Polysaccharides of Aspergillus cristatus attenuate obesity by regulating gut microbiota and gut microbiota-related metabolites
Mingzhi Zhu, Boho Shng, Fng Zhou, Yong Yun, Feiyn Yin, Jin Co,Jinn Hung, Kunbo Wng, Xin Zeng, Miqun Li, Zhonghu Liu,
a College of Horticulture, Hunan Agricultural University, Changsha 410128, China
b College of Life Sciences, Huaibei Normal University, Huaibei 235000, China
c Hunan Tea Group Co., Ltd., Changsha 410128, China
d School of Food Science and Technology, Hunan Agricultural University, Changsha 410128, China
Keywords: Golden-f lower fungus Aspergillus cristatus Polysaccharides Obesity Gut microbiota
ABSTRACT Golden-flower fungus, the only dominant microorganism determining the Fu-brick tea quality through fermentation and the important microbe in Liupao tea, is considered a potential probiotic fungus based on its anti-obesity effect. However, the classif ication of golden-f lower fungi is still controversial; the anti-obesity effect of golden-f lower fungus polysaccharides remains unknown. In this study, we identify a golden-f lower strain as Aspergillus cristatus based on morphological characteristics and multigene phylogeny analysis, which resolves the controversy of classif ication. Moreover, we f ind A. cristatus polysaccharides (ACPS) attenuate obesity in rats. ACPS modulate gut bacterial composition, in which Akkermansia, Akkermansia muciniphila,Bacteroides, Romboutsia, Blautia, and Desulfovibrio are considered the core microbes regulated by ACPS.ACPS increase fecal total short-chain fatty acid content and serum, hepatic, and fecal total bile acid content.Furthermore, ACPS-induced gut microbiota alteration plays a causal role in the protection from obesity,according to a fecal transplantation experiment. Thus, ACPS ameliorate obesity by regulating gut microbiota and gut microbiota-related metabolites.
1. Introduction
Obesity, a chronic multifactorial disorder characterized by an increase in body fat volume, has affected over 600 million people worldwide[1]. Numerous studies have shown that gut microbiota plays a crucial role in the development of obesity[2-5]. Maintaining the stability and heterogeneity of gut microbiota is essential for host health[6]. Indigestible dietary polysaccharides (PS) derived from fungi (e.g.,OphiocordycepssinensisandGanodermalucidum) have received a substantial interest as functional ingredients in recent years because of their potential role in modulating the gut microbiota[7-8].As the primary substrate and carbon source for bacterial fermentation,indigestible PS could modulate gut microbiota structure by elevating the community diversity. Additionally, PS directly affects gut microbiota by promoting the synthesis of short-chain fatty acids(SCFAs)[9]. In addition, indigestible dietary PS may improve bile acid(BA) metabolism by regulating gut microbiota, which contributes to the alleviation of obesity[10].
Fu-brick tea is a famous brick-style compressed tea exclusively produced in China, with a consumption history of over 600 years[11].It is a microbial-fermented tea that differs considerably from green tea, oolong tea, and black tea[12-15]. It is processed by controlled solid-state fermentation of tea leaves (Camellia sinensisL.), with one kind of golden-colored fungus as the only dominant microorganism[16].The golden-colored fungus, known as the golden-flower fungus in China, is regarded as the most crucial factor in processing Fu-brick tea[17]. The golden-colored fungus is also the important microbe in Liupao tea. The golden-flower fungus, characterized by its megascopic yellow cleistothecia, is tentatively identified asAspergilluscristatus,Aspergillus pseudoglaucus, orAspergillus amstelodamibased on its internal transcribed spacer (ITS) sequence[13,18-19]. Golden flower fungi metabolize the chemical components of raw tea materials(e.g., polyphenols, alkaloids, proteins, amino acids, cellulose, pectin,and aromatic components) during fermentation. This leads to the generation of a particular aroma, liquor color, taste, and beneficial health effects in Fu-brick tea[12]. The golden-flower fungus proliferates during fermentation and overspreads the surface and inside of Fu-brick tea after fermentation. The quantity and quality of golden flower fungi are critical indicators of the quality of Fu-brick tea[12,20].
Fu-brick tea has been proven to have therapeutic effects in obesity, hyperglycemia, hypercholesterolemia, metabolic disorders,and gastrointestinal disorders in recent years[21]. The biological activities of Fu-brick tea were attributed to the tea’s chemical components rather than to the golden-flower fungus in the past. This results in little research on biological activities of the golden-flower fungus. However, a boom in the research of edible health-promoting fungi, especially fungal PS, has recently driven bioactive studies of golden-flower fungi[7]. Our previous studies have found that the water extract of golden-flower fungus significantly inhibited lipid deposition inCaenorhabditis elegans[22]. Kang et al.[13]also found that the golden-flower fungus ameliorated obesity in mice. Furthermore,we found that PS was the main functional component of the goldenflower fungus[20]. Thus, we believe that the PS of golden-colored fungus deserves special attention. However, little is known about the anti-obesity effect of PS and its underlying mechanism in goldencolored fungi.
In this study, a strain of golden-flower fungus was isolated and identified asA.cristatusbased on morphological and molecular biological identification. The effect ofA.cristatusPS (ACPS) on weight loss in a high-fat diet (HFD)-fed obese rats was assessed.The effects of ACPS on gut microbiota and gut microbiota-related metabolites, including SCFAs and BAs, were also investigated.Moreover, a fecal transplantation (FT) assay was conducted to determine whether ACPS-regulated gut microbiota contributes to the anti-obesity function of ACPS. This study will offer new insights into the potential anti-obesity mechanism of ACPS.
2. Methods
2.1 Isolation and identification of golden-flower fungus
The yellow cleistothecia of golden-flower fungus in the Fu-brick tea (Jinxiangyi-tezhilipincha; Hunan Yiyang Tea Factory Co., Ltd., Hunan, China) were manually screened. Yellow cleistothecia were washed with sterile water to remove impurities(e.g., tea crumbs and infectious microbes). The yellow cleistothecia of golden-flower fungus was inoculated on potato dextrose agar plates and then repeatedly purified to obtain a single colony of golden-flower fungus. The morphological identification was performed using the yeast extract agar with 40% sucrose (M40Y) plates. The reproductive structures of golden-flower fungus were examined by light microscope(Scope.A1; Zeiss, Germany) and scanning electron microscope (SEM;JSM-6380LV, JEOL, Tokyo, Japan), as described in previous studies[17,23].
The golden-flower fungus was further identified using the multilocus sequence typing (MLST) method described by Peterson[24]and Hubka et al.[25]. The 4 loci of ITS,β-tubulin (BT2), calmodulin(CF), and RNA polymerase II (RPB2) were used for multilocus sequence identification[23]. Molecular evolutionary genetics analysis across computing platforms (MEGA X) was used to align the DNA sequences for phylogenetic analysis using ClustalW. A phylogenetic tree was constructed for the combined alignment with parsimony analysis using MEGA X[23,25]. The golden-flower fungus was identified asA.cristatusand stored in the China Center for Type Culture Collection (CCTCC, Wuhan, Hubei, China; storage number:M20211325).
2.2 Preparation of PS from A. cristatus
The strain ofA.cristatus(CCTCC No. M20211325) isolated in this study was grown on M40Y medium to harvest cleistothecia.Then, cleistothecia were inoculated on the sterile primary dark tea to expand the culture according to the method of scattered tea flowering[26]. The cleistothecia were collected by sieving after expanding the culture and then used for the extraction of PS as follows. The sporoderm-broken cleistothecia ofA.cristatuswere first obtained by grinding with liquid nitrogen. Then, the sporodermbroken cleistothecia were extracted with deep eutectic solvents(DESs) (1:20,m/V) for 40 min at 70 °C. The DESs were prepared by mixing choline chloride and oxalic acid in a molar ratio of 1:1 and heating at 80 °C for 30 min[27]. The extract was filtered and then precipitated overnight at 4 °C after adding quadruple volumes of 95% ethanol solution. The solution was centrifuged at 4 500 r/min for 15 min at 4 °C, and the precipitates were washed with 95% ethanol solution repetitively. The precipitates were dissolved in distilled water and de-proteinised using the Sevag method. The resulting solution was dialyzed with distilled water and freeze-dried to obtain purified PS for subsequent experiments[27]. Furthermore, PS contained(39.54 ± 2.16)% of natural carbohydrate, (17.23 ± 1.38)% uronic acid, and (26.37 ± 2.01)% protein, as determined by the phenolsulfuric acid colorimetric approach, 3,5-dimethyl phenol colorimetric method, and Bradford method, respectively[28]. The average molecular weight of PS was determined to be 21.16 kDa by high-performance gel permeation chromatography method[28]. PS consisted of ribose,glucose, galactose, and mannose in a molar ratio of 1:1.7:4.4:5.2,respectively, which was measured by high-performance liquid chromatography after precolumn derivatization[29].
2.3 Animal treatments
All experimental procedures were approved by Hunan Agricultural University (Biomedical Research Ethics Committee;permission number: HAU/BREC-2018136; Changsha, Hunan, China).The male Sprague-Dawley rats of specific pathogen free grade were purchased from Silaikejingda Experimental Animal Co., Ltd.(Changsha, Hunan, China). All rats, which had free access to food and water, were kept under the standard environment (23−25 °C, 12/12 h light/dark cycle). The normal diet (ND, 3.6 kcal/g, 10% kcal energy from fat, TP23302) and HFD (5.1 kcal/g, 45% kcal energy from fat,TP23000) were obtained from TROPHIC Animal Feed High-Tech Co., Ltd. (Nantong, Jiangsu, China). ND contains 19.4% protein(casein,L-cystine), 67.3% carbohydrate (dextrin, sucrose), 4.0% fat(soybean oil, lard), 4.8% fiber (cellulose), and 4.5% mineral and vitamin mixture, while HFD is composed of 24.8% protein (casein,L-cystine), 40.3% carbohydrate (dextrin, sucrose, corn starch), 23.0%fat (soybean oil, lard), 6.1% fiber (cellulose), and 5.8% mineral and vitamin mixture. After a week of adjustment, the 4-week-old rats were grouped into three groups (n= 8) as follows: (1) ND group, fed an ND; HFD group, fed an HFD; (3) ACPS group, fed an HFD with ACPS (400 mg/(kg∙day), intragastric administration). The experiment lasted for 12 weeks, and fresh feces were collected daily from the rats in the 5thweek and continued for eight weeks for the FT experiment.
The collected feces were made into fresh transplant material, and the FT experiment was conducted based on our previous method[30].Eight-week-old rats were used as recipients for receiving fresh transplant material daily via oral gavage. These recipient rats were fed an HFD and randomly assigned to three groups (n= 8): (1) NDFTHFD group (received fresh transplant material from the ND group); (2)HFDFTHFD group (received fresh transplant material from the HFD group); (3) ACPSFTHFD group (received fresh transplant material from the ACPS group). The FT trial lasted for 8 weeks. All rats were sacrificed with sodium pentobarbital (50 mg/kg of body weight, i.p.)after an overnight fast. Blood, perirenal, and epididymal adipose tissues, colon, liver, and feces were obtained for further investigation.
2.4 Biochemical assessment and histological and Western blotting analysis
Biochemical assessment and histological analysis were performed using previously described methods[30]. Histology score of colon sections was evaluated according previously described method[31].Western blot analysis was carried out as described previously[27].
2.5 Gut microbiota profiling
The 16S rRNA gene sequencing of fecal samples was carried out by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China) to obtain the gut microbiota profile according to a previously established protocol[30]. The linear discriminant analysis (LDA) effect size (LEfSe)algorithm was used to assess the critical gut microbiota[30]. The threshold was set to > 4.0 for the logarithmic score of LDA.
2.6 Determination of SCFAs and BAs
The fecal SCFAs were measured using a gas chromatographymass spectrometer (GC-MS; 7890A/5975C; Agilent Technologies,Inc., Santa Clara, California, USA), as described previously[32]. The levels of 28 BAs in the fecal, serum, hepatic, and colonic samples were detected by ultra-performance liquid chromatography (UPLC;Acquity UPLC system, Waters; Milford, MA, USA) coupled with a triple quadrupole mass spectrometer (Q-Trap 4000 MS; AB Sciex, Framingham, MA, USA) (UPLC-QQQ-MS), as described previously[32]. These 28 BAs were cholic acid (CA), beta-cholic acid(β-CA), alpha-muricholic acid (α-MCA), beta-deoxycholic acid(β-DCA), chenodeoxycholic acid (CDCA), hyodeoxycholic acid(HDCA), lithocholic acid (LCA), allolithocholic acid (AlloLCA),isolithocholic acid (IsoLCA), 12-ketolithocholic acid (12-ketoLCA),7-ketolithocholic acid (7-ketoLCA), ursocholic acid (UCA),allocholic acid (ACA), glycocholic acid (GCA), beta-muricholic acid (β-MCA), deoxycholic acid (DCA), glycochenodeoxycholic acid (GCDCA), tauro-alpha-muricholic acid (T-α-MCA), taurobetamuricholic acid (T-β-MCA), glycodeoxycholic acid (GDCA),glycoursodeoxycholic acid (GUDCA), glycochenodeoxycholic acid(GHDCA), glycolithocholylglycine acid (GLCA), taurocholicacid(TCA), taurochenodeoxycholic acid (TCDCA), taurodeoxycholic acid (TDCA), taurohyodeoxycholic acid (THDCA), and tauroursodeoxycholic acid (TUDCA) (Steraloids Inc., Newport,Rhode Island, USA).
3. Results
3.1 Morphological and molecular identification of goldenflower fungus from Fu-brick tea
Many yellow cleistothecia of golden-flower fungus were observed on the surface and inside of Fu-brick tea with the naked eye(Figs. 1A-B). The isolated strain of golden flower fungus (CCTCC No. M20211325) was cultivated on an M40Y medium at 28 °C for morphological observation (Fig. 1C). A white colony was observed on the 2ndday of incubation; then the color of the colony turned light yellow on 3rd–4thday; on the 7thday, the colony showed a sub-circular shape with a floccose texture and a diameter of 40–50 mm. The colony established a cream-yellow center with a light-yellow edge.Yellow pigments were observed without exudates, but brown exudates were secreted at the center of the colony if they continued to grow.Massive yellow cleistothecia and sparse conidia were observed on the colony surface. Furthermore, light microscope analysis revealed the presence of fungal conidia, conidiophores, and cleistothecia(Figs. 1D-F). The conidia gemmated from the top of mature phialidesa, and the shape of the conidia head changed from sub-globose to radiate at the maturity phase (Figs. 1D-E). Cleistothecia were globose to sub-globose and released asci during the maturity phase(Fig. 1F). Moreover, 8 ascospores embraced to a sphere were discharged from one ascus by autolysis of the pseudoparenchyma according to SEM analysis (Fig. 1G). The ascospores were convex with a rough surface and conspicuous furrows bordered by ridges (Figs. 1H-I). The results of the golden-flower fungus observed in this study conformed to the morphological characterization ofA.cristatus[25].
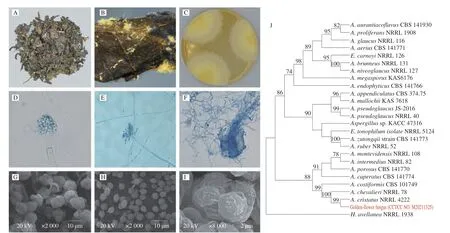
Fig. 1 Morphological characteristics (A-I) and phylogenetic tree analysis (J) of golden-flower fungus isolated from Fu-brick tea. (A) The yellow cleistothecia of golden-flower fungus of Fu-brick tea visible to the naked eyes; (B) appearance of yellow cleistothecia of golden-flower fungus on the surface of Fu-brick tea by Canon digital camera; (C) colonies of purified golden-flower fungus strain plating on M40Y medium for 7 days at 28 °C; morphological characteristics under(D-F) light microscope and (G-I) SEM, respectively; (D-E) conidia; (F) cleistothecia; (G) asci; (H-I) ascospores.
Based on the morphological characteristics, the MLST technique was further used for the molecular taxonomy of golden-flower fungi, in which sequences are amplified at 4 genetic loci: ITS, BT2,CF, and RPB2. The sequences of ITS, BT2, CF, and RPB2 from golden-flower fungus were submitted to GenBank with accession numbers OP021862, ON 971906, ON971907, and ON971908,respectively. These sequences were analyzed using MEGA X to generate a phylogenetic tree using the maximum likelihood algorithm model. The genetic relationship between the targeted strain and other strains is depicted in the phylogenetic tree (Fig. 1J). We found that the strain of golden-flower fungus had 100% similarity withA.cristatus. Thus, the strain of the golden-flower fungus isolated in this study was defined asA.cristatusbased on the combined results of morphological and molecular phylogenetic analyses.
3.2 Effects of ACPS on obesity in HFD-induced obese rats
We investigated the anti-obesity effect of ACPS in HFDinduced obese rats via intragastric gavage of ACPS for 12 consecutive weeks (Fig. 2A). Compared to ND feeding, HFD feeding significantly increased body weight gain (Fig. 2B). Furthermore, hepatic steatosis and fat accumulation were also observed in the HFD group (Figs. 2B-E).However, ACPS alleviated the HFD-induced increase in body weight gain, adipose tissue weight, and the liver/body weight ratio(Fig. 2B). HFD-induced hepatic steatosis and epididymal adipocyte hypertrophy were prevented by ACPS intervention (Figs. 2C-E). In addition, ACPS supplementation also improved serum parameters in HFD-induced obese rats, including aspartate aminotransferase(AST), alanine aminotransferase (ALT), homeostatic model assessment for insulin resistance (HOMA-IR), total cholesterol (TC),low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG) (Figs. 2F-H). A normal pathological morphology without inflammation was observed in the colon of the ND group by analysis of hematoxylin and eosin(H&E)-stained colonic sections. Nevertheless, villi destruction,inflammatory infiltration, and disappearance of goblet cells in the colon tissue were found in the HFD group. However, the pathological damage to the colon was effectively alleviated in HFD-induced obese rats by ACPS supplementation (Fig. 2I). Furthermore, the number of goblet cells and periodic acid-Schiff (PAS)-positive areas in the colon of HFD-induced obese rats increased significantly after ACPS intervention based on PAS staining (Figs. 2J-K). In addition,ACPS effectively improved the colonic expression of three tight junction proteins, including occludin, zonula occludens-1 (ZO-1),and claudin-1 in HFD-induced obese rats (Fig. 2L). Moreover,serum lipopolysaccharide (LPS) and inflammatory cytokines were also measured in this study (Figs. 2M-N). ACPS decreased the serum levels of LPS, monocyte chemotactic protein-1 (MCP-1),tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6), and simultaneously increased the serum level of interleukin-10 (IL-10) in HFD-induced obese rats.
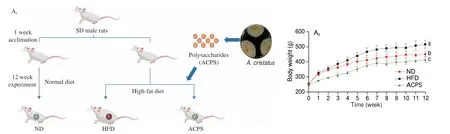
Fig. 2 ACPS ameliorated obesity in HFD-induced obese rats (donor). (A) Rats were treated for 12 weeks via intragastric gavage as indicated; (B) body weight,weight gain, liver/body weight ratio, and epididymal and perirenal fat weight; (C-D) liver microsections stained with H&E and Oil red O; (E) epididymis adipose microsections stained with H&E; (F-H) serum parameters; (I-J) histopathology of H&E-stained and PAS-stained colon sections. Red circle: inflammatory cell infiltration; black arrow: PAS-positive goblet cells. (K) Histology score of H&E-stained colon sections and the proportion of PAS-positive area in colon sections;(L) expression of tight junction proteins in colon tissues; (M) serum LPS; (N) serum inflammatory cytokines. Different lowercase letters indicate significant differences (P < 0.05).
3.3 Effects of ACPS on gut microbiota of HFD-induced obese rats
The alpha diversity of the gut microbiota community was evaluated after HFD feeding and ACPS intervention (Fig. 3A).Compared with ND feeding, HFD feeding resulted in a low Shannon index but a high Simpson index, indicating a low alpha diversity of the gut microbiota. However, the HFD-induced decline in low alpha diversity was partially recovered by ACPS intervention. Furthermore,we observed a distinct clustering of gut microbiota composition for the ND, HFD, and ACPS groups according to the PCA score plot and hierarchical clustering (Fig. 3B). This indicated a significant variation in the beta diversity of the gut microbiota among the three groups.Thus, ACPS supplementation substantially reversed the HFD-induced decrease in gut bacterial community diversity.
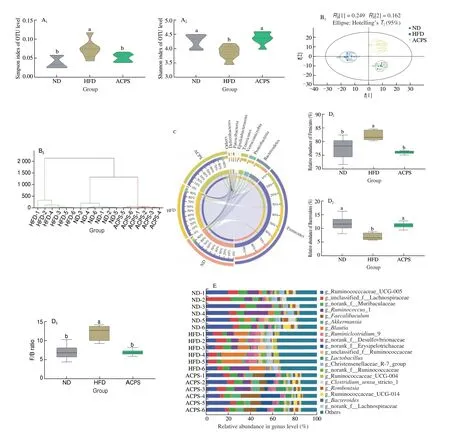
Fig. 3 ACPS restored gut microbiota dysbiosis in HFD-induced obese rats (donor). (A) Shannon and Simpson indices; (B) PCA score plot and hierarchical clustering tree; (C) Circos plot displaying the 8 dominant bacterial phyla in each group; (D) Firmicutes and Bacteroidetes abundance and Firmicutes/Bacteroidetes(F/B) ratio; (E) genera-level bacterial taxonomic characterization; (F) representative genera, F1: Akkermansia, F2: Bacteroides, F3: Blautia, F4: Clostridium sensu_stricto_l,F5: Desulfovibrio, F6: Faecalibaculum, F7: Lachnoclostridium, F8: Parabacteroides, F9: Ruminococcaceae_UCG-005, F10: Romboutsia, F11: Roseburia,F12: Streptococcus; (G) A. muciniphila in all groups; (H) LEfSe analysis. Different lowercase letters indicate significant differences (P < 0.05).
Taxonomic analysis was conducted to reveal specific changes in gut microbiota after the ACPS intervention. Although Firmicutes and Bacteroidetes mainly dominated the gut microbiota in the ND, HFD, and ACPS groups, ACPS intervention significantly altered the composition of gut microbiota at the phylum level(Fig. 3C). Specifically, compared with ND, HFD results in a high Firmicutes abundance and a low Bacteroidetes abundance, which causes a high F/B ratio. ACPS intervention protected against the effects of HFD. Moreover, there was no statistically significant difference in the abundance of Firmicutes and Bacteroidetes and the F/B ratio between the ACPS and ND groups (Fig. 3D). ACPS also observably changed gut microbiota composition at the genus level (Figs. 3E-F). HFD increased the abundance ofBlautia,Desulfovibrio,Lachnoclostridium,Roseburia, andStreptococcuscompared with ND,which was recovered by ACPS. In contrast, HFD reduced the abundance ofAkkermansia,Bacteroides,Clostridium_sensu_stricto_1, Ruminococcaceae_UCG-005, andRomboutsiacompared with ND,which was restored by ACPS (Fig. 3F).Furthermore, HFD reduced the abundance ofA.muciniphilain HFDinduced obese rats compared to ND, which was recovered by ACPS supplementation (Fig. 3G). Moreover, the abundance of the generaAkkermansiaandA.muciniphilain the ACPS group exceeded that in the ND group (Figs. 3F-G). In addition, no significant differences inFaecalibaculumandParabacteroidesabundance were found between the HFD and ND groups. Nevertheless, ACPS increased the abundance ofFaecalibaculumandParabacteroidescompared with HFD and ND (Fig. 3F).
The results of LEfSe were used to determine the gut microbiota responsible for the greatest changes across the ND, HFD, and ACPS groups (Fig. 3H). 4, 6, and 4 operational taxonomic units were dominant in the ND, HFD, and ACPS groups, respectively.Consistently, the HFD group was found to have a highBlautiaabundance, whereas the ACPS group had a highAkkermansia,Bacteroides, andFaecalibaculumabundance. Together, these results prove that ACPS alleviates gut microbiota dysbiosis in HFD-induced obese rats.
3.4 Effects of ACPS on SCFAs and BAs in HFD-induced obese rats
To evaluate metabolic changes in response to ACPS-remodeled gut microbiota, we performed targeted metabolomic profiling of SCFAs in feces using GC-MS (Fig. 4A). Our results showed that HFD feeding decreased the total SCFA content in feces compared with ND feeding. Specifically, HFD reduced the content of 6 SCFAs, including acetic, propionic, butyric, isobutyric, valeric, and caproic acids, in feces compared to ND. Nevertheless, ACPS increased the content of these 6 SCFAs and total SCFAs compared to HFD. No significant difference in isovaleric acid content was found among the ND, HFD,and ACPS groups. The BAs profiles in the feces, serum, liver, and colon were also measured by UPLC-QQQ-MS to assess the effect of ACPS on the metabolism and enterohepatic circulation of BAs.HFD feeding resulted in a decrease in total BA loss in feces compared to ND feeding (Fig. 4B). Meanwhile, HFD feeding significantly decreased unconjugated BA content in the serum and unconjugated and conjugated BAs contents in the liver compared with ND feeding,which led to a reduction in serum and hepatic total BA content(Figs. 4C-D). Notably, the ACPS supplementation partially reversed this effect. Fecal BA content was elevated by ACPS supplementation in HFD-induced obese rats. ACPS supplementation also dramatically elevated the content of unconjugated and conjugated BAs in the serum and liver tissue of HFD-induced obese rats, thereby increasing the serum and hepatic total BA content. Specifically, in the hepatic BA profiles, ACPS supplementation significantly increased CA,CDCA, DCA, LCA, TCA, TCDCA, and all glycine-conjugated BA contents. It reduced T-α-MCA and T-β-MCA contents (Figs. 4B-D).In addition, although no significant differences in the colonic total BA content were found among the ND, HFD, and ACPS groups,ACPS supplementation significantly decreased the TCA content. Still,it increased the T-α-MCA and T-β-MCA contents in HFD-induced obese rats (Fig. 4E).
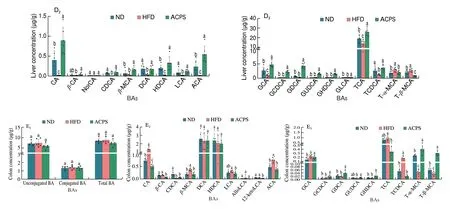
Fig. 4 (Continued)
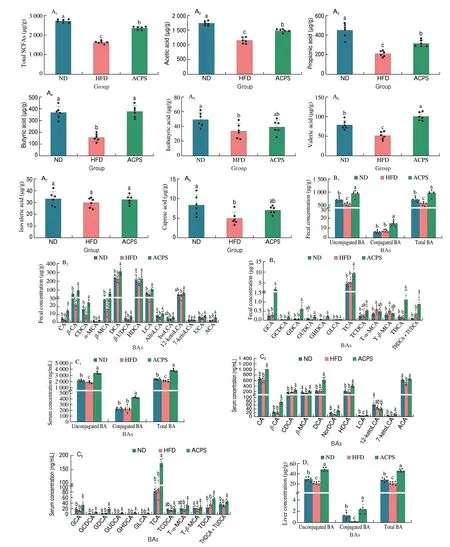
Fig. 4 Effects of ACPS on the metabolism of SCFAs (A) and BAs (B-E) in HFD-induced obese rats (donor). Different lowercase letters for the same metabolism indicate significant differences (P < 0.05).
3.5 Effects of ACPS on obesity are transferable by FT
To further illustrate the role of gut microbiota in regulating the positive effect of ACPS on obesity, gut microbiota from ACPStreated rats (donor) were transplanted into HFD-fed rats (recipient)by FT (Fig. 5A). The body weight gain, adipose tissue weight, and liver/body weight ratio in the ACPSFTHFD group were lower than those in the HFDFTHFD group but higher than those in the NDFTHFD groups (Fig. 5B). Furthermore, HFDFTHFD treatment resulted in the deterioration of hepatic steatosis and epididymal adipocyte hypertrophy compared with NDFTHFD treatment, according to the histological characterizations of liver tissue and epididymal fat tissue. ACPSFTHFD treatment attenuated this deterioration compared to HFDFTHFD treatment (Figs. 5C-E). Furthermore, HFDFTHFD treatment deteriorated some serum parameters compared with NDFTHFD treatment, which was restored by ACPSFTHFD treatment.These serum parameters included AST, ALT, HOMA-IR, TC,LDL-C, HDL-C, and TG levels (Figs. 5F-H). Notably, the regulatory effect of ACPSFTHFD treatment on obesity-related traits resembled that of ACPS supplementation alone.
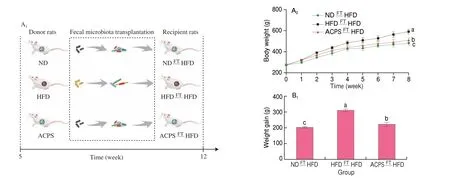
Fig. 5 Effects of ACPS on obesity of HFD-fed recipient rats by fecal transplantation (FT). (A) Recipient rats were treated for 8 weeks as indicated; (B) body weight, weight gain, liver weight/body weight ratio, and epididymal and perirenal fat weight; (C-D) liver microsections stained with H&E and Oil red O;(E) epididymis adipose microsections stained with H&E; (F-H) serum parameters; (I-J) histopathology of H&E-stained and PAS-stained colon sections. Red circle:inflammatory cell infiltration; black arrow: PAS-positive goblet cells. (K) histology score of H&E-stained colon sections and the proportion of PAS-positive area in colon sections; (L) expression of tight junction proteins in colon tissues; (M) serum LPS; (N) serum inflammatory cytokines. Different lowercase letters indicate significant differences (P < 0.05).
The PAS-positive area and goblet cell count of the colon in the ACPSFTHFD group were superior to those in the HFDFTHFD group (Figs. 5I-K). Furthermore, the colonic expression of tight junction proteins, including occludin, ZO-1, and claudin-1, in the ACPSFTHFD group was higher than that in the HFDFTHFD group(Fig. 5L). Moreover, some serum parameters in the ACPSFTHFD group were superior to those in the HFDFTHFD group. These serum parameters included MCP-1, TNF-α, LPS, IL-6, and IL-10(Fig. 5M-N). Collectively, the ACPS-treated gut microbiota attenuated obesity in recipient rats, indicating that gut microbiota mediate the metabolic protection of ACPS.
3.6 Effect of ACPS-altered gut microbiota on the gut microbiota composition of HFD-fed recipient rats
This study evaluated the regulatory effect of ACPS-altered gut microbiota on the gut microbiota composition of recipient rats (Fig. 6).ACPSFTHFD treatment significantly increased the alpha diversity of gut microbiota compared to HFDFTHFD treatment, as illustrated by the Simpson and Shannon indices. Furthermore, no significant difference was observed between the ACPSFTHFD and NDFTHFD groups in terms of alpha diversity (Fig. 6A). In addition, we observed a notable structural discrepancy between the ACPSFTHFD and HFDFTHFD groups, according to the PCA results (Fig. 6B). Moreover, the effects of ACPS-altered gut microbiota on the Shannon index, Simpson index, and PCA score plot were similar to those of ACP treatment.Significant differences were observed between the ACPSFTHFD and HFDFTHFD groups regarding gut microbiota composition at the phylum level (Fig. 6C). Specifically, compared with HFDFTHFD treatment, ACPSFTHFD treatment increased Bacteroidetes abundance and reduced Firmicutes abundance and F/B ratio. Furthermore,Bacteroidetes in the ACPSFTHFD group were higher than that in the NDFTHFD group (Fig. 6D). Notably, the ACPSFTHFD treatment yielded results similar to those of ACPS supplementation in terms of Shannon index, Simpson index, PCA score plot, and microorganism community composition at the phylum level.
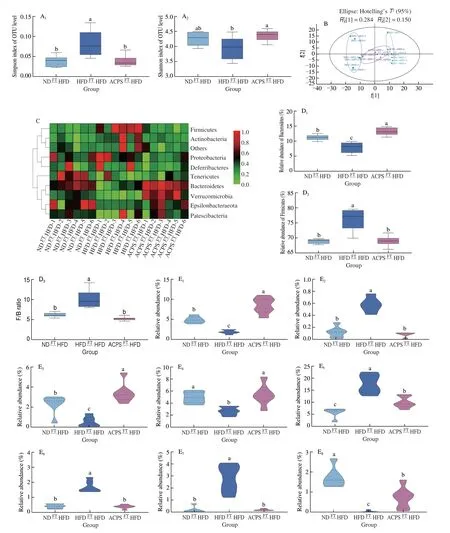
Fig. 6 Effect of ACPS-altered gut microbiota on the gut microbiota composition of HFD-fed recipient rats. (A) Shannon and Simpson indices; (B) PCA score plot; (C) heatmap of gut microbiota at the phyla level; (D) Firmicutes and Bacteroidetes abundance and F/B ratio; (E) representative genera, E1: Akkermansia,E2: Allobaculum, E3: Alloprevotella, E4: Bacteroides, E5: Blautia, E6: Desulfovibrio, E7: Escherichia-Shigella, E8: Faecalibaculum,E9: Romboutsia, E10: Rikenella, E11: Ruminococcaceae UCG-005, E12: Ruminococcaceae UCG-014, E13: Roseburia, E14: Streptococcus; (F) LEfSe analysis.
Significant differences in gut microbiota composition at the genus level were also observed between the ACPSFTHFD and HFDFTHFD groups. ACPSFTHFD treatment reduced the abundance ofBlautia,Desulfovibrio, andStreptococcuscompared to HFDFTHFD treatment,which was comparable to the results found in the oral ACPS-feeding experiment. In contrast, ACPSFTHFD treatment increased the abundanceofBacteroidesandRomboutsiacompared to HFDFTHFD treatment, which was comparable to the effect of ACPS (Fig. 6E).Moreover, the abundance ofA.muciniphilain the ACPSFTHFD group was higher than that in the HFD group and even higher than that in the NDFTHFD group. The regulatory effect of ACPSFTHFD onAkkermansiawas similar to that of ACPS (Figs. 6E). Moreover, the genusAkkermansiawas also enriched in the ACPSFTHFD group based on LEfSe analysis (Fig. 6F). This suggests that the genusAkkermansiacan be used as a biomarker. Interestingly, the ACPSFTHFD treatment showed similar results to ACPS supplementation in terms of modulation of the generaAkkermansia,Bacteroides,Romboutsia,Blautia,Desulfovibrio, andStreptococcus.Besides, ACPSFTHFD increased theAlloprevotella,Faecalibaculum,Rikenella,andRoseburiaabundance,but reduced theAllobaculumandEscherichia-Shigellaabundance when compared to HFDFTHFD(Fig. 6E). ACPS-altered gut microbiota improved the gut microbiota composition in HFD-fed recipient rats. Furthermore, the generaAkkermansia,Bacteroides,Romboutsia,Blautia,Desulfovibrio, andStreptococcuswere considered core microbes regulated by both ACPS- and ACPS-altered gut microbiota.
4. Discussion
Tea is one of the most widely consumed non-alcoholic beverages around the world[33-40]. In China, Fu-brick tea has historically been used to treat obesity and metabolic disorders[41]. As the most important bacteria in Fu-brick tea, the golden-flower fungus was preliminarily found to have an anti-obesity effect[13,22]. However, the categorization of golden-flower fungi remains debatable; the role of PS extracted from the golden-flower fungus in ameliorating obesity and modulating gut microbiota has not been reported. In this study, we isolated a strain of golden-flower fungus from Fu-brick tea and identified it asA.cristatus, rather thanA.pseudoglaucusorA.amstelodami,based on morphological and molecular phylogenetic analyses, which is consistent with our previous studies[23]. Our results will end the controversy over the classification of golden-flower fungi. Moreover,we demonstrate for the first time that ACPS has a protective effect against obesity.
Given that PS have a high molecular weight and poor absorption[42], we believe that the anti-obesity activity of ACPS is mainly attributed to the interaction between ACPS and gut microbiota. Lower community diversity of gut microbiota is a typical feature of “obese microbiota”[43]. We observed that the decrease in the community diversity of gut microbiota in HFD-fed obese rats was restored by ACPS supplementation, which was similar to the effects ofG.lucidumPS andO.sinensisPS[7-8].Moreover, ACPS supplementation restored HFD-induced alterations in the composition of gut microbiota in this study. Specifically,ACPS supplementation increased the Bacteroidetes abundance but lowered the Firmicutes abundance and F/B ratio. High Firmicutes abundance and F/B ratio and low Bacteroidetes abundance have been reported to result in the development of obesity[2-3,6]. Therefore, the anti-obesity function of ACPS is directly linked to variations in the abundance of Firmicutes and Bacteroidetes, and the F/B ratio. We observed an increased abundance ofAkkermansia,Bacteroides,Clostridium_sensu_stricto_1,Faecalibaculum, Ruminococcaceae_UCG-005, andRomboutsiain HFD-induced obese rats after ACPS supplementation. Notably, there has been a surge in interest regarding the functional significance of these bacteria in the gut.Akkermansia,Faecalibaculum, Ruminococcaceae_UCG-005, andBacteroides, which are the probiotics, are essential contributions to obesity prevention by regulating glucose metabolism, immunological response, and amino acid and lipid metabolism[3,9,44-45]. In particular,A.muciniphilahas been shown to reduce body weight gain and improve glucose tolerance, insulin sensitivity, and metabolic health in the obese body, and is considered a promising next-generation probiotic[5]. ACPS supplementation increasesA.muciniphilaabundance in HFD-induced obese rats. Thus,A.muciniphilamay be one of the core microbes of ACPS owing to its anti-obesity effects[46]. In addition, ACPS supplementation reduced the abundance of some potentially harmful bacteria in obese rats, includingBlautia,Desulfovibrio,Lachnoclostridium,Roseburia, andStreptococcus.Desulfovibriohas the ability to produce hydrogen sulfide in the intestine, which has pro-inflammatory and DNA-damaging effects,eventually degrading the intestinal epithelial barrier[47]. A high abundance ofBlautia,Desulfovibrio,Lachnoclostridium,Roseburia,andStreptococcushave been associated with obesity and its related metabolic phenotype[48-50]. The reduction in these bacteria contributes to the inhibition of LPS generation and alleviation of HFD-induced inflammation[6,49-50]. Thus, the anti-obesity effect of ACPS correlated with altered gut microbiota.
PS are usually metabolized and fermented into SCFAs by gut microbiota[51]. In addition to being a source of energy for the host,SCFAs have a crucial role in improving intestinal homeostasis and glycolipid metabolism[52]. Oral acetate gavage were found to inhibit body weight gain in obese rats[53]. The rise in the acetic acid content of feces caused by ACPS supplementation in HFD-induced obese rats may be a crucial role in obesity prevention. In addition, decreased fasting blood glucose and increased insulin levels were observed in healthy humans after 24 weeks of propionate supplementation in the previous study[54]. The decreased HOMA-IR in this study may be partly due to the rise in the propionic acid content of feces caused by ACPS supplementation. ACPS supplementation also elevated the fecal content of butyric acid, valeric acid, and total SCFAs in HFD-induced obese rats. Butyric acid has a positive impact on ameliorating obesity, alleviating inflammation, and improving oxidative stress and gut barrier function[55]. Flaxseed PS supplementation restored the HFD-induced reduction in the total SCFAs content, which was consistent with the effect of ACPS supplementation[56]. Moreover, the elevated SCFAs content in the feces of ACPS-treated rats was consistent with the enhanced abundance of some SCFA-producing bacteria, such asA.muciniphila,Bacteroides,Clostridium_sensu_stricto_1, andFaecalibaculum[57-59].Therefore, the rise in the SCFA content of feces caused by ACPS supplementation may be a key reason for alleviating obesity and related metabolic diseases.
FT experiment is a critical way to evaluate whether the functional ingredient-remodeled gut microbiota is required for its therapeutic effect[60]. The anti-obesity effects of resveratrol and ginger have been confirmed to be dependent on the regulation of gut microbiota by FT[61-62]. In this study, gut microbiota from ACPS-treated donor rats attenuated obesity and its related phenotypes in recipient rats.Moreover, gut microbiota from ACPS-treated donor rats also attenuated gut microbiota dysbiosis in the recipient rats. Specifically,some microbes, includingAkkermansia,A.muciniphila,Bacteroides,Romboutsia,Blautia,Desulfovibrio, andStreptococcus,can be improved by supplementation with both ACPS and ACPS-remodeled gut microbiota. Thus, these microbes are considered core microbes regulated by ACPS. In addition, based on the regulation of SCFAs and BAs in HFD-induced donor rats, we speculated that ACPS-remodeled gut microbiota have the potential to regulate SCFAs and BAs metabolism in recipient rats. Therefore, gut microbiota, and gut microbiota-related metabolites are required for the anti-obesity effects of ACPS.
5. Conclusion
We isolated a strain of golden-flower fungus from Fu-brick tea. We identified it asA.cristatusbased on morphological and molecular phylogenetic analysis, which will end the controversy over the classification of golden-flower fungi. This study is the first to provide clear evidence that ACPS effectively attenuates obesity in HFD-induced obese rats. Furthermore, ACPS modulates gut bacterial composition. Some microbes, includingAkkermansia,A. muciniphila,Bacteroides,Romboutsia,Blautia,Desulfovibrio, andStreptococcus,are considered the core microbes regulated by ACPS. ACPS improves the metabolism of gut microbiota-derived SCFAs and BAs, including increasing the acetic acid, propionic acid, butyric acid, valeric acid,and total SCFA contents, and elevates the serum, hepatic, and fecal total BA content. Moreover, ACPS-induced gut microbiota alteration played a causal role in the protection of HFD-induced obese rats from obesity, according to the FT experiment. Collectively, ACPS ameliorated obesity in HFD-induced rats by regulating the gut microbiota and gut microbiota-related SCFAs and BAs, indicating that ACPS could serve as a prebiotic to treat obesity and modulate gut microbiota. Further studies on human subjects are required to evaluate the effects of long-term consumption of ACPS on reducing body weight gain and regulating ACPS gut microbiota.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
This research was financially supported by Natural Science Foundation of China (32002095 and 32172217), Major Project of Science and Technology of Guangxi Zhuang Autonomous Region(AA20302018), Key Research and Development Program of Hunan Province (2020WK2017), Hunan “Three Top” Innovative Talents Project (2022RC1142), Natural Science Foundation of Hunan Province for Outstanding Young Scholars (2022JJ20028), Training Program for Excellent Young Innovators of Changsha (kq2107015),and Scientific Research Fund of the Hunan Provincial Education Department (20A241).
- 食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango

