Lactose-free milk powder can effectively relieve diarrhea symptoms in weaning SD rats and children
Mnmn Liu, Shiwen Hn, Boy Li, Cheng Chen, Lu Yo, Jung-il Kwon, Jun Jin, Huilin Che,
a Beijing Advanced Innovation Center for Food Nutrition and Human Health, College of Food Science and Nutritional Engineering, China Agricultural University, Beijing 100083, China
b Maeil Innovation Center, Maeil Dairies Co., Ltd., Seoul 03142, South Korea
Keywords: Diarrhea Lactose-free Milk powder Intestinal barrier Gut microbiota
ABSTRACT Diarrhea has become the leading cause of illness and death among infants and young children in developing countries. Clinically, patients with diarrhea showed damaged intestinal epithelial villi, usually accompanied by lactase def iciency. In this study, we evaluated the therapeutic effects of lactose-free milk powder on rats and children with diarrhea. Antibiotic-associated diarrhea (AAD) model was established by gavage with antibiotic mixture in SD rats, followed by administration of milk powder containing lactose or not. The results showed that lactose-free milk powder ameliorated AAD-related diarrhea symptoms, and accelerated the recovery from diarrhea. And 16S sequencing results indicated lactose-free milk powder contributed to increase the α- and β-diversity of intestinal f lora, and restore the intestinal microbiota disorder. In conclusion, our data demonstrate that lactose-free milk powder could alleviate diarrhea by restoring gut microbiota and intestinal barrier function.
1. Introduction
As the second major cause of death of children under 5 years old[1], diarrhea caused more than 0.5 million deaths of children[2]and affected up to 5% of adults[3]. Characterized by thin feces and increased fecal moisture in clinical[4], diarrhea may induce dehydration, reduce the quality of life, and even stunt the growth of children[5]. Eighty percent of diarrhea in infants and children is caused by virus, mainly rotavirus[6], whose virus strains include G1P,G2P, G3P and G9P[7-8]. According to report from the world health organization’s centers for disease control and prevention (WHO/CDC), as many as 215 800 children under the age of f ive succumbed to rotavirus infection globally in 2013[9]. Another principal virus leading to diarrhea is adenovirus including subtypes of Ad41, Ad3,Ad7 and Ad31, whose degree of infection is positively correlated with intussusception symptoms[10]. Multiple pathogens tend to induce diarrhea mainly by infecting the inner surface of intestinal epithelium.
Rotavirus is a medium-sized non-enveloped virus, containing 11 distinct genome segments of double-stranded RNA wrapped in a triple-shelled capsid[11], which will target the villous tips of mature intestinal epithelial cells[12]. The extensive replication of virus causes atrophy of intestinal epithelial villous, loss of microvilli, mononuclear cell inf iltration, endoplasmic reticulum and mitochondrial engorgement in enterocytes[13-14]. Although rotavirus directly interacts with intestinal epithelial cells, it has endangered the function of various organs beyond the intestine[13], and been linked to systemic diseases such as acute cerebellitis and autoimmune pathology[15]. Under normalcy, healthy enterocytes secrete lactase into the small intestine that helps in lactose metabolism, but changes in small intestine morphology must lead to loss of intestinal brush border enzymes such as lactase when rotavirus infected[16]. When indigestible lactose reaches the large intestine, it will be metabolized by microorganisms in the colon, increasing the osmotic pressure of the intestine and inhaling water into intestinal lumen[17]. In addition,Organic acids and irritating gases such as hydrogen and methane produced by gut microbes subject the organism to abdominal distension, abdominal pain, etc.[18]. So, children with rotavirus infection are unable to tolerate milk, who may experience recurrence of diarrhea after milk reintroduction into the child’s diet as a result of bacterial fermentation of the lactose in the gut[19]. Therefore, for those children who have already developed diarrhea, special attention should be paid to the secondary stimulation of the fragile intestinal tract after the intake of lactose-enriched milk powder.
The intestinal tract is colonized by abundant microbiota,which was regarded as “second genome” of the human body. The composition and function of intestinal flora is closely bound up with the development of immune system, regulation of intestinal nutritional metabolism and the occurrence of diseases[20]. The food what we take is fermented by gut flora to produce short-chain fatty acids (SCFAs), which are pivotal signaling factors between the flora and host[21]. Diarrhea tend to disturb the balance of intestinal flora,causing metabolic disorders, intestinal inflammatory diseases, immune dysfunction and other diseases[22]. However, the impact of lactose-free milk powder on the gut microbiota during diarrhea remains unknown.
In order to evaluate the effect of lactose-free milk powder on the recovery from diarrhea, antibiotic-associated diarrhea (AAD) model was constructed in SD rats by a mixture of clindamycin, ampicillin and streptomycin. Then, the symptoms of diarrhea and intestinal function were compared after supplemented by ordinary milk powder and lactose-free milk powder, respectively. Furthermore, we studied the effects of two milk powders on the duration of diarrhea in children, which may facilitate the development of measure to prevent or alleviate diarrhea.
2. Material and methods
2.1 Animals and management
Newly weaned SD rats used in this study were purchased from Vital River Laboratories, Inc. (Beijing, China) and raised in the specific pathogen-free (SPF) animal laboratory with temperature of(22 ± 1) °C, humidity of (55 ± 5)%, a 12 h light/dark cycles and air exchanges at 15 times/h. Free food and water intake, and adaptive feeding for week prior to initiation.
In total 60 rats aged 3 weeks were randomly divided into 3 groups including normal control group (NC), normal milk powder AAD group (NA) and lactose-free milk powder AAD group (LA), with 20 rats in each group, half male and half female. Antibiotic-induced diarrhea model was conducted according to the method reported previously[23]. As depicted in Fig. 1A, rats in NC group were orally administrated with saline (20 mL/(kg BW·day)) from day 1 to day 15,and continued SPF rodent maintenance feeding over the following 5 days. And rats in NA group and LA group were administrated 2 mL of antibiotic mixture (50 mg/mL clindamycin, 55.5 mg/mL ampicillin and 27.75 mg/mL streptomycin, dissolved in saline) for 15 days, then treated with 17.5 g/(kg BW·day) of normal milk powder and lactose-free milk powder from day 16 to day 20, respectively.During the experiment, the weight, food intake and activity status of rats were recorded daily. And food utilization rate is calculated by the percentage of body weight gain to total food intake. Fecal samples on day 15 and 20 were collected for subsequent analysis.
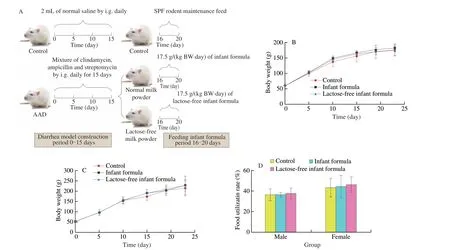
Fig. 1 Lactose-free milk powder did not affect the normal growth of weaned SD rats. (A) Diarrhea rat models were constructed orally with antibiotic mixture for 15 days, followed by the administration of normal or lactose-free milk powder. Effect of normal formula and lactose-free formula on body weight of (B) male and(C) female rats (n = 10). (D) Effects of normal formula and lactose-free formula on food availability in SD rats.
All animal experiments complied with the relevant guidelines and was approved by China Agricultural University Animal Experimental Welfare and Ethical Inspection Committee.
2.2 Evaluation of diarrhea
On day 20, metabolic cages were used to collect fecal samples for diarrhea evaluation. The degree of loose stool, weight of single fecal and fecal water content were employed as the indexes of diarrhea according to previously described methods[23]. The score of loose stools was graded as follows: (1) normal stool retaining shape, and brown; (2) soft and semi-shaped stool with yellow color; (3) loose and unformed stool, yellow. Weight of single fecal were determined by all feces collected in the metabolic cage and weighing. Then the feces collected were thoroughly dried at 95 °C, until the weight change of the sample less than 0.5 g within 30 min. And the fecal water content was calculated as follows:
2.3 Bioassay of lactase activity
Small intestinal submucosa was snipped off under asepsis condition. Then the activity of lactase was determined by incubating enzymes with 20 mmol/LO-nitrophenyl-β-galactopyranoside (ONPG)at 37 °C for 10 min, stopping the reaction by adding 0.5 mol/L Na2CO3, and measuring theA420nmof the solution[24]. One unit of activity was defined as the amount of enzyme decomposing 20 mmol/L ONPG per min.
2.4 Scanning electron microscopy (SEM) observation
Jejunum samples were fixed in 2.5% glutaraldehyde after removal of the internal contents by washing with the saline, then the segments were incubated with 1% tetroxide osmium for 2 h at 4 °C, dehydrated in an increasing gradient of ethanol, and dried at critical point in carbon dioxide[25]. The final samples were coated with gold-palladium and examined using a digital SEM (Hitachi S-3000N, Japan).
2.5 Hematoxylin-eosin (HE) staining
Jejunum of rats were dissected and fixed in 2.5% glutaraldehyde.Then the sampled tissues were embedded in paraffin and sliced into 4-μm thick sections prior to staining with HE and measuring under microscopy.
2.6 16S rDNA Sequencing
Total microbial genomic DNA was extracted from stool samples by using cetyltrimethylammonium bromide (CTAB) protocol. DNA quantity and concentration were measured using gel electrophoresis.The bacterial 16S rDNA V4 variable region was amplified with 515F and 806R. The DNA library was quantified using 2% agarose gel electrophoresis, and then sequenced on the Thermofisher Ion S5TMXL sequencer carried out by the Beijing Novogene Technology Co., Ltd. The specific method of intestinal microflora determination was described previously[26].
2.7 Clinical specimens
Infants of both sex groups with ages ranged from 0 to 24 months and suffering from diarrhea were recruited from diarrhea were recruited from the Third Affiliated Hospital of Xinxiang Medical University. The experiment complied with the relevant regulations and was approved by Human Research Ethics Committee. Diarrhea was defined as the discharge of three or more loose or liquid stools within 24 h, lasting for at least 24 h[27]. Infants with any of the following conditions will be excluded: any hematochezia, lactose intolerance, cow’s milk allergy or immune deficiency.
2.8 Fecal microbiology culture
Stool was collected on the day of hospitalization and discharge for microbiological analysis. In brief, 0.1 g fecal samples were diluted in 0.9 mL sterile deionized water, and mixed in vortex at a speed of 300 r/s. Five plates were inoculated with 0.2 mL sample of three appropriate dilutions (10–1, 10–3, 10–5) at different conditions:potato dextrose agar (PDA) for 48 h at 37 °C for yeast, LB agar for 48 h at 37 °C forEscherichia coli, MRS agar for 48 h at 37 °C under anaerobic conditionforLactobacillus, NA agar for 48 h at 37 °C forBacillusor bacteria, and CLO agar for 24 h at 37 °C under anaerobic condition forClostridium[28]. All results were expressed as logarithm of colony forming unit (CFU) per gram of feces.
2.9 SCFAs determination
Concentrations of five SCFAs including lactic, acetic, propionic,butanoic, formic acids were measured by high performance liquid chromatography (HPLC)[29]. In brief, 0.2 g fecal samples were accurately weighed in centrifuge tubes, and 1 mL of 0.15 mmol/L H2SO4and 1 mL pure water were added in centrifuge tubes. After homogenizing with a vortex for 2 min until uniformly dispersed,the samples were centrifuged for 10 min at 12 000 r/min. The supernatants were extracted and filtered by 0.22 µm organic filter for the subsequent HPLC analysis.
2.9 Statistical analysis
Statistical analysis and result visualization were performed by GraphPad Prism (v5.01) or R software (v3.6.0), and presented as the mean ± standard deviation (SD) from at least three independent measurements. Data were analyzed using one-way analysis of variance (ANOVA) when data conformed to normal distribution,otherwise Mann Whitney test was used. Differences were considered significant atP< 0.05.
3. Results
3.1 Lactose-free milk powder did not affect the normal growth of weaned SD rats
In a 20-day trial, rats were orally administrated with antibiotics from day 1 to day 15, followed by the treatment of normal or lactosefree milk powder over the next 5 days. As shown in Figs. 1B–D, there was no significant difference in body weight, food utilization rate,both male and female, suggesting that lactose-free milk powder can meet the needs of daily growth, as the normal milk powder. The blood routine and blood biochemistry of rats at the end of experiment were shown in Tables 1 and 2. Among them, the number of leukocytes in the female LA group was considerably increased compared with AAD group, but there was no significant difference with NC group and the NA group, and there was no gender dependence. The remaining indexes are in the normal range which had no difference in either group.

Table 1 Blood routine between diarrhea rats fed with different kinds of milk powder.
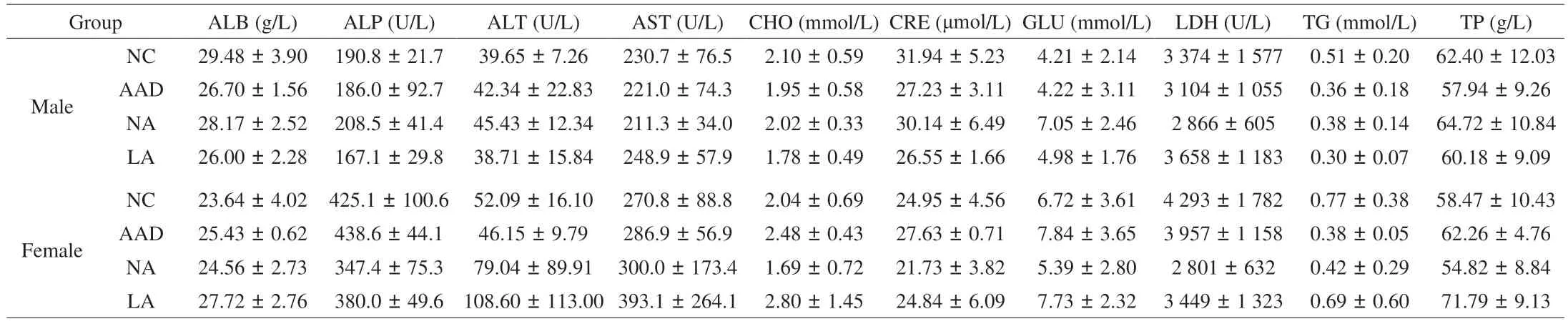
Table 2 Blood biochemistry between diarrhea rats fed with different kinds of milk powder.
3.2 Lactose-free milk powder effectively ameliorated diarrhea caused by mixed antibiotics
We observed obvious diarrhea symptoms in rats after 15-day antibiotic treatment, especially loose stool, increased fecal weight and water content, suggesting the successful establishment of diarrhea model. As shown in Fig. 2A, ranging between dry and loose, the stools excreted by rats in NA group showed soft and semi-shaped, whereas rats subjected to lactose-free milk powder recovered completely on day 20. Randomly weigh the single feces of the rats in each group,and there was no significant difference between the NA group and AAD model. However, there was a greater drop in the fecal weight of rats in LA group, as with control rats (Fig. 2B). Consistently, lactosefree milk powder supplementation reduced the antibiotic enlargement of fecal water content (Fig. 2C), which indicated that lactose-free milk powder significantly relieved the symptoms of diarrhea caused by antibiotics.
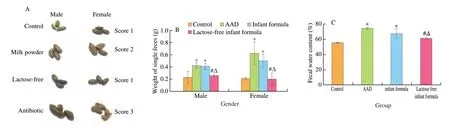
Fig. 2 Lactose-free milk powder ameliorated diarrhea symptoms of AAD rats. (A) Representative stool morphology and loose stool score of rats in each group.(B) Weight of single piece of feces in each group. (C) Comparison of fecal water content among diarrhea rats fed with different formula. All values were expressed as mean ± SD. *P < 0.05 vs. control group; #P < 0.05 vs. AAD group; ∆P < 0.05 vs. normal infant formula group. The same below.
3.3 Lactose-free milk powder relieved intestinal irritation caused by lactose accumulation
Gastrointestinal dysbiosis tend to cause disorders in lactase enzyme, which are related to lactose intolerance or carbohydrate malabsorption. So, we explored the recovery of lactose under different dietary patterns. The overall activity of enzyme revealed significant variation between control group and antibiotic-treated group, as shown in Fig. 3A. Antibiotic cocktail significantly lessened the activity of lactase, which was in a gradual rehabilitation after formula feeding.However, the mean value of lactase activity in lactose-free formula group was higher than that of normal formula group (P< 0.05).SEM was used to examine the morphology of jejunum as shown in Fig. 3B. The weaning rats from the antibiotic treatment exhibited more damaged and atrophic intestinal villi than normal rats, which were alleviated by lactose-free milk powder. As shown in Fig. 3C,obvious swelling, shortening occurred in AAD rats, accompanied with damaged intestinal mucosa, and decrease in villi height. The pathological symptoms of jejunum villi in the lactose-free and normal formula group were significantly ameliorated. These results suggested that lactose-free milk powder mitigated intestinal injury induced by antibiotic cocktail via elevating lactase activity and ameliorating intestinal morpho logy.
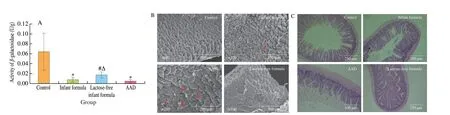
Fig. 3 Lactose-free milk powder relieved intestinal irritation in diarrhea rats. (A) Effect of antibiotics and different formula treatments on lactase activity.(B) Representative scanning electron microscopes of intestinal; a, Rupture of small intestinal villi; b, Atrophy of small intestinal villi; c, Slight damage to small intestinal villi. (C) HE staining of jejunum
3.4 Lactose-free milk powder improved intestinal flora of diarrhea rats
Gut microbial disturbance can often be observed in diarrhea patients. So, we performed 16S rRNA sequencing analysis on the intestinal flora to verify whether lactose-free milk powder could improve the intestinal microenvironment. It can be found thatα-diversity was remarkably decreased in AAD rats from Fig. 4A,while formula and lactose-free formula could reverse this reduction.Compared with normal formula, lactose-free milk powder showed a more augmented Shannon and Simpson indexes, although there is no significant difference. Theβ-diversity of microbiota profiles was assessed by the principal coordinates analysis (PCoA) and nonmetric multidimensional scaling (NMDS), which revealed that the representing cluster of AAD rats was far from that of the control group. And rats in normal formula and lactose-free formula exhibited a similar clustering with closer to control group (Figs. 4B–C).Bacteroidetes, Firmicutes, and Proteobacteria comprised the dominant microbiota in the intestines. As shown in Fig. 4D, the AAD group showed a significant decrease in the proportion of Bacteroidetes but increase in the proportion of Firmicutes and Proteobacteria compared with the control group, increasing the probability of diarrhea. After subjected to the lactose-free milk powder, the relative abundance of Bacteroidetes significantly improved, while the abundance of Proteobacteria returned to a lower level. And compared with the antibiotic-treated rats, lactose-free milk powder notably reduced the level of Firmicutes approach to that of the control group. Rats in lactose-free formula group exhibited gut microbiota composition similar to that of the control group, which is more conducive to disease recovery. The function of the intestinal flora was predicted by the Tax4Fun package in R, and a total of 273 signaling pathways were involved. The heatmap of top 25 enrichment pathways was shown in Fig. 4E, which enables to distinguish rats subjected to formula from antibiotic treatment. The significant factors in prediction function abundance, such as RIG-1-like receptor signaling pathway,FcγR-mediated phagocytosis and other inflammatory pathways, were decreased in the lactose-free group compared to AAD treatment. In addition, metabolic-related pathways containing glycosaminoglycan degradation, glycosphingolipid and synaptic vesicle cycle, were restored after lactose-free milk powder intake. All together, lactosefree milk powder increased the richness of intestinal flora and optimized the flora community structure, thus restoring normal intestinal function.

Fig. 4 Lactose-free milk powder improved the intestinal microbiota in diarrhea rats. (A) α-Diversity analysis of the gut microbiota, including Shannon and Simpson index. β-Diversity analysis based on OTUs of gut microbiome was demonstrated by (B) the principal coordinates analysis (PCoA) and (C) non-metric multidimensional scaling (NMDS). (D) Relative abundance of fecal flora at the phylum level. (E) Effect of milk powder on the metabolic processes. Values were expressed as mean ± SD (n = 3). *P < 0.05 vs. control group.
3.5 Lactose-free milk powder shortened the course of diarrhea in children
In this study, a total of 93 children were recruited, including 30 without diarrhea, 30 with normal milk powder, and 33 with lactosefree formula. As shown in Table 3, majority (n= 73, 78%) of the infants were aged between 1 and 12 months, and there were 68 (73%)male and 25 (27%) female patients without significant difference in male to female ration among groups. The feeding mode is breast milk, formula or formula combined with normal diet. Only one case of shrimp allergy occurred in the lactose-free milk powder group, and no food allergy occurred in the rest. In terms of fecal evaluation, the degree of diarrhea in the end was obviously lower than that at first.Comparing the frequency of visits between the two diarrhea groups,there were 6 cases in the diarrhea normal milk powder group with more than 3 visits, while 4 cases in the diarrhea lactose free group with more than 3 visits. The mean period of diarrhea was notably shorter in infants receiving lactose-free formula (12.70 days) as compared to those taking normal formula (17.07 days).

Table 3 Clinical case information.
3.6 Lactose-free milk powder significantly increased the contents of bacilli and formic acid in the feces of children with diarrhea
To explore the effect of lactose-free milk on the enteric organisms, colony count technique on selective media was utilized for enumerating of yeast,Escherichia coli, bacteria,Lactobacillus,BacillusandClostridium. Above microbes were evaluated on the first day and last day after receiving formula. As shown in Table 4,Bacilluscounts were (2.55 ± 0.69) (lg (CFU/g)) in the first detection of lactose-free milk group, but increased significantly to (3.31 ±0.99) (lg (CFU/g)) last. Compared with normal formula feed infants,supplementation with lactose-free milk decreased the contents ofE. coli, which was considered as causative agent of intestinal infections. The results of plate counting indicated that lactose-free formula appeared to enrich probioticBacillusand inhibit the growth ofE. coli, promoting healthier intestinal.
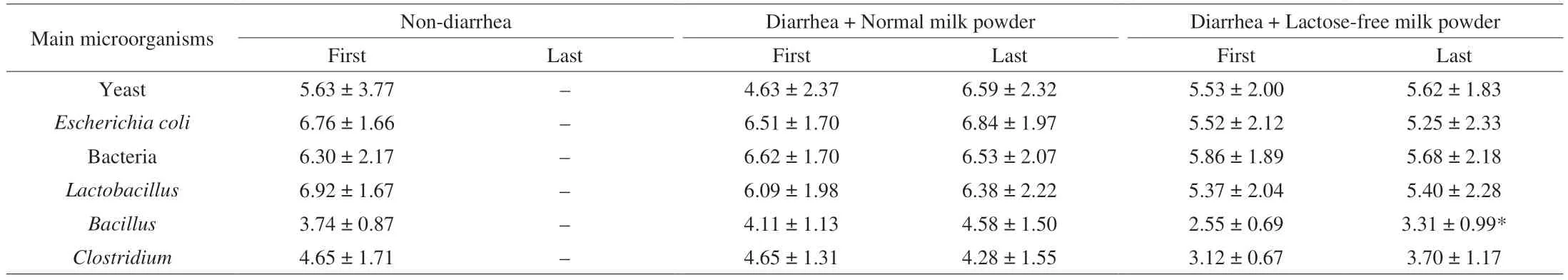
Table 4 Count of main microorganisms in feces (lg (CFU/g)).
The effects of lactose-free milk powder on microbial metabolites were investigated by measuring the concentrations of SCFAs, which was exhibited in Table 5. Infants subjected to diarrhea showed decreased contents of lactic, acetic, propionic and formic acids in varying degrees, which were recovered in subjects receiving lactosefree milk powder. Most notably, the formic acid concentration increased significantly before and after the treatment in lactosefree milk powder group, and moved closer to non-diarrhea group.However, the other index did not differ among groups, which may be related to significant individual difference.
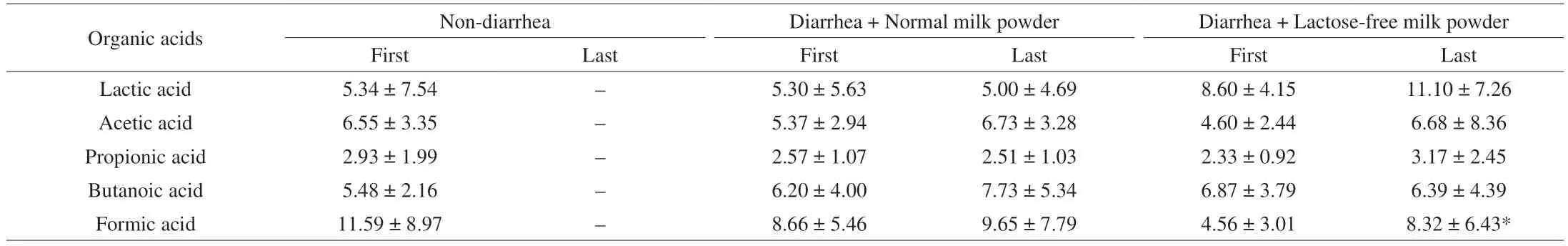
Table 5 Content of main organic acids in feces (mg/g).
4. Discussion
There were nearly 1.7 billion cases of diarrhea among children annually[30]. Diarrhea is a clinically common multifactorial gastrointestinal disease, which has negative effects on the quality of life. In most cases patients could recover spontaneously, but targeted intervention would promote self-healing and reduce their pain[31]. In the present study, we discussed the feasibility of lactose-free milk powder to cure diarrhea. Mixture of clindamycin, ampicillin and streptomycin was used to establish a rat model of diarrhea. Then,AAD rats were intervened by normal formula and lactose-free formula respectively, using enteric pathology and gut microbiota to determine the influence and mechanism of lactose-free milk powder on diarrhea. Our results revealed that administration of lactose-free milk powder sped up the restoration of antibiotic-associated diarrhea,attributed to the improvement of gut microbiota and intestinal barrier integrity.
During the experiment, a steady increase and no significant difference in body weight among all the groups, along with the similar food utilization, which suggesting that lactose-free milk powder can meet the needs of daily growth. On the 5thday administrated lactosefree formula, the conspicuous amelioration of diarrhea symptoms including stool morphology, weight and water content emerged in comparison with AAD rats, implicating that lactose-free formula possessed the potential to remedy diarrhea, which was supported by some previous studies. Xu et al.[32]found duration for diarrhea remission was significantly shorter in the lactose-free formula group ((3.17 ± 1.04) days) compared to conventional formula group((5.25 ± 1.58) days). And another study showed the median duration of diarrhea was shortened by 20.5 h in the lactose-free group, with higher weight gaining and lower moderate acidosis[33].
Several studies have reported that intestinal barrier injury is an important pathological characteristic of diarrhea. The intestinal villi were essential to prevent pathogens from entering bloodstream and provided a living environment for intestinal flora. Damage to the villus of the small intestine has been seen when diarrhea happened by SEM, which was ameliorated by lactose-free formula treatment.The growth of intestinal villi and restoration of gut barrier can be seen in the group englobed lactose-free formula. Meanwhile, the destroy of intestinal morphology was contemporaneous with a falling of lactase, which may explain why formula lactose-containing formula aggravates diarrhea symptoms. Lactose failed to catabolize, accessed to colon directly and was used to produce acid and gas by intestinal microorganisms, thereby leading to lactose-related secondary damage.Therefore, lactose free diet seems to be more suitable for people with diarrhea, which may attribute to the improvement of intestinal barrier.
The disorders of gut microbiota have been reported to be relevant to the development of intestinal disease[34-35]. In the present study,16S rRNA sequencing results revealed that lactose-free milk powder significantly increased theα- andβ-diversity of diarrhea rats. As is known to all, an abundance of microbiota plays a vital role in maintaining immune homeostasis, inhibiting pathogenic bacteria and involving in metabolism[36]. In addition, there was a significant difference in the gut microbiota composition in phylum level.Lactose-free milk powder decreased the abundance of Proteobacteria,which hinders the nutrition absorption and body metabolism.Simultaneously, rats subjected to lactose-free milk powder exhibited a significant increase of Bacteroidetes and decrease of Firmicutes,which made the bacterial composition closer to the that of control group at the phylum level, thereby supporting the role of lactose-free formula on rebuilding the balance of the gut microbial environment.
To verify our results from rat models, infants between 0–24 months of age with diarrhea was recruited and fed with normal formula and lactose-free formula to observe the effect on diarrhea.We found that the average course of diarrhea was 4.37 days less than that in normal formula. In addition,Bacillusin lactose-free group was measurably higher compared with that in the first fecal examination by culturing microorganismsin vitro. Studies have confirmed the positive roles ofBacilluson growth performance, feed efficiency of piglets[37], regulating host symbiotic microbiota and inhibiting the growth of pathogenic bacteria[38]. So, the supplement ofBacillusseems an attractive therapeutic approach for diarrhea. Furthermore,lactose-free milk powder supplementation notably up-regulated the concentrations of SCFAs, which have been reported to participate in the host metabolism as important signaling molecules[39]. We speculated that the lactose-free milk powder may affect the activity of different bacteria in the intestinal tract to produce different SCFAs.Our results manifested lactose-free formula was able to ameliorate the intestinal microenvironment, which may offer new insights into the mechanism involved in relieving diarrhea.
5. Conclusion
In summary, we found lactose-free milk powder ameliorated diarrhea symptoms of antibiotic induced weaning rats without affecting their normal growth, by modifying the composition and structure of gut microbiota, thereby restoring epithelial barrier function. In addition, Duration was shortened by lactose-free milk powder in children with diarrhea, accompanied with boosted contents ofBacillusand SCFAs. This study revealed the relationship between lactose and intestinal flora, which will lay a foundation for further investigations of microbiota-based diarrhea therapies.
Conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Acknowledgments
This work was supported by the project of two children’s dairy products (201704810610483).
Data availability
The data presented in the study will be deposited in the NCBI database.
- 食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango

