 were assigned as isorhamnetin-3-Orutinoside-7-O-glucoside, isorhamnetin-3-O-rutinoside and isorhamnetin 3-rutinoside-7-rhamnoside[9]. Peaks FC5 (tR27.27 min,m/z755.206 3),FC10 (tR36.62 min,m/z593.131), FC12 (tR39.59 min,m/z593.131) and FC15 (tR44.81 min,m/z739.168 7) contained characteristic ion atm/zof 285 for kaempferol, and were identified as kaempferol 3-rutinoside 7-glucoside, kaempferol-3-rutinoside,kaempferol 3-O-neohesperidoside and kaempferol 3-O-rhamninoside by comparison with the MassBank database and references[9]. It was found that peaks FC7, FC8, FC9 and FC10 were phenolamine compounds, which exist in the form of derivatives formed by the amidation of spermidine and phenolic acids. Peak FC7 exhibited a[M−H]−atm/zof 614.252 0 and major ion fragmentation atm/z478.201 4 obtained by missing caffeoyl residue C8H8O2,m/z452.221 4 obtained by missing caffeoyl moiety C9H6O3,m/z322.144 7 obtained by losing two caffeoyl residue 2C8H8O2, andm/z322.163 5 obtained from the loss of a caffeoyl residue C8H8O2and a coumaroyl moiety C9H8O2. The fragmentation pathway (Fig. S3B) indicated that peak FC7 was identified as dicaffeoyl coumaroyl spermidine. Same fragmentation patterns were also observed for the compounds in peaks FC8, FC9 and FC10 (Figs. S3C–E) with respective differences correlated with the type of feruloyl,p-coumaroyl, or caffeoyl, linked to the different amino groups in the molecules. Peaks FC8, FC9 and FC10 correspond to caffeoyl dicoumaroyl spermidine, tricoumaroyl spermidine and feruloyl dicoumaroyl spermidine, respectively[28-30].
The total ion chromatogram of soluble phenolic compounds from lotus bee pollen was shown in Fig. S4, and a total of 8 main components were putatively identified (Table S5). Based on the elution gradient and mass spectrum data compared with the standard and literature, peaks FL1, FL2 and FL3 (precursor ionm/z179, 137 and 121) were identified as caffeic acid,p-hydroxybenzoic acid and benzoic acid, respectively. Peaks FL4, FL6, FL7 and FL8 had the characteristic fragment ions of kaempferol aglycone atm/z227,255 and 285, which were identified as kaempferol-3-rutinoside,kaempferol-3-O-glucoside, kaempferol-3-O-glucuronide and kaempferol-3-O-rhamnoside. Peak FL5 was identified as quercetin 3-O-rhamnoside-7-O-glucoside, which mass spectrum data indicated a loss of 470 Da corresponding to a rutinoside-glucoside linked together.
3.3 Effects of wall-disruption on phenolic compounds and antioxidant activity in bee pollen
TPC and TFC of free phenolic extracts of camellia and lotus bee pollen were shown in Table 3. Due to the difference of phenolic compounds in camellia and lotus bee pollen, their TPC, TFC and antioxidant activities showed significant differences. The TFC, TPC and antioxidant activities of camellia bee pollen were much higher than those of lotus bee pollen. The TPC and TFC of CA and LA were significantly higher than those of CB and LB, especially the TPC in LA was nearly twice than that of LB. A total of 15 phenolic compounds were identified in the camellia bee pollen (Table S3). Among them,caffeic acid and galloyl glucose were only found in CA. As shown in Table S4, a total of 8 phenolic compounds were identified in the lotus bee pollen, among which caffeic acid was only found in LA.Results indicated that the wall-breaking treatment facilitated the release of free phenolic compounds in bee pollen. Furthermore, as shown in Table S5, the TPC and TFC in the bound phenolic extracts of CA and LA were significantly lower than those in CB and LB(P< 0.05). A total of 7 phenolic compounds were identified in the bound phenolic extracts of camellia bee pollen, and 4 phenolic acids were identified in lotus bee pollen (Tables S3 and S4, Figs. S5 and S6). Compared with CB and LB, the contents ofp-coumaric acid,ferulic acid and isoferulic acid were significantly lower in CA and LB(P< 0.05). What’s more,p-hydroxybenzoic acid was only found in LB, but not detected in LA. According to previous reports, the TPC in wall-broken rape bee pollen by ultrasonic and enzymatic methods was higher than that in untreated bee pollen[22]. The current results were the same as those in the above literature. After destroying the pollen wall, the internal substances including phenolic compounds flowed out, thus benefiting the extraction of phenolic compounds. Bound phenolic compounds were usually bound to stable macromolecules in plant cells, such as cellulose, hemicellulose or cytoskeleton proteins on the cell wall, and cannot be extracted by organic solvents[31]. On the one hand, phenolic compounds could be directly released under the wall-breaking treatment. On the other hand, the wall-disruption treatment converted more bound phenolic compounds into free phenolic compounds.
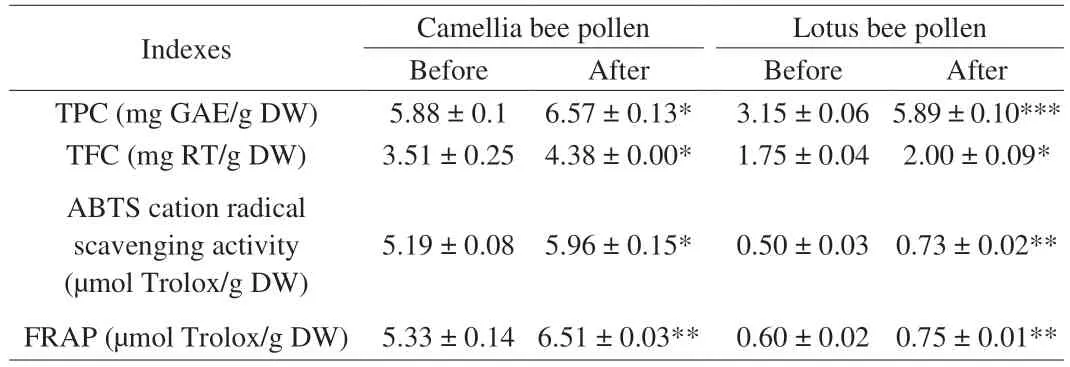
Table 3 Total phenolic contents, total flavonoid contents and antioxidant activities of free phenolic extracts from bee pollen.
FRAP and ABTS methods were used to determine the antioxidant activity of phenolic extracts in bee pollen before and after wall disruption (Table 3). Similar to the trend of TPC and TFC, compared with CB and LB, CA and LA showed higher ABTS cation radical scavenging activity and FRAP in phenolic extracts (P< 0.05). It further proved that the wall-breaking treatment had an important contribution to the antioxidant activity of bee pollen. The increased release of antioxidants, especially phenolic compounds, was conducive to the improvement of antioxidant capacity.
3.4 Identification of the metabolites of camellia and lotus bee pollens in the in vitro digestion model
3.4.1 Camellia bee pollen
Table S8 and Figs. 2A-B showed the identified compounds in CB and CA digestive juices at each stage of theinvitrosimulation. A total of 41 compounds, including 3 carbohydrates, 11 phenolic acids and derivatives, 10 flavonoids, and 17 fatty acids, were identified or preliminary characterized. Compared with the compounds of CB, 13 compounds were only found in the CA, including 8 phenolic acids,1 flavonoid, and 4 fatty acids. In addition, Fig. S7 illustrated the proposed fragmentation patterns of some identified compounds.
Peaks C1, C2 and C3 were tentatively identified as glucose,gluconic acid and sucrose, respectively, by comparing with standards and their fragmentation patterns[32]. A total of 11 phenolic acids were characterized and these compounds can be further classified into hydroxycinnamic acid derivatives. Peak C6, with a product ion atm/z119.051 which was suggestive of ap-coumaroyl moiety, was tentatively identified as dicoumaroyl putrescine (Fig. S7A)[33]. Peaks C7, C8, C9 and C17 had the same fragmentation pattern based on the observed loss of a feruloyl, orp-coumaroyl, or caffeoyl moiety, and were identified as caffeoyl dicoumaroyl spermidine, tricoumaroyl spermidine, dicaffeoyl coumaroyl spermidine and feruloyl dicoumaroyl spermidine, respectively. With the same molecule ion and fragment ions, peaks C10 and C12 were characterized as isomers of peak C7, and peaks C11, C14 and C16 were characterized as isomers of peak C8. By matching its fragmentation pattern(Fig. S7B), peak C35 with [M–H]–atm/z312.254 was tentatively identified as 2-(4-(diethylamino)-2-hydroxybenzoyl) benzoic acid.Peak C36 showed a precursor ion atm/z339.229 6, and was suggested as 6,7-dihydroxycoumarin-6-glucoside based on the product ion atm/z177.211 5 [M–H–162]–as well as the observed loss of a hexose moiety[34].
A total of 10 flavonoids and derivatives were found in CB and CA digestive juice. Peaks C4, C5, C13, C15, C18, C19, C21 and C22 were unambiguously identified as isorhamnetin-3-O-rutinoside-7-Oglucoside, kaempferol-3-O-rutinoside-7-O-glucoside, isorhamnetin-3-O-rutinoside, kaempferol-3-O-rutinoside, isorhamnetin-3-Orutinoside, kaempferol-3-O-neohesperidoside, kaempferol-3-O-robinoside-7-O-rhamnoside and quercetin-3-O-rhamnoside,respectively, which have also been described in before. Peak C20 with molecular ion atm/z769.173 5 showed the product fragment ion atm/z315.049 2, from the loss of molecules from galactoside and rhamnoside, and the quercetin aglycone fragment ion atm/z300.027 9 was identified as 7-O-methylquercetin-3-O-galactoside-6’’-rhamnoside[35]. Peak C23 showed a precursor ion atm/z474.216 4,and the MS2study produced fragment ions atm/z311.296 8 was from the loss of a hexose moiety. Based on the fragmentation patterns and data from previous studies, peak C16 was tentatively identified as betavulgarin glucoside (Fig. S7C)[36].
As shown in Table S8, a total of 16 fatty acids were detected in the camellia bee pollen digestive juice. Two SFAs, arachidic acid(peak C24, Fig. S7D) and palmitic acid (peak C39), were tentatively identified based on their mass-to-charge ratios (311.2218 and 255.2326) and predicted molecular formulas (C18H32O4and C16H32O2)and by matching data in the literature[37]. Similarly, 3 unsaturated fatty acids,α-linolenic acid (peak C37, Fig. S7E), linoleic acid(peak C38) and elaidic acid (peak C40) were identified by matching with literature data[37]. Peak C26 were tentatively characterized as 9-hydroxy-10,12,15-octadecatrienoic acid based on accurate mass results, fragmentation patterns (Fig. S7F) and data reported in the literature[38]. Peak C25 (tR31.46 min) with molecular ion atm/z783.573 2 and fragment ions atm/z391.283 was identified as FAHFA 52:3 (26:1/26:2) by matching with the MassBank of North American Database (https://mona.fiehnlab.ucdavis.edu/) and literature data[39-40].Peak C27 (tR34.22 min,m/z783.573 2) exhibiting the same molecular and fragment ions but different retention time with peak C25 was tentatively characterized as the isomer of compound 25. Furthermore,peaks C28, C29, C30, C32 and C33 were tentatively assigned as phosphatidic acids and their derivatives. Peak C29 (tR34.6 min,m/z636.310 3) and peak C32 (tR36.35 min,m/z614.331) showed aglycone fragment ions atm/z474.258 9 [M–H–C6H10O5]–and 452.276 6[M–H–C6H10O5]–, respectively, which were from losing a molecular of hexosyl. Peak C29 and peak C32 were identified as hexosyl LPE 18:3 (Fig. S7G) and hexosyl LPE 16:0 (Fig. S7H), respectively. By comparing with accurate molecular weight, fragmentation patterns and reported MSndata[39,41], peaks C28, C30 (Fig. S7I), C33 (Fig. S7J)and C34 (Fig. S7K) were confirmed as PA (16:0/16:0), LPC 18:3 and LPE 16:0, respectively. Finally, according to the precise molecular weight and the fragment pattern (Figs. S7L and S7M), peaks C31 and C41 were preliminarily identified as 9-pentadecanoyloxytricosanoic acid and (Z)-8-[(9Z,12Z,15Z)-octadeca-9,12,15-trienoyl] oxynonadec-9-enoic acid, respectively.
3.4.2 Lotus bee pollen
A total of 21 compounds, including 1 carbohydrate, 2 flavonoids and 18 fatty acids and derivatives, were identified or tentatively characterized in the digestive juices of LB and LA afterinvitrodigestion. Figs. 2C and 2D showed the total ion chromatogram, and the information of identified compounds was summarized in Table S9.The characterizations of 21 peaks were described as follows.
Peak L1 with fragment ions atm/z130.966 7 [C6H8O6–CO2–H]−and 86.976 4 [C6H8O6–CO2–CO2–H]−was identified as glucuronolactone by searching the Human Metabolome Database and MassBank of North America. Peaks L6 and L8 showed [M–H]−ion atm/z609.407 8 and 593.276 1 were therefore suggested as quercetin 3-O-rhamnoside-7-O-glucoside and kaempferol 3-rutinoside, respectively. Moreover, based on their masses and the above results, three unsaturated fatty acids (γ-linolenic acid (peak L14),α-linolenic acid (peak L18) and arachidonic acid (peak L19))and two SFAs (arachidic acid (peak L3) and palmitic acid (peak L20)) were tentatively identified. By comparing with the Human Metabolome Database, two hydroxy fatty acids, 3-hydroxydodecanoic acid (peak L2) and 9-hydroxyoctadeca-10,12,15-trienoic acid (peak L5) were tentatively assigned. Peaks L4, L7 and L13 showed the same molecular weight and fragment ions, thus they were isomers of each other and were preliminarily characterized as FAHFA 52:3.Peak L21 with molecular ion atm/z585.488 and fragment ions atm/z281.240 9 was identified as FAHFA 38:4 (18:1/20:3) by matching with the MassBank of North American Database and literature data[42].Additionally, peaks L9 (hexosyl LPE 18:3), L10 (LPG 18:3), L11(LPC 18:3), L12 (LPE 16:0), L15 (LPC 16:0), L16 (LPC 18:3) and L17 (hexosyl LPE 16:0) were tentatively assigned as phosphatidic acids and their derivatives. Peak L10 gave a fragment ion atm/z152.996 8 and 277.218 2, which was tentatively proposed as LPG 18:3[43].
3.5 Influence of wall-disruption treatment on main compounds of bee pollen and bioaccessibility of phenolic compounds after in vitro gastrointestinal digestion
Figs. 3A and B showed the dissolved TPC of camellia and lotus bee pollen with or without wall disruption. The TFC of bee pollen gradually increased during the simulated digestion, especially after intestinal digestion. It may be due to the further release of bound polyphenols in bee pollen through the hydrolysis of the acidic environment of gastric juice and the alkaline environment of intestinal juice, or the enzymatic hydrolysis of digestive enzymes. In addition,the TPC dissolved in the wall-broken bee pollen after digestion was significantly higher than that of the unbroken bee pollen (P< 0.05). The TPC of CB after digestion was in the range of 3.30–7.19 mg GAE/g,while that of CA was 3.49–9.17 mg GAE/g, respectively. For lotus bee pollen, the TPC digestedinvitroafter wall-breaking treatment was 4.26–8.57 mg GAE/g, while that in untreated one was 3.43–7.47 mg GAE/g,respectively. During simulatedinvitrodigestion, the release pattern of TFC was similar to that of TPC, which showed an increasing trend(Figs. 3C and D). Wall-breaking treatment of bee pollen could cause significant changes in TFC duringinvitrodigestion. In more detail,compared with CB, the TFC totally released from CA was increased by 43.84% in oral digestion, 57.22% in gastric digestion, and 112.16%in intestinal digestion. And after the wall-disruption treatment with lotus bee pollen, the release of TFC increased by 26.19% in oral digestion compared to LB, 34.43% in gastric digestion, and 57.99%in intestinal digestion. Overall, the release of total phenols and flavonoids duringinvitrodigestion of camellia and lotus bee pollen was similar. TPC and TFC values were also significantly increased(P< 0.05) duringinvitrodigestion of wall-broken treated bee pollen.This result might be because the phenolic compounds released by the pollen with unbroken walls were incomplete during the digestive process, and the wall-broken bee pollen could be more conducive to the release of phenolic compounds.
The content of phenolic compounds released by bee pollens into intestinal juice after wall disruption was extremely higher than those of bee pollens before wall disruption. Therefore, as shown in Table 4,the average bioaccessibility of total phenolics and flavonoids of wall-broken camellia and lotus bee pollens were significantly higher than those of wall-unbroken camellia and lotus bee pollens(P< 0.001). In particular, the bioaccessibility of flavonoids from wallbroken bee pollens was nearly twice higher than those of wall-broken bee pollens. It thus suggested that wall disruption could increase the bioaccessibility of phenolic compounds in bee pollen. The larger SSA allowed particles to interact more with other molecules and improving bioavailability and intestinal absorption[7]. From the point of view of dialyzable rate after dialysis, the dialysable rates of the phenolics were 20%–30%, indicating that the sum of absorbable phenolics(out of dialysis membrane) for further absorption (availability)were lower than those of bioaccessible phenolics. Furthermore, the dialyzable rates of phenolics in CA and LA increased by 32.87%and 30.71%, compared to those of CB and LB after dialysis. The dialyzable rates of flavonoids in CA and LA were higher than those in CB and LB, but there were no significant differences(P> 0.05). The above results confirmed that the bioaccessibility and bioavailability of phenolics in bee pollen can be modulated by the treatment of wall disruption. Bee pollen processing could promote the bioaccessibility of bioactive compounds by disrupting the cell walls with ultrafine pulverization.
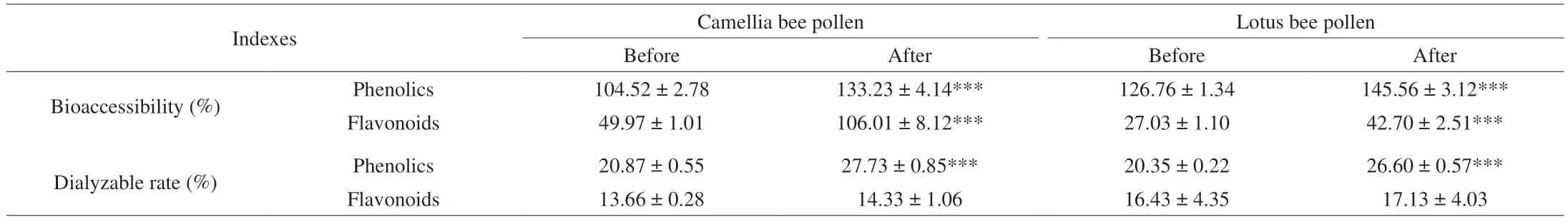
Table 4 The bioaccessible and dialyzable phenolic compounds of bee pollen samples (before and after wall-disruption) after simulated gastrointestinal digestion and dialysis.
It can be seen that the compositions of the bee pollen digestive juices with and without wall disruption were very different (Fig. 2 and Tables S8–9). A total of 28 major substances were identified in the CB digestive juices, and they were all detected in CA digestive juices. Moreover, there were 12 other phenolics and fatty acids only detected in CA digestive juices. Twenty-two main components were identified during theinvitrosimulated digestion of lotus bee pollen.Among them, 4 fatty acids and their derivatives were only detected in LB digestive juice, and 6 fatty acids and their derivatives were only detected in LA digestive juices. In addition, flavonoid compounds(quercetin-3-O-rhamnoside-7-O-glucoside and kaempferol-3-O-rutinoside) were detected only in LA digestive juices. Fewer phenolics were detected in theinvitrodigestion of bee pollen than those in the extracts, probably because the major lipophilicity of the aglycone flavonoids resulted in greater retention in the residue and lower solubilization in the aqueous phase. The water solubility of flavonoids is very low, which may be related to the presence of glycosidic residues in their molecular structures[44]. In addition,phenolic compounds, including phenolic acids and flavonoids, often interact with other food components (proteins, carbohydrates, and lipids) released during digestion, reducing their concentrations below the detection limit[45]. Our results demonstrated that a wall-breaking treatment could improve the bioavailability of phenolic compounds in bee pollen by increasing their extractability and dissolving them during oral and gastrointestinal digestion.

Fig. 2 Total ion chromatogram in the negative ion mode of camellia and lotus bee pollen during in vitro digestion. (A) CB and (B) CA after simulated digestion in vitro through (1) oral, (2) stomach and (3) intestine, respectively, (C) LB and (D) LA after simulated digestion in vitro through (1) oral, (2) stomach and(3) intestine, respectively.

Fig. 3 Changes of the (A, B) total phenolics, (C, D) flavonoids, (E, F) ABTS cation radical scavenging activity and (G, H) ferric reducing antioxidant power in(A, C, E, G) camellia and (B, D, F, H) lotus bee pollen samples during the in vitro digestion (n = 3). Different letters mean significant difference was observed,which was carried out using one-way analysis of variance (ANOVA), followed by Duncan’s multiple range tests.
Theinvitroantioxidant effects in different digestion stages of CB, CA, LB and LA were determined by ABTS and FRAP assays. As shown in Figs. 3E–H, the samples showed greater scavenging effects after wall-disruption treatment, especially in the gastrointestinal stage. The effects of scavenging the ABTS radical in bee pollen improved significantly after wall-breaking treatments. Compared to the FRAP values of the CB and LB groups, those of the CA and LA groups increased significantly, by approximately 22.14% and 25%,respectively. It can be seen from Table S10 that there was a significant correlation between the antioxidant values and the TPC/TFC in bee pollen digestive juice (P< 0.01). Besides, the correlation between the antioxidant values and TPC/TFC of the untreated bee pollen digestive juices at each stage were all lower than those of the bee pollen digestive juice with wall-disruption treatment. The antioxidant components dissolved in bee pollen digestive juices were not only phenolics but also contained other antioxidants such as unsaturated fatty acids, phosphatidic acids and their derivatives (Tables S8-9). The wall-disruption treatment was effective for the release and dissolution of phenolic components in bee pollen during digestion, and the TPC/TFC and antioxidant values had a higher correlation.
4. Conclusion
In our study, the effects of superfine grinding technology on nutrient release, antioxidant activity, andinvitrodigestion of bee pollen were investigated. The results showed that after the walldisruption treatment, a large number of nutrients in bee pollen were released, including protein, fat, starch, soluble sugar, and free amino acids. Compared with the untreated bee pollen, the variety of fatty acids, amino acids and phenolic compounds of wall-disruption bee pollen were increased. It was also found that wall disruption was conducive to the release and extraction of total phenolics and flavonoids, and enhanced their FRAP and ABTS cation radical scavenging antioxidant activities. Meanwhile, the wall disruption was shown to not only improve the bioaccessibility, dialysis rate and antioxidant activities of TPC and TFC, but also facilitate the release of nutrients (such as phenolics and fatty acids) in bee pollen during theinvitrosimulated digestion.
Conflict of interest
The authors declare no competing interests.
Acknowledgment
This study was financially supported by the Double Thousand Plan of Jiangxi (jxsp2019201077) and the Program of State Key Laboratory of Food Science and Technology, Nanchang University(SKLF-ZZB-202119).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.26599/FSHW.2022.9250132.
- 食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango

