Insight into the spoilage heterogeneity of meat-borne bacteria isolates with high-producing collagenase
Hodong Wng, Lingting Sho, Jinho Zhng, Xinglin Xu, Jinjun Li, Huhu Wng,,
a Jiangsu Collaborative Innovation Center of Meat Production and Processing, Quality and Safety Control, College of Food Science and Technology,Nanjing Agricultural University, Nanjing 210095, China
b Yantai XiWang Meat Food Co., Ltd., Yantai 264000, China
Keywords: Bacteria Chilled chicken Heterogeneity Collagenase Spoilage
ABSTRACT Chilled chicken is inevitably contaminated by microorganisms during slaughtering and processing, resulting in spoilage. Cutting parts of chilled chicken, especially wings, feet, and other skin-on products, are abundant in collagen, which may be the primary target for degradation by spoilage microorganisms. In this work, a total of 17 isolates of spoilage bacteria that could secrete both collagenase and lipase were determined by raw-chicken juice agar (RJA) method, and the results showed that 7 strains of Serratia, Aeromonas, and Pseudomonas could signif icantly decompose the collagen ingredients. The gelatin zymography showed that Serratia liquefaciens (F5) and Pseudomonas saponiphila (G7) had apparent degradation bands around 50 kDa,and Aeromonas veronii (G8) and Aeromonas salmonicida (H8) had a band around 65 and 95 kDa, respectively.The lipase and collagenase activities were detected isolate-by-isolate, with F5 showing the highest collagenase activity. For spoilage ability on meat in situ, F5 performed strongest in spoilage ability, indicated by the total viable counts, total volatile basic nitrogen content, sensory scores, lipase, and collagenase activity. This study provides a theoretical basis for spoilage heterogeneity of strains with high-producing collagenase in meat.
1. Introduction
The chicken industry has developed rapidly in recent years, and the consumption of chicken has increased. China has become the second largest chicken producer in the world. In China, the industry was forced to upgrade due to the H7N9avian influenza epidemic in 2013, and restrictions on live poultry transactions were introduced as new regulations around China. With the transformation of consumption patterns, chilled chicken is becoming more and more popular among consumers[1]. Thus, microbial control and shelf life have become the primary issue to be addressed. However, the current research mainly focuses on the spoilage effect of microorganisms on myofibrillar protein. For example, studies have confirmed that the strong spoilage effect ofPseudomonas fragi[1-2]andAeromonas salmonicida[3]is mainly caused by the extracellular proteases degradation of myofibrillar protein. However, chilled chicken is abundant in collagen, especially on wings, feet, and other skin-on products of cutting parts. Overall, the rich nutrient compositions make it extremely susceptible to microbiological contamination during production and distribution[1,4-5].
Storage of chilled chicken under refrigerated conditions will produce negative sensory attributes in microbiological, pH, color and descriptive sensory, leading to consumers’ perception changing[6].Various bacteria have been conf irmed to be involved in the spoilage process of chicken, such as lactic acid bacteria, Enterobacteriaceae,Pseudomonas,Acinetobacter,SerratiaandShewanella[5,7-8]. Most of them can secrete diverse enzymes that promote the spoilage of meat.Pseudomonas fragican produce extracellular protease AprD to contribute to the spoilage of chilled meat[2]. Meat-borneBacillus cereusisolated from beef exerted genes related to protease and lipase production resulting in food spoilage[9].PseudomonasandShewanellacan produce lipases that hydrolyze triglycerides into fatty acids, glycerol, and proteases that degrade muscle and connective tissue, inducing bacterial proliferation[8]. However, the collagen in the chilled chicken products is the first object to be degraded by spoilage microorganisms. So far, there has been limited studies of spoilage bacteria with strong collagenase activity and the relevant research has been limited.
This study aimed to screen for spoilage bacteria isolates with high-producing collagenase from chilled chicken parts enriched with collagen, and elucidate their spoilage potential. This will provide a deep insight into the spoilage process of chilled chicken, which is of great significance for preserving chilled chicken.
2. Materials and methods
2.1 Bacteria and culture conditions
A total of 17 strains capable of producing collagenase and lipase were previously isolated from spoiled chilled chicken feet. The chilled chicken feet were originally purchased from local supermarkets in Nanjing, and the samples were aseptically transferred to the laboratory in an ice box within 2 h. Then the samples were stored at 10 °C until spoilage before isolation. All strains were stored at–80 °C in trypticase soy broth (TSB, Qingdao Hope Bio-Technology Co., Ltd., China) supplemented with 25% glycerin. These strains were activated on tryptic soya agar (TSA, Qingdao Hope Bio-Technology Co.,Ltd., China) plates and incubated at 30 °C for 24 h. Thereafter, a single colony of each strain was incubated in 6 mL TSB at 30 °C for 24 h.
2.2 Evaluation of spoilage ability
The raw-chicken juice agar (RJA) plate referred to the method of Wang et al.[1]with appropriate modifications. The chilled boneless chicken feet were homogenized with 0.1 mol/L phosphate buffered saline (PBS, pH 6.2) at a ratio of 1:100, and filtered by gauze. Then the homogenate was mixed with 1% agar (m/V) to produce the culture medium. The solidified plate was sterilized (Hangyu Irradiation Technology Co., Ltd., Nanjing, China) using60Co irradiation at an 8 kGy dose to eliminate background flora[1]. The prepared RJA plate is shown in Fig. S1. The spoilage bacteria were incubated in TSB at 30 °C for 24 h, and the optical density (OD600nm) of the bacterial solution was adjusted to 0.4. A 2.5 μL of the bacterial solution was inoculated on the RJA plates at 30 °C. This experiment was carried out using three replicates. The bacteria colony diameters (BCDs)and decomposition zone diameters (DZDs) were recorded daily,and the spoilage bacteria which produced decomposition zone on RJA were chosen. Subsequently, they were sent to the Biozeron(BiozeronTechnologies Co. Ltd., Shanghai, China) for 16S rDNA identification and the amplified sequences of each strain were analyzed by the nucleotide blast (http://blast.ncbi.nlm.nih.gov/Blast.cgi) in the NCBI database.
2.3 Gelatin zymography
The spoilage bacteria were incubated in 6 mL of TSB at 30 °C for 2 days, then centrifuged at 12 000 ×gto remove the bacteria,and filtered by 0.22 μm membranes to obtain the extracellular crude enzyme solution. The gelatin zymography referred to the method described by Talebi et al.[10]. The crude enzyme solution and 4 × SDS loading buffer were mixed evenly, and loaded with 10 μL in the gelatin zymogram gels (Thermo Fisher Scientific Co., Ltd.,China). The collagenase type I and IV were loaded with 10 μL. The negative control was a blank growth medium. The loading buffer was a non-denaturing reducing condition. Electrophoresis was run at 80 V for 30 min and then followed at 110 V for 100 min.
The gel was shaken for 40 min in elution buffer (pH 7.5)which contained 50 mmol/L Tris-HCl, 5 mmol/L CaCl2and 2.5%TritonX-100 to remove SDS and renature the enzyme, then rinsed in buffer (pH 7.5) which contained 50 mmol/L Tris-HCl and 5 mmol/L CaCl2for 20 min. The elution process and rinsing process were both performed twice. Subsequently, the gel was put in buffer (pH 7.5)containing 50 mmol/L Tris-HCl, 5 mmol/L CaCl2and 150 mmol/L NaCl for 42 h incubation at 30 °C. The gel was then put in Coomassie blue fast staining solution for 20 min and was scanned by the Universal HOOD II Gel Imaging System (Bio-Rad, USA).
2.4 Collagenase activity
The preparation of the extracellular crude enzyme solution referred to section 2.3. The collagenase activity was determined using collagenase assay kits (Shanghai Milbo Biotechnology Co., Ltd.,China) following the manufacturer’s instructions. This experiment was performed using four replicates.
2.5 Lipase activity
The preparation of the extracellular crude enzyme solution was referred to section 2.3. The determination of lipase activity referred toChina National Food Safety Standard methods-Lipase Preparations(GB 23535–2009). Polyvinyl alcohol solution (4%) was mixed with olive oil at a ratio of 3:1 (V/V) and homogenized for 10 min at 12 000 ×gto make the substrate solution. Substrate solution (4 mL)and phosphate buffer (5 mL, pH 7.5) were pre-incubated at 40 °C for 5 min in a water bath. Crude enzyme solution (1 mL) was mixed and counted immediately for reaction in the water bath. The reaction was terminated with 15 mL ethanol (95%) after 15 min. The blank group was first added with 15 mL of 95% ethanol, pre-incubated in a water bath for 5 min, and then added to the crude enzyme solution for 15 min.Two drops of phenolphthalein indicator were added to the blank and sample solutions after the reaction was completed. The reacting solution was titrated with 0.05 mol/L NaOH until slightly red for 30 s without fading. The volume of NaOH consumed was recorded to calculate lipase activity. This experiment was performed using four replicates. One unit (U) of lipase activity was defined as the amount of lipase which liberated 1 μmol titratable fatty acid per minute and calculated as follows:
WhereV1represented the amount of NaOH solution consumed by the experimental group (mL);V2represented the amount of NaOH solution consumed by the blank group (mL);cNaOHrepresented the concentration of the NaOH (mol/L);trepresented the reaction time (min).
2.6 Growth curve
Four spoilage bacteria were diluted to 102CFU/mL, and 200 μL of bacteria solution was added to cell culture plates before using the automatic growth curve analyzer (FP-1100-C, Bioscreen, Finland).The growth curves were measured at 10 and 30 °C respectively,and the results were expressed as OD600nm. The Logistic, Gompertz and Hill equations were fitted respectively by Origin 2018 (Origin Lab Corporation, Northampton, MA, USA) to establish the optimal primary model. The equations of Logistic, Gompertz and Hill models are shown in formula (2–4), respectively.
Where in formula (2)yrepresented the OD600nmof bacterial solution atxmin,A1represented the maximum OD600nmof the bacterial solution,A2represented the OD600nmof the initial bacterial solution,x0represented the time taken to reach the maximum growth rate (min),prepresented the maximum specific growth rate atx0min;where in formula (3)yrepresented the OD600nmof bacterial solution atxmin,awas the difference between OD600nmof the maximum bacterial solution and OD600nmof the initial bacterial solution,xcrepresented the time it takes for reaching its maximum growth rate (min),krepresented the maximum growth ratio corresponding to timexc; where in formula (4)ywas the OD600nmof bacterial solution atxmin,Vmaxrepresented the maximum growth rate, andkrepresented the time taken to reach the maximum growth rate (min),nwas the maximum specific growth rate at timek.
2.7 Assessment of spoilage potential in situ
2.7.1 Inoculation of spoilage bacteria in chicken feet
The boneless chicken feet were put into sterile homogenized bags and sent for irradiation sterilization with ice bags[1]. One milliliter of bacterial suspension (103CFU/mL) was added to the sterilized samples, while the control group was inoculated with 1 mL of 0.85%sterile saline. All samples were stored at 10 °C for 7 days, and taken daily to determine microbial and biochemical analyses. These experiments below were all performed using five replicates.
2.7.2 Colony counts and total volatile basic nitrogen (TVB-N) analysis
Total viable counts of samples during storage referred toChina National Food Safety Standard methods-Microbiological examinationof food: aerobic plate count(GB 4789.2–2016). Briefly, sterile saline(180 mL, 0.85%) was added to 20 g samples in sterile homogenization bags and thoroughly homogenized by a stomacher blender (BagMixer 400VW, Interscience, France) for 2 min. The initial inoculations were about 3 (lg (CFU/g)). The homogenized solutions were diluted at a 1:10 gradient; 1 mL of diluent was added to plate count agar plates (PCA, Qingdao Hope Bio-Technology Co., Ltd., China) and incubated at 30 °C for 48 h. All results were expressed as lg (CFU/g).The TVB-N in the samples was determined by referring to the semimicro nitrogen determination ofChina National Food Safety Standard methods-Determination of total volatile basic nitrogen in food(GB 5009.228–2016). For TVB-N, a sample of 4 g added to 30 mL of distilled water was shaken for 30 min and filtered through gauze.Then 10 mL of filtrate was added with 1 g of MgO before analyzing by FOSS 2300 automatic apparatus (FOSS, Denmark). The TVB-N content was expressed as mg TVB-N per 100 g sample.
2.7.3 Sensory evaluation
The sensory evaluation referred to the method described by Patsias et al.[11]and Zhang et al.[12]. A scoring team of five laboratorytrained researchers rated the overall acceptability of samples, with whole acceptability including odor, color, elasticity, viscosity, and leachate status. A 9-point Hedonic scale was used, with the scoring scale (1 to 9) denoted as follows: 9, excellent; 8, very good; 7, good;6, acceptable; 5, between acceptable and unacceptable; 4, slightly unacceptable; 3, moderately unacceptable; 2, very much unacceptable;1, extremely unacceptable. Poor indicated that a sample was considered spoiled when the sensory score reached 6 or less.
2.7.4 Enzyme activity in situ
The samples were placed in sterile homogenization bags with 15 mL of 0.85% sterile saline and shaken by a stomacher blender(BagMixer 400VW, Interscience, France) for 2 min. The obtained eluate was centrifuged at 12 000 ×gand passed through 0.22 μm membranes to remove bacteria. The determination of collagenase and lipasein situreferred to sections 2.4 and 2.5, respectively.
2.8 Data analysis
SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA)was used for the statistical analysis of the data, and Duncan’s multiple range test was used for the analysis of variance (ANOVA) (the level of statistical significanceP< 0.05). The Origin 2018 (Origin Lab Corporation, Northampton, MA, USA) software was used for figures,and the results were displayed as mean ± standard deviation (SD).
3. Results and discussion
3.1 Evaluation of spoilage ability
Seventeen strains were explored whether they could utilize the plentiful collagen nutrients of chicken feet according to the decomposition zones on the RJA plates. This was a simple and effective method to evaluate the spoilage ability of bacteria. There were significant differences in DZDs among tested strains (Fig. 1).Seven strains that could form decomposition zones on RJA were selected, which were F5, G3, G4, G6, G7, G8, and H8 (Fig. 1).The DZDs of the other strains that did not produce decomposition zone was 0 mm. The above 7 strains were identified by 16S rDNA sequencing, and the obtained sequences were compared with those in the GenBank database by BLAST. And the results were shown in Table 1. The DZDs of F5 and H8 were significantly larger than those of the other tested strains, indicating that F5 and H8 had the strongest spoilage ability. The DZD of G7 was the largest one inPseudomonas,and G8 was the smallest among all the isolates. Spoilage bacteria,such asPseudomonas fragi[13]andAeromonas[1], were confirmed to produce extracellular proteases, resulting in the degradation of chicken proteins into smaller peptides. This can promote the spoilage process of the chilled chicken. Thus, the results of the RJA test indicated that the 7 isolates may also secrete certain extracellular enzymes that promote spoilage.
Table 1 Identification of the isolates.

Fig. 1 The DZDs and BCDs profiles of RJA plate on 7th day (n = 3).Different letters between BCDs and between the DZDs indicate significant differences (P < 0.05). Values are means ± SD. F5 was Serratia liquefaciens,H8 was Aeromonas salmonicida, G3, G4, G6, G7 were Pseudomonas saponiphila, and G8 was Aeromonas veronii. The same below. Other bacteria were not identified.
3.2 Gelatin zymography
As demonstrated in Fig. 2, the bands with collagenase were detected. F5 and G3, G4, G6, G7 had obvious degradation bands around 50 kDa, while G8 and H8 had degradation bands around 65 and 95 kDa, respectively. The bands indicated that the collagenase activities and molecular weights differed. Microbial collagenase can hydrolyze natural triple helix or denatured collagen, a tightly coiled triple helical molecule composed of threeα-chains, mainly repetitive Gly-X-Y sequences (X and Y are usually proline and hydroxyproline)[14-15].Gelatinolytic bands were obvious inAeromonasby gelatin zymography and the presence of metalloprotease was around 84 and 93 kDa[16], which was similar to the band of H8 in this study.
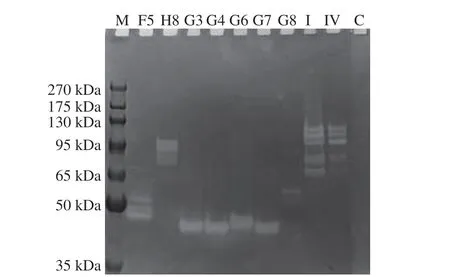
Fig. 2 Gelatinase activity electrophoresis analysis. M, Molecular weight markers; C, The blank medium; I, Type I collagenase; IV, Type IV collagenase.

Fig. 3 (A) Collagenase activity and (B) lipase activity of F5, H8, G7 and G8(n = 4). Different letters indicate significant differences (P < 0.05). Values are means ± SD.
3.3 Activities of collagenase and lipase
The DZDs of F5 and H8 were the largest two strains in RJA testing, and G7 was the largest one amongPseudomonas, and G8 was the smallest one among all the isolates. The results of collagenase and lipase activities of the spoilers are shown in Figs. 3A and B. Although lipase activity between the 4 strains did not vary, a significant difference was observed in collagenase activities between them(P< 0.05). Specifically, F5 had significantly greater collagenase activity than H8, G7 and G8. Microorganisms can secrete various enzymes linked to meat spoilage, including lipase and protease.Bacillusspp.[9],Brochothrix thermosphacta[17], andSerratia[18-20]isolated from meat and meat products could secret lipase and protease,exacerbating meat spoilage.
3.4 Growth curve
The growth curves of F5, H8, G7 and G8 are illustrated in Fig. 4.G8 grew fastest at 30 °C, and G7 grew slowest. However, H8 growth was the fastest, and the OD600nmof F5 was the highest at 10 °C,suggesting that F5 and H8 were more conducive at low temperatures.The primary model of microorganisms was mainly used to describe the growth law of microorganisms that form an S-shaped change trend[21]. The fit effect of the model was analyzed by comparing the correlation coefficientR2. The closerR2was to 1, the better the fitting effect of the model. The growth curves obtained at 30 and 10 °C were fitted with the Logistic, Gompertz and Hill equation to establish and determine the optimal primary model (Fig. 4). TheR2of the model at 30 °C, fitted by the Logistic equation of all four strains, was greater than that of the Gompertz and Hill equations (Tables S1 and S2),indicating that the former model was closer to the true value. Hence,the Logistic equation could be chosen as the optimal primary model at 30 °C. Similarly, TheR2of the Logistic model had a better fit at 10 °C than other models. Logistic growth models are widely used to describe bacterial growth, and it is necessary to understand the growth abilities of these bacteria we screened. Logistic was also reported that it had reasonably good predicted results for the observed data[22].Therefore, the model developed in this experiment can be used as a quantitative basis for evaluating of the growth ability of these spoilage bacteria, which can then be used as a reference value for controlling spoilage bacteria growth.
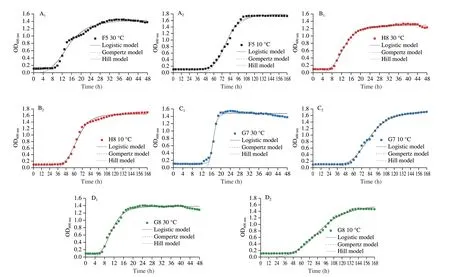
Fig. 4 Kinetic models of (A) F5, (B) H8, (C) G7 and (D) G8 at (A1–D1) 30 and (A2–D2) 10 °C.
3.5 Assessment of spoilage ability in situ
The growth and proliferation of spoilage microorganisms can lead to rapid spoilage processes. Based on the RJA results, F5,H8, G7 andG8 were inoculated into raw chicken feet to access the spoilage potential, and the change in the total viable count during storage is presented in Fig. 5A. The total viable counts in the blank group remained unchanged, indicating the efficacy of irradiation in eliminating the samples. F5 showed more rapid growth than the other strains, while the growth of H8 and G8 was relatively similar throughout the storage period, with G7 growing the slowest.The total viable counts of F5, H8, and G8 reached 7.37, 7.06, and 7.01 (lg (CFU/g)) on the 3rdday exceeding 6 (lg (CFU/g)). They exceeded the limit for fresh meat and poultry products according to the National Standard GB 2749–2015 and were considered spoiled.However, G7 was over 6 (lg (CFU/g)) on the 4thday. This result also corroborated with the growth ability at 10 °C.
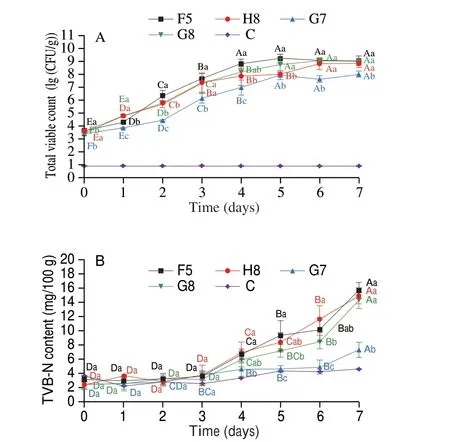
Fig. 5 Changes of (A) total viable count and (B) TVB-N content of chilled chickens during storage at 10 °C for 7 days. Values are means ± SD (n = 5).Different uppercase letters indicate significant differences of the same strain between different days (P < 0.05). Different lowercase letters indicate significant differences between different groups on the same day (P < 0.05).C was blank control.
Furthermore, the growth trend of total viable counts demonstrated an S-shape over time. The primary model was established and determined by fitting the Logistic, Gompertz and Hill equations to the total viable countsin situ. Fig. 6 and Table S3 highlight the fitted curves and growth kinetic models, respectively. The primary models were comprehensively analyzed, and the Logistic model was closer to 1,indicating the most suitable model to describe the growth kinetic parameters.
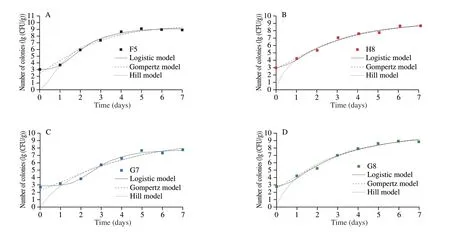
Fig. 6 Kinetic models of (A) F5, (B) H8, (C) G7 and (D) G8 in situ.
TVB-N refers to volatile nitrogen-containing substances such as organic amines produced by the degradation of proteins and other nitrogen-containing compounds during food spoilage[23]. The TVB-N content of the control group remained relatively constant throughout the storage period (Fig. 5B). The result did not differ significantly from those in the control group in the first 3 days. The TVB-N generation process is usually accompanied by a delay period that slight change in TVB-N concentration[3,23]. The TVB-N contents of G7 and G8 were significantly lower than those of F5 and H8 in the later storage period. Although F5 and H8 did not vary, they were significantly higher than G7, indicating that F5 had a stronger ability to break down proteins. The higher value of H8 compared to G8 indicated a stronger decomposition capability of H8. The rapid growth of bacteria induces the secretion of extracellular enzymes to decompose proteins and other nutrients, exacerbating TVB-N contents[24].
Furthermore, TVB-N content often reaches adapt an exponential increasing phase after a lag phase because bacteria need to produce various enzymes to adapt to the environment. Hence, the spoilage levels of different meats were unlikely by specifying a single TVB-N acceptable threshold of ≤ 15 mg/100 g which is the limit of the National Standards of China for ‘fresh’ meat and poultry products[23].The significant differences variation in TVB-N produced in meats is due to changes in TVB-N precursor concentrations and microbiota composition[23]. Besides, the decomposition rate and consumption of nutrients in meat were also different in the growth stages of microorganisms, resulting in changes in TVB-N content[25]. Thus,the spoilage of meats enriched with collagen may be different from ordinary meat.
The changes in sensory scores during storagein situare shown in Fig. 7. The sensory scores of F5, H8 and G8 were already below 6 on the 3rdday, indicating that the samples were spoiled, while G7 was below 6 on the 4thday. The sensory assessment results were generally consistent with the total viable counts as a criterion for assessing spoilage. Microorganisms use nutrients such as protein, fat and carbohydrates in the growth and metabolism of meat matrix to produce spoilage metabolites. This resulted in deteriorated sensory profiles such as bad odor, surface stickiness and darker color,which are related to the biological characteristics and metabolic characteristics of spoilage bacteria[26]. Moreover, irradiation sterilization could also affect the color and odor and lead to the decline of the sensory score[27-28].
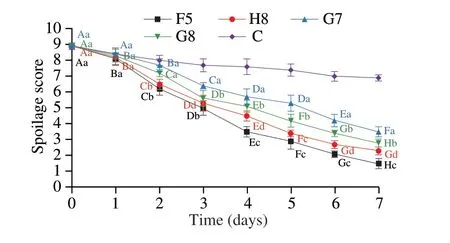
Fig. 7 Spoilage scores of chilled chickens during storage at 10 °C for 7 days.Values are means ± SD (n = 5). Different uppercase letters indicate significant differences of the same strain between different days (P < 0.05). Different lowercase letters indicate significant differences between different groups on the same day (P < 0.05).
Changes in lipase activity of spoilage strains duringin situshowed a gradual uptrend with the increase of storage time (Fig. 8A).The lipase activities of F5 and H8 were significantly higher than that of G7 and G8. Also, the changes in collagenase activityin situ(Fig. 8B) demonstrated a gradual increase in F5, significantly higher than other strains. Extracellular enzymes from microorganisms generally play an essential role in inducing and accelerating spoilage processes, including texture softening and TVB-N production[29].PseudomonasandShewanellawere reported that they could hydrolyze triglycerides into fatty acids and glycerol by lipases and degrade muscle and connective tissue by proteases, which could cause stronger spoilage and contribute to growth of bacteria when the nutrients on the surface of meat were completely used[8,30-31].SomePseudomonasspecies can even produce proteases and lipases even under low temperatures[32-33]. Also, metalloprotease activity and expression inPseudomonas fluorescensat 4 °C was higher than at 25 °C[34], and similar observations were reported for lipase activity[35]. Hence, F5 and H8 could exhibit stronger enzyme activity at low temperatures, leading to spoilage of the meat.
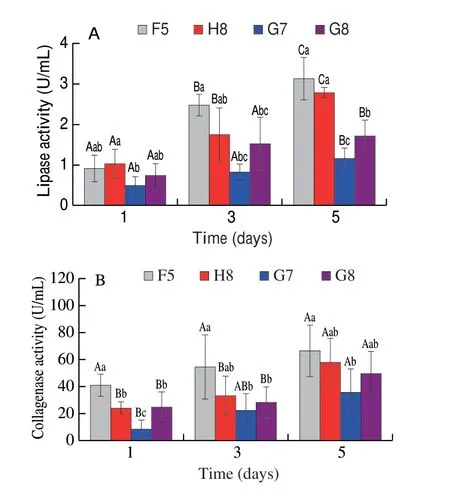
Fig. 8 Changes of (A) lipase activity and (B) collagenase activity of 4 spoilage bacteria during meat storage in situ. Values are means ± SD(n = 5). Different uppercase letters indicate significant differences of the same strain between different days (P < 0.05). Different lowercase letters indicate significant differences between different groups on the same day (P < 0.05).
4. Conclusion
In this study, 7 strains could produce decomposition zones on RJA plates, includingS. liquefaciens(F5),A. salmonicida(H8), 4Pseudomonas(G3, G4, G6, G7) andA. veronii(G8),in which F5 and H8 expressed stronger decomposition ability.Although the lipase activities did not differ among the strains,the collagenase activities of strain F5 were stronger than others.F5 and G3, G4, G6, G7 had apparent degradation bands around 50 kDa, while G8 and H8 had degradation bands around 65 and 95 kDa respectively. F5 performed stronger spoilage capacity including total viable counts, TVB-N, sensory scores, lipase activity and collagenase activity, which was consistent with RJA results. The finding suggested that spoilage bacteria that have strong collagenase in chilled chicken should be paid close attention. Future studies will focus on elucidating the spoilage mechanisms of collagenase secreted by high-producing bacteria, which will provide a great insight into extending the freshness and shelf life of chicken meat.
Declaration of competing interest
The authors declare that they have no known competing financial interests.
Acknowledgements
This work has been financed by grants from the Natural Science Foundation of Jiangsu Province in China (BK20221515) and the National Natural Science Foundation of China (32172266).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.26599/FSHW.2022.9250118.
- 食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango

