Stability and transepithelial transport of oligopeptide (KRQKYD) with hepatocyteprotective activity from Jinhua ham in human intestinal Caco-2 monolayer cells
Wen Nie, Feirn Xu, Ki Zhou, Jieying Deng, Ying Wng, Boci Xu,
a Engineering Research Center of Bio-process, Ministry of Education, School of Food and Biological Engineering, Hefei University of Technology, Hefei 230601, China
b School of Biological and Food Engineering, Chuzhou University, Chuzhou 239001, China
Keywords: Jinhua ham KRQKYD (Lys-Arg-Gln-Lys-Tyr-Asp)Environmental stability Gastrointestinal digestive Transport mechanism
ABSTRACT The study evaluated the stability of an oligopeptide (Lys-Arg-Gln-Lys-Tyr-Asp, KRQKYD) and its transport mechanism by simulating gastrointestinal digestion and a model of human intestinal Caco-2 monolayer cells in vitro. In this study, the effects of environmental factors (temperature, pH and NaCl concentration) and simulated gastrointestinal digestion on the stability of KRQKYD were evaluated by indicators of the levels of alanine transaminase (ALT), aspartate transaminase (AST) and malondialdehyde (MDA) in an alcoholinduced hepatocyte injury model. The results showed that KRQKYD still maintained satisfactory hepatocyteprotective activity after treatment with different temperatures (20−80 °C), pH (3.0−9.0), NaCl concentration(1%−7%) and simulated gastrointestinal digestion, which indicated that KRQKYD showed good stability to environmental factors and simulated gastrointestinal digestion. Furthermore, the intact KRQKYD could be absorbed in a model of Caco-2 monolayer cells with a Papp value of (9.70 ± 0.53) × 10−7 cm/s. Pretreatment with an energy inhibitor (sodium azide), a competitive peptide transporter inhibitor (Gly-Pro) and a transcytosis inhibitor wortmannin did not decrease the level of transepithelial KRQKYD transport, indicating that the transport mechanism of KRQKYD was not associated with energy dependent, vector mediated and endocytosis. The tight junction disruptor cytochalasin D signif icantly increased the level of transepithelial KRQKYD transport (P < 0.05), suggesting that intact KRQKYD was absorbed by paracellular transport.
1. Introduction
Alcoholic liver disease (ALD), a major cause of morbidity and mortality worldwide of chronic liver disease, is a serious threat to human health[1]. Currently, the bioactive peptides derived from food proteins have been considered to have no undesirable adverse effects as compared to the synthetic drug (metadoxin, amoxicillin clavulanate, rifaximin and ciprof loxacin), which are attracting more and more interest as alternatives to manage ALD[2-3]. In the past decades, various bioactive peptides, such as chicken breast-derived peptide[4], chicken liver peptide[5], Xuanwei ham peptide[6]and Jinhua ham peptide[7], had been found in protein hydrolysates and fermented products with hepatocyte-protective activityin vivoandin vitro,providing an approach for the therapy of ALD. However, there are still many problems and challenges in the practical application of these peptides.
Generally, peptide activity may be affected by various environmental factors (such as temperature, pH and NaCl concentration) during processing into functional products (such as beverages and candy containing peptides)[8]. The resistance of different peptides to environmental factors and gastrointestinal digestion is different, which is related to their structure[9-10]. Therefore,it is necessary to clarify the influence of environmental factors on functional peptides, which can effectively guide the production of functional products containing peptides. In addition, to exert action in the target organ, bioactive peptides have to overcome two important physiologic barriers, extensive enzymatic degradation in the gastrointestinal tract and being absorbed intact through intestinal epithelium into blood circulation and target sites after oral administration[11]. There are three possible pathways to transport bioactive peptides across the intestinal mucosa, including paracellular route via the tight junction, transcytosis via endocytosis, and active route via peptide transporter 1 (PepT1), which is widely distributed on human intestinal membranes[12]. However, the transport of different bioactive peptides may have different mechanisms.
Our previous study had shown that KRQKYD (Lys-Arg-Gln-Lys-Tyr-Asp) is a bioactive oligopeptide derived from Jinhua hams with the ability to prevent alcohol-induced liver damage[7].Unfortunately, the stability of KRQKYD for temperature, pH, NaCl concentration and gastrointestinal digestion is unknown. In addition,the permeability of KRQKYD has not been studied. This study aimed to evaluate the stability of KRQKYD based on temperature, pH, NaCl concentration and gastrointestinal digestion in an HHL-5 cells model of alcohol-induced damage. Moreover, the mechanism of KRQKYD absorption was investigated in a human intestinal Caco-2 monolayer cell model. It is of much significance to understand the transport mechanism of KRQKYD for their pharmacological application and bioavailability in the future.
2. Materials and methods
2.1 Chemicals
2-Chlorotrityl chloride resin, product No.: HC5501-1-1, was purchased from Tianjin Nankai Hecheng S&T Co., Ltd. (China).;Fmoc-Asp (OtBu)-OH, CAS: 71989-14-5; Fmoc-Tyr (tBu)-OH, CAS: 71989-38-3; Fmoc-Gln (Trt)-OH, CAS: 132327-80-1;Fmoc-Lys (Boc)-OH, CAS: 71989-26-9; Fmoc-Arg (Pbf)-OH, CAS: 154445-77-9; 1-hydroxy-7-azabenzotriazole (HoAt),CAS: 39968-33-7; 2-(7-azabenzotriazol-1-yl)-N,N,N’,N’-tetramethyluronium hexafluorophosphate (HATU), CAS:148893-10-1;O-(6-chloro-1-hydrocibenzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphat (HCTU), CAS: 330645-87-9;N-methylmorpholine (NMM), CAS: 109-02-4;N,N-diisopropyl ethylamine (DIEA), CAS: 7087-68-5; methylene chloride (DCM),dimethylformamide (DMF), pepsin, trypsin and NaCl, all the above reagents were analytically pure, purchased from Aladdin Reagent(Shanghai) Co., Ltd. (China). Fetal bovine serum protein, DMEM-F12 cell culture medium, penicillin (10 kU/mL)-streptomycin (10 mg/mL)were purchased from Shenggong Bioengineering (Shanghai) Co., Ltd.(China). Main antibodies Claudin-1 and Occludin were bought from Abcam (MA, USA), and antibodies against GAPDH were obtained from Sigma-Aldrich (USA). The secondary anti-rabbit IgG (H + L)and anti-mouse IgG (H + L) were obtained from Cell Signaling Technology (Beverly, MA, USA).
2.2 Synthesis of KRQKYD
The KRQKYD was synthesized by Fmoc-solid phase synthesis method according to the study of Nie et al.[7]. The specific synthesis steps are shown in Supporting Information 1.
2.3 Stability analysis of KRQKYD
The method used to measure the stability of KRQKYD based on temperature, pH and NaCl concentration was according to the research of Zhu et al.[8]with some modifications. Briefly, KRQKYD powder was used to prepare a 3 μmol/mL peptide solution with ultrapure water that was placed in 10 mL centrifuge tubes. Then, the centrifuge tubes were heated with a hot water bath at 20, 40, 60, 80 and 100 °C for 2 h. After being removed, the centrifuge tubes were placed in an ice-water bath for rapid cooling. For further analysis, the peptide solution was stored at 4 °C. KRQKYD powder was used to prepare 3 μmol/mL peptide solutions with different pH values (pH 3.0,5.0, 7.0, 9.0 and 11.0) that were placed in 10 mL centrifuge tubes.The pH values of the solutions were adjusted with 1 mol/L HCl and 1 mol/L NaOH solutions. Then, the centrifuge tubes were heated in a 37 °C water bath for 2 h. After being removed, the centrifuge tubes were placed in an ice-water bath for rapid cooling. Finally, the peptide solutions were desalted with a polar enhanced polymer (PEP) column(500 mg/6 mL) and stored at 4 °C. KRQKYD powder was used to prepare a 3 μmol/mL peptide solution with ultrapure water that was placed in a 10 mL centrifuge tube. Then, NaCl solutions with mass fractions of 1%, 3%, 5% and 7% were added and incubated for 2 h at room temperature. Finally, the peptide solutions were desalted with a PEP column (500 mg/6 mL) and dried in a vacuum freeze-dryer.
2.4 Simulation of gastrointestinal digestion in vitro
The method used to measure the stability of KRQKYD to pepsin and trypsin during simulated gastrointestinal digestionin vitrowas based on the research of Xie et al.[13]. A total of 2 g NaCl and 3.2 g pepsin (3 000 U/mg pro) were accurately weighed and dissolved in 800 mL of ultrapure water, and the pH value was adjusted to 3.0 with a 6 mol/L HCl solution, after which the volume was adjusted with ultrapure water to 1 L (to simulate gastric juice). Then, 0.68 g of potassium dihydrogen phosphate was dissolved in 70 mL of ultrapure water, 7.7 mL of a 0.2 mol/L NaOH solution was added, the solutions were mixed well, and 1 g of trypsin (285 U/mg protein) and 6 g bile salt were added and dissolved (to simulate intestinal fluid). A 3 μmol/mL peptide solution was prepared, and the pH was adjusted to 3.0 with a 1 mol/L HCl solution, after which 5 mL of simulated gastric juice was added to the solution, and the mixture was shaken in a 37 °C water bath for 2 h. Then, simulated gastric digestion was terminated by heating at 100 °C for 10 min, and the pH of the mixture was adjusted to 7.2 with a 0.2 mol/L NaOH solution. A total of 5 mL of simulated intestinal juice was added to the solution, and the mixture was shaken in a 37 °C water bath for 2 h. Finally, the digestive liquid was transferred to an ultrafiltration centrifuge tube(1 kDa) after centrifugation at 8 500 ×gfor 20 min at 4 °C, and the filtered solutions were collected. Finally, the filtered solutions were desalted with a PEP column (500 mg/6 mL) and dried in a vacuum freeze-dryer.
2.5 HHL-5 cell culture and treatments
HHL-5 cells (American Type Culture Collection) were cultured and treated according to Nie et al.[6]with some modifications.HHL-5 cells were grown in DMEM-F12 medium (1:1) containing 3.15 g/L glucose, 0.365 g/LL-glutamine and 3.57 g/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)supplemented with 10% fetal bovine serum and 1% penicillin(10 kU/mL)-streptomycin (10 mg/mL) at 37 °C in an atmosphere with 5% CO2and 90% relative humidity. The HHL-5 cells were seeded on 96-well culture plates at a density of 4 × 103cells/well and cultured for 24 h. Culture media containing different concentrations of alcohol(0, 100, 200, 300, 400 and 500 mmol/mL) were added and incubated for 48 h. Then, cell viability was evaluated by Cell Counting Kit-8 assay to determine the optimal concentration of alcohol to induce HHL-5 cell injury. The culture media containing different concentrations of KRQKYD (0, 1, 2 and 3 μmol/mL) were added and incubated for 48 h to evaluate the cytotoxicity of KRQKYD by the strategy described above. The ability of KRQKYD to prevent the alcohol-induced HHL-5 hepatocytes damage was evaluated by the following strategies. In the KRQKYD + EtOH group, a culture medium supplemented with 3 μmol/mL KRQKYD was added and incubated for 48 h, and the culture medium was then substituted with a culture medium supplemented with 500 mmol/L alcohol and incubated for 48 h. In the EtOH group, a normal culture medium was added and incubated for 48 h, and the culture medium was then substituted with a culture medium supplemented with 500 mmol/L alcohol and incubated for 48 h. In the CTRL group, a normal culture medium was added and incubated for 48 h, and then the culture medium was substituted with a culture medium supplemented with an equal volume of PBS and incubated for 48 h. Finally, the culture media were collected to evaluate the level of aspartate transaminase(AST), alanine transaminase (ALT) and malondialdehyde (MDA)with commercial kits (Nanjing jiancheng Biotechnology Institute,Nanjing, China).
2.6 Animal experiments
The male SPF C57BL/6 mice (8 weeks old, weight (20 ± 2) g)used in this study were provided by Changzhou Kavins Experimental Animal Co. (license No.: SCXK (Su) 202009152). The mice were reared in a standard environment with a 12-h alternating darkness/light cycle. The ambient temperature was (25 ± 2) °C, and the relative humidity was (60 ± 5)%. Animal experiments were performed according to the guidelines of the institutional animal ethics committee and were approved by the biomedical ethics committee of Hefei University of Technology (HFUT 20201026001). All mice were fed one week in advance for acclimation to the experimental environment.
The chronic alcohol-fed mouse model was established as previously described[7]. The mice were randomly divided into different groups (8 per group), including the control group, alcohol group and alcohol + KRQKYD group, and fed for 35 days. The alcohol +KRQKYD group was oral gavaged 239 mmol/kg bw KRQKYD and then fed Lieber DeCarli 3% (V/V) alcohol liquid diet by oral gavage after 2 h from the 1stto the 14thday and was oral gavaged 239 mmol/kg bw KRQKYD and then fed Lieber DeCarli 5% (V/V) alcohol liquid diet by oral gavage after 2 h from the 15thto the 35thday (EtOH +KRQKYD group,n= 8). The alcohol group was oral gavaged an equal volume of normal saline and then fed Lieber DeCarli 3% (V/V)alcohol liquid diet by oral gavage after 2 h from the 1stto the 14thday and was oral gavaged an equal volume of normal saline and then fed Lieber DeCarli 5% (V/V) alcohol liquid diet by oral gavage from the 15thto the 35thday (EtOH group,n= 8). The control group was oral gavaged an equal volume of normal saline and then fed the normal Lieber DeCarli without alcohol liquid diet after 2 h by oral gavage from the 1stto the 35thday (CTRL group,n= 8). The dose of KRQKYD was determined based on our previous study[7]. The details of the alcohol liquid diet formula are described in Table S1. All mice were fasted for 9 h. In the last 2 h, mice in all groups were fed 239 mmol/kg bw KRQKYD by oral gavage. Finally, all mice were euthanized in a CO2chamber, followed by the collection of small intestine and liver. Blood was sampled from mice eyes and centrifuged at 4 000 ×g(7 583 r/min in a No. 1 rotor, centrifuge-H1850R, Xiangyi Co., Changsha, Hunan, China) at 4 °C for 10 min to obtain serum. 0.5 g of liver tissue was homogenized in 1 mL 1× PBS.After centrifugation at 4 000 ×gfor 10 min at 4 °C, the supernatants were collected.
2.7 Transport experiments
The transepithelial transport of KRQKYD was evaluated according to Hubatsch et al.[14]with some modifications. Caco-2 cells (American type culture collection) were grown in DMEM containing 3.5 g/L glucose, 3.7 g/L NaHCO3,and 1.3 g/L HEPES supplemented with 10% fetal bovine serum, 1% nonessential amino acid and penicillin (100 U/mL)-streptomycin (100 μg/mL) at 37 °C in an atmosphere of 5% CO2with 90% relative humidity.Caco-2 cells from generations 10−20 were split using 0.25% trypsin with 0.02% EDTA. After centrifugation at 2 000 ×gfor 2 min at 4 °C, the supernatant was removed. Then, Caco-2 cells were used to prepare a cell suspension with a density of 1 × 105cells/mL using a complete medium, and 0.5 mL of the cell suspension was seeded on the apical (AP) side of Transwell®12-well permeable supports(0.4-μm pore polyester membrane, 12-mm diameter, 1.12-cm2cell growth surface; Costar, Corning, Birmingham, UK). Additionally,1.5 mL of complete medium was added to the basolateral (BL) side.The culture medium was replaced every 2 days in the first week, and the culture medium was then replaced every day until the end of the 21-day culture period. The integrity and tightness of the monolayer of Caco-2 cells were evaluated by measuring transepithelial electrical resistance (TEER), with a value higher than 300 Ω∙cm2used for the transport experiments. Alkaline phosphatase (AKP), a representative enzyme of the brush edge of Caco-2 cells, reflects the differentiation and growth status of epithelial cells. The activity of APK on the AP side and BL side was measured with an AKP kit. The differentiated Caco-2 cell monolayer membrane was washed with HBSS buffer three times and incubated for 30 min. Then, HBSS buffer supplemented with 1 mmol/L KRQKYD was added to the AP side (200 μL) of the different wells, and 1.5 mL of HBSS buffer without KRQKYD was added to the BL side. Samples (200 μL) were taken from the BL side at 30, 60, 90, and 120 min, and an equivalent volume of HBSS buffer was added to the BL side to maintain the volume. The concentrations of KRQKYD in the samples were measured by reversed phase highperformance liquid chromatography-time of flight mass spectrometry(RP-HPLC-TOF/MS).
2.8 KRQKYD measurements by RP-HPLC-TOF/MS
The concentration of KRQKYD was determined by RP-HPLC-TOF/MS (ACCHROM HPLC system equipped with a Waters symmetry®C18reversed-phase chromatography column(4.6 mm × 250 mm, 5 μm), part No.: WAT 054275, Ireland, serial No. 03193906413886). The sample was filtered with a 0.22-μm microfiltration membrane and injected into sample bottles. The chromatographic conditions were as follows: column temperature:25 °C; detection wavelength: 280 nm; fluidity A: 0.1% TFA aqueous solution; fluidity B: 0.1% TFA acetonitrile solution; elution procedure: 0−40 min 2%−45% B; 40−45 min 45%−2% B; flow rate:0.8 mL/min; injection volume: 20 μL. The flow is entered directly into the TOF/MS system for multiple reaction measurements. The mass recording range of precursor ions wasm/z200–4 000. Mass Lynx V4.1 was used to operate the instrumental and analyze the mass spectrogram information.
2.9 Statistical analysis
All results are expressed as the mean ± SD. A linear mixed model(LMM) was used to describe all fixed and random effects on different traits. All statistically significant results were analyzed by F-ratio tests(P< 0.05). The apparent permeability coefficient (Papp, cm/s) was calculated as follows:
Where ΔQ/Δtis the permeability rate of KRQKYD per unit time (mmol/s),Ais the surface area of the Transwell membrane (cm2),andC0is the initial concentration of KRQKYD on the AP side of the Caco-2 monolayer cell model (mmol/L). The concentration of KRQKYD on the BL side decreased after each sampling because a new HBSS buffer was added to maintain the volume. Therefore,the accumulation of KRQKYD on the BL side was corrected by the following equation:
WhereQis the amount of KRQKYD that accumulated on the BL side (mmol),Cnis the concentration of KRQKYD in samplen,Vis the volume of liquid in the BL chamber,Ciis the concentration of KRQKYD in samplei, andViis the volume of samplei.
3. Results and discussion
3.1 Cytotoxicity of KRQKYD in HHL-5 and Caco-2 Cells
As shown in Supporting Information 1, KRQKYD was successfully synthesized, and the purity of the KRQKYD was greater than 95% after purification with reversed-phase chromatography. This result suggested that the synthesized KRQKYD could be used for subsequent experiments. The cytotoxicity of alcohol and KRQKYD for HHL-5 and Caco-2 cells was evaluated by CCK-8 kits. As shown in Fig. 1A, the viability of HHL-5 cells significantly decreased(P< 0.05) as the concentration of alcohol increased. The viability of HHL-5 cells was approximately 49.61% at the 500 mmol/L alcohol,which was a semi-lethal concentration of alcohol for the HHL-5 cells(IC50). This result implied that the model of alcohol-induced HHL-5 cell damage had been successfully established and confirmed the concentration of alcohol (500 mmol/L) chosen for subsequent experiments. As shown in Figs. 1B and D, the viability of the HHL-5 and Caco-2 cells was not significantly decreased (P> 0.05) with an increase in the concentration of KRQKYD, which confirmed that KRQKYD is not cytotoxic to HHL-5 or Caco-2 cells. As demonstrated in Fig. 1C, with increasing concentrations of KRQKYD pre-treatment, the viability of HHL-5 cells was significantly (P< 0.05)higher than that in EtOH group. However, the viability of HHL-5 cells was not significantly (P> 0.05) difference between pre-treatment with 3 and 4 μmol/mL concentrations of KRQKYD. Therefore, the concentrations of KRQKYD (3 μmol/mL) were chosen for subsequent experiments. Moreover, the effective dose of KRQKYD (3 μmol/mL)was lower than that of NPPKFD (Asn-Pro-Pro-Lys-Phe-Asp)(4.18 μmol/mL)[6], KPC (Lys-Pro-Cys) (5 mmol/mL)[4], PGWNQWFL(Pro-Gly-Trp-Asn-Gln-Trp-Phe-Leu) (100 μmol/mL) and VEVLPPAEL(Val-Glu-Val-Leu-Pro-Pro-Ala-Glu-Leu) (100 μmol/mL)[15], which have all been previously described.
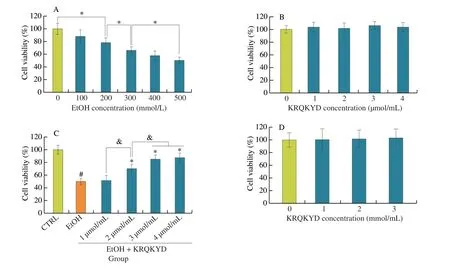
Fig. 1 (A) Effects of alcohol concentration on HHL-5 cell viability; (B) Effects of KRQKYD concentration on HHL-5 cell viability; (C) Effects of the KRQKYD on HHL-5 cell viability in an model of alcohol-induced HHL-5 cells damage (alcohol concentration: 500 mmol/L, KRQKYD concentration: 1, 2, 3 and 4 μmol/mL);(D) Effects of KRQKYD concentration on Caco-2 cell viability. #P < 0.05 vs. the CTRL group, *P < 0.05 vs. the EtOH group, &indicates significant difference between sample groups (P < 0.05).

Fig. 2 (A-C) Effects of the KRQKYD pre-treatment with different temperatures (20−100 °C) on the levels of AST, ALT and MDA on the model of alcoholinduced HHL-5 cells damage; (D-F) Effects of the KRQKYD pre-treatment with different pH (3.0−11.0) on the levels of AST, ALT and MDA on the model of alcohol-induced HHL-5 cells damage; (G-I) Effects of the KRQKYD pre-treatment with different concentrations of NaCl (1%−7%) on the levels of AST, ALT and MDA on the model of alcohol-induced HHL-5 cells damage; #P < 0.05 vs. the CTRL group, *P < 0.05 vs. the EtOH group, &indicates significant difference between sample groups (P < 0.05).
3.2 Effects of temperature, pH and NaCl on the stability of KRQKYD
In general, temperature, pH and salt concentration are the key factors that affect the activity of bioactive peptides. These factors in the external environment may destroy the structure of peptides,resulting in the loss of their biological activity. The levels of AST, ALT and MDA are considered major indicators of alcoholinduced HHL-5 cell damage[6]. In this study, the levels of ALT, AST and MDA were measured to investigate the stability of KRQKYD with the environmental factors, such as temperature, pH and NaCl concentration in a model of alcohol-induced HHL-5 cell damage. As shown in Figs. 2A-C, with increasing temperature from 20 °C to 80 °C,the levels of ALT and MDA showed a trend of increasing, but they were still significantly lower than that of the EtOH group (P< 0.05).The level of AST was not significantly changed (P> 0.05). As the temperature further rose to 100 °C, the levels of AST, ALT and MDA were significantly increased (P< 0.05), indicating that KRQKYD was unstable at 100 °C. Simultaneously, the effect of different pH environments on the stability of KRQKYD was shown in Figs. 2D-F.The results suggested that KRQKYD had strong stability in neutral and acidic environments. Even if KRQKYD was treated with an extremely acidic environment (pH 3.0) still maintained satisfactory stability. However, when the pH was elevated to 11.0, the stability of KRQKYD was significantly decreased (P< 0.05). This implied that KRQKYD is unstable in extreme alkaline environments.Consequently, KRQKYD should not be used or stored in extreme alkaline (pH 11.0) or high temperature (≥ 100 °C) conditions.Moreover, compared to the CTRL group, the levels of ALT, AST and MDA were not significantly increased (P> 0.05) in the KRQKYD group treated with different concentrations of NaCl (1%−7%)(Figs. 2G-I). These results demonstrated that KRQKYD showed satisfactory stability at salt concentrations (1%−7%), high temperatures (20−80 °C) and low pH (3.0−9.0), but its stability was significantly decreased at an extremely high temperature (100 °C) and pH (pH 11.0).
The content and structure changes of KRQKYD with pre-treatment under high temperature and extreme alkaline conditions were investigated for elucidating the cause of KRQKYD instability under 100 °C and pH 11.0. As shown in Fig. 3A, with increasing temperature from 20 °C to 100 °C, the contents of KRQKYD were not significantly difference (P< 0.05). However, KRQKYD showed significant aggregation with increasing temperature (Fig. 3C).Therefore, the instability of KRQKYD under 100 °C may be attributed to self-assembling aggregation of KRQKYD, which led the active group in KRQKYD to be embedded in the aggregates. Aggregation greatly decreased the surface area of KRQKYD in contact with HHL-5 cells, thereby restraining the hepatocyte-protective activity of KRQKYD. As shown in Fig. 3B, the content of KRQKYD with pre-treatment under pH 11.0 was significantly decreased (P< 0.05).As shown in Figs. 3D-F, about 68.14% of the amino acid residue Gln in KRQKYD was deaminated to form Glu, altering the peptide’s main sequence to KREKYD. Previous studies have shown that theαcarbon atoms of amino acid residues are prone to deamination reactions to form ketoacids in alkaline environments[16-17]. This is similar to the results of this study. From these findings, it can be proposed that in an excessively alkaline environment, the mechanism of KRQKYD may be unstable.

Fig. 3 (A) Effect of pre-treatment at different temperatures on content of KRQKYD; (B) Effect of pre-treatment with different pH on content of KRQKYD;(C) Effects of the KRQKYD pre-treatment with different temperatures on morphology by transmission electron microscope; (D) The total particles of RP-HPLC of KRQKYD pre-treatment at pH 11.0; (E-F) Identification of the molecular weight and amino acid sequence of the peak at 7.06 and 9.74 min by TOF/MS spectrum.&indicates significant difference between sample groups (P < 0.05).

Fig. 4 (A-C) Effects of the KRQKYD pre-treatment with pepsin/trypsin-simulated gastrointestinal digestion on the levels of AST, ALT and MDA on the model of alcohol-induced HHL-5 cells damage; (D) Effects of the KRQKYD pre-treatment with pepsin/trypsin-simulated gastrointestinal digestion on the content of KRQKYD; (E) The total particles of RP-HPLC of KRQKYD pre-treatment with trypsin-simulated intestinal digestion; (F-K) Identification of the molecular weight and amino acid sequence of the peak at 3.39, 6.08, 9.74, 17.21, 20.34 and 22.03 min by TOF/MS spectrum; (L-N) Effects of KRQKYD on serum concentrations of ALT, AST and MDA in male mice fed a control diet or an ethanol-containing diet with or without KRQKYD for 35 days. #P < 0.05 vs. the CTRL group, *P < 0.05 vs. the EtOH group, &indicates significant difference between sample groups (P < 0.05).
3.3 Effects of pepsin/trypsin-simulated gastrointestinal digestion on the stability of KRQKYD
As demonstrated in Figs. 4A-C, the contents of ALT, AST, and MDA were not significantly increased in the EtOH + KRQKYD group after digestion with pepsin for 2 h (P> 0.05), indicating that KRQKYD shows strong resistance to pepsin. However, the contents of ALT, AST and MDA were significantly increased in the EtOH +KRQKYD group after digestion with trypsin for 2 h (P< 0.05), which indicated that KRQKYD was unstable to trypsin. The fragments of KRQKYD digestion with trypsin were analyzed by LC-MS/MS. As shown in Figs. 4D-K, approximately 36.60% of the KRQKYD was split into smaller fragments after trypsin treatment; these fragments included YD (Tyr-Asp, 9.61%), KR (Lys-Arg, 6.43%), QKYD(Gln-Lys-Tyr-Asp, 8.34%), KRQK (Lys-Arg-Gln-Lys, 7.50%) and QK (Gln-Lys, 4.72%), indicating that trypsin specifically cleaved the carboxy-terminal peptide bond between lysine and arginine in KRQKYD. This may be the reason of KRQKYD instability to trypsin hydrolysis. Although 36.60% of KRQKYD was hydrolyzed by trypsin into smaller polypeptide fragments, 63.40% of KRQKYD remained intact and showed strong hepatocyte-protective activity.Simultaneously, the effect of KRQKYD on alcohol-induced liver damage in mice was investigated. As shown in Figs. 4L-N, the contents of ALT, AST and MDA in EtOH group were significantly higher than that of CTRL group (P< 0.05), which implied that the mouse model of alcohol-induced liver damage was successfully developed. Compared to the EtOH group, the contents of ALT,AST and MDA were significantly decreased in EtOH + KRQKYD group, which further confirm that KRQKYD could remain effective active for preventing alcohol-induced liver damagein vivoeven after gastrointestinal digestion. This discovery suggested that KRQKYD could be used as a potential functional factor to ameliorate alcoholic liver injury.
3.4 Transport of KRQKYD
The Caco-2 cell monolayer model is usually used to investigate the absorption of drugs or functional factors into human small intestinal epithelial cells because of its good homology and many features that are similar to those of the of the human small intestine epithelium[18-20]. In this study, the Caco-2 cell monolayer model was selected to investigate the efficiency and mechanism of KRQKYD transmembrane transport. TEER is formed by the flow of ions through the paracellular space, which is positively correlated with the density of cell monolayers. As demonstrated in Fig. 5A, the TEER value of Caco-2 cells was approximately (425.1 ± 7.6) Ω∙cm2on the 21thday, indicating that the Caco-2 cells were closely connected as a dense monolayer. Generally, a dense monolayer of Caco-2 cells with a TEER value higher than 300 Ω∙cm2can be used for transport experiments[21-22]. The enzyme AKP is considered a major biomarker of Caco-2 cell polarization and is mainly expressed in the brush edge.The activity of AKP on the brush edge can be used to determine whether the cells have established polarity. The AKP activity ratio(AP/BL) increased with time. After 21 days, the enzyme activity ratio on the two sides increased to 1.00:6.81 (Table S2), indicating polarization of the Caco-2 membrane. The above results indicated that the Caco-2 cell model was successfully established and could be used to study the intestinal absorption of KRQKYD in this study. The stability of KRQKYD on Caco-2 cell monolayer was investigated by evaluating the recovery of KRQKYD in the apical surface Caco-2 cell monolayers. As shown in Fig. 5B, the recovery of KRQKYD in the apical surface Caco-2 cell monolayers was 96.07% after 2 h incubated time, which confirmed that KRQKYD had a good stability to Caco-2 cell monolayers. This result was associated with the strong resistance of KRQKYD to gastrointestinal proteases. ThePappvalue is the general criterion used to determine the difficulty of drug absorption in Caco-2 cell monolayers. A value ofPapp< 1.0 × 10−6cm/s indicates poor absorption and an absorption rate of 0−20%. A value of 1.0 × 10−6cm/s <Papp< 10.0 × 10−6cm/s indicates moderate absorption and an absorption rate of 20%–70%. A value ofPapp> 10 × 10−6cm/s indicates good absorption and an absorption rate of 70%–100%. As demonstrated in Fig. 5C, thePappvalue of KRQKYD at 1 mmol/L increased from (2.08 ± 0.09) × 10−7cm/s to (9.70 ± 0.53) × 10−7cm/s with transport from the AP side to the BL side from 30 min to 120 min, which indicated that KRQKYD could be absorbed into the Caco-2 cell monolayer, although the absorption efficiency is low.Moreover, the transepithelial permeability of KRQKYD was higher than that of GLLLPH (Gly-Leu-Leu-Leu-Pro-His)[23], KVLPVP(Lys-Val-Leu-Pro-Val-Pro)[24], VYIHPF (Val-Tyr-Ile-His-Pro-Phe)[25],and SRYPSY (Ser-Arg-Tyr-Pro-Ser-Tyr)[26], which have all been previously described. The higher absorptivity of KRQKYD might be due to the presence of Gln with hydrophobic uncharged residues.
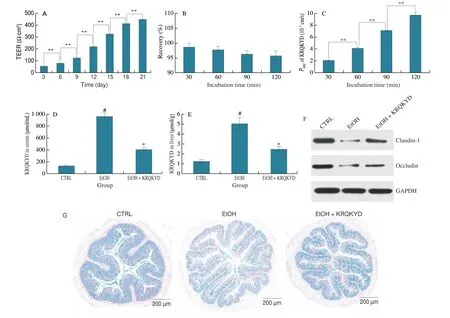
Fig. 5 (A) Transepithelial electrical of Caco-2 monolayers at different growth times; (B) Stability of KRQKYD (1 mmol/L in HBSS, pH 7.4) in AP side of Caco-2 cell monolayers within 2 h; (C) Effects of incubation time on the apparent permeability coefficient of KRQKYD across Caco-2 cell monolayers;(D-E) The concentrations of KRQKYD in serum and liver; (F) The small intestine tight junction protein expression levels of Claudin-1 and Occludin were detected by Western blot analysis; (G) A biopsy of the small intestine was stained with Alcian blue. #P < 0.05 vs. the CTRL group, *P < 0.05 vs. the EtOH group,**in figures indicate significant differences (P < 0.05).
In addition, the absorption of KRQKYDin vivowas investigated by the model of mice. The concentrations of KRQKYD in serum and liver were measured for evaluating the absorption of KRQKYDin vivo. As shown in Figs. 5D-E, the concentrations of KRQKYD in serum ((129.53 ± 13.46) μmol/mL, the absorption rate was about 2.71%) and liver ((1.25 ± 0.17) μmol/g, the absorption rate was about 0.26‰) were lowest in CTRL group. However, this result suggested that the practical absorptivity of KRQKYD in the small intestine (2.71%) was higher than that in the Caco-2 cell monolayer(< 2%), which might be because the Caco-2 cell monolayer is tighter than the human intestine[27]. Compared to the CTRL group, the concentrations of KRQKYD in serum ((967.35 ± 57.37) μmol/mL,the absorption rate was about 20.23%) and liver ((5.07 ± 0.62) μmol/g,the absorption rate was about 1.06‰) were significantly higher(P< 0.05) in EtOH group. As shown in Figs. 5F-G, the expression level of tight junction protein in the CTRL group was significantly higher than that in EtOH group (P< 0.05). This result showed an opposite trend to the absorption of KRQKYDin vivo. Therefore, it is reasonable to speculate that the trans-epithelial transport mechanism of KRQKYD may be associated with tight connections between intestinal cells.
3.5 Mechanism of KRQKYD transport across the Caco-2 cell monolayer
To further verify the above conjecture, the mechanism of KRQKYD transepithelial transport in human intestinal Caco-2 monolayer cells was investigated by adding inhibitors to the Caco-2 cell monolayer model. As shown in Fig. 6, pretreatment with an energy inhibitor (sodium azide, 10 mmol/L) and a PepT1 competitive inhibitor (Gly-Pro, 10 mmol/L) for 30 min did not decrease the level of transepithelial KRQKYD permeability, suggesting that KRQKYD transmembrane transport is not energy dependent or vector mediated.Previous studies have confirmed that IRW (Ile-Arg-Trp)[28]and YPI (Tyr-Pro-Ile)[29]undergo trans-epithelial transport in Caco-2 monolayer cells mainly by PepT1, but PepT1 was not responsible for KRQKYD transport in this study. Moreover, the transcytosis inhibitor wortmannin (500 nmol/L, 30 min) did not decrease the level of transepithelial KRQKYD permeability, indicating that the transport mechanism of KRQKYD is not associated with endocytosis. In addition to active transport mediated by transporters and endocytosis,potential pathways for the intestinal transport of bioactive peptides include transcellular passive diffusion of hydrophobic molecules and paracellular transport of small water-soluble compounds.Transcellular passive diffusion is associated with the liposolubility of substances to the lipid bilayers. However, KRQKYD is a small watersoluble oligopeptide, and this type of molecule cannot be transported in the intestinal tract by transcellular passive diffusion. Therefore, the transport mechanism of KRQKYD is not associated with transcellular passive diffusion. Cytochalasin D, a tight junction disruptor,can significantly decrease the tight junctions between Caco-2 monolayer cells. As shown in Fig. 6, cytochalasin D (0.5 μg/mL,30 min) significantly increased the transepithelial permeability of KRQKYD (P< 0.05), suggesting that intact KRQKYD was absorbed by paracellular transport in human intestinal Caco-2 monolayer cells.The mechanism of transepithelial transport of the oligopeptides VLPVL (Val-Leu-Pro-Val-Pro)[30], KVLPVP (Lys-Val-Leu-Pro-Val-Pro)[24]and VPP (Val-Pro-Pro)[31]are similar to the mechanism of KRQKYD transepithelial transport defined in this study.

Fig. 6 Effects of various compounds (wortmannin, cytochalasin D, Gly-Pro and sodium azide) on the apparent permeability coefficient of KRQKYD across Caco-2 cell monolayers; **in figures indicate significant differences (P < 0.05).
4. Conclusion
KRQKYD showed strong resistance to temperature, pH and NaCl concentration. Additionally, KRQKYD showed good stability against simulated gastrointestinal digestionin vitro. Trypsin specifically cleaved the carboxy-terminal peptide bond between lysine and arginine in KRQKYD. This discovery provides a theoretical basis for designing target peptides with specific molecular structures to avoid enzyme digestion and reduce peptide hydrolysis. Furthermore, intact KRQKYD could be absorbed through Caco-2 cell monolayers, with aPappvalue of (9.70 ± 0.53) × 10−7cm/s, by paracellular transport.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This study was supported by the Major special project of Anhui Province (2021d06050001); the Major Science and Technology Project of Anhui Province (201903b06020004); the Natural Science Foundation of Anhui Province (2308085QC115); the Special Fund for Anhui Province Agricultural Products Processing Industry Technology System (340000211260001000420). We would like to thank Shanghai Biotree Biotech Co., Ltd. for providing us with peptide mass spectrometry identification services.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.26599/FSHW.2022.9250127.
- 食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango

