Veratrilla baillonii Franch alleviate the symptoms of diabetes in type 2 diabetic rats induced by high-fat diet and streptozotocin
Chunlan Yan, Zhihao Zhang, Liqun Ma, Xinyi Xu, Muhamma Azhar,Xianju Huang,e,, Jianxun Shi, Jun Li
a College of Life Sciences, South-Central Minzu University, Wuhan 430074, China
b College of Pharmaceutical Science, South-Central Minzu University, Wuhan 430074, China
c Department of Biosciences, COMSATS University Islamabad, Sahiwal 57000, Pakistan.
d Zhejiang Pharmaceutical University, Ningbo 315100, China
e Ethnopharmacology Level 3 Laboratory, National Administration of Traditional Chinese Medicine, South-Central Minzu University, Wuhan 430074, China
Keywords: Veratrilla baillonii Franch Type 2 diabetes mellitus Liver injury Skeletal muscle GLUT4
ABSTRACT Our previous research studies have shown that Veratrilla baillonii Franch, a food supplement used by ethnic minorities in Southwest China, has multiple pharmacological activities, such as detoxif ication, antiinf lammatory, antioxidant, and anti-insulin resistance. However, the detailed signal pathways for its salutary effect on damages in multiple organs due to type 2 diabetes mellitus (T2DM) remains unclear. The current study is to evaluate the therapeutic effects of V. baillonii on T2DM rats and to explore the underlying mechanisms. The T2DM rat model was successfully established by a high-sugar and high-fat diet (HFD)combination with intraperitoneal injection of a small dose of streptozotocin (STZ, 35 mg/kg). Biochemical analysis and histopatholgical examinations were conducted to evaluate the anti-diabetic potential of water extracts of V. baillonii (WVBF). The results showed that the WVBF treatment can improve hyperglycemia and insulin resistance, ameliorate the liver, kidney and pancreas injuries via decreasing inf lammatory cytokines such as IL-6 and TNF-α, and oxidative damages. Further investigation suggested that WVBF modulates the signal transductions of the IRS1/PI3K/AKT/GLUT4 and AMPK pathways. These f indings demonstrate potentials of WVBF in the treatment of T2DM and possible mechanisms for its hepatoprotective activities.
1. Introduction
Type 2 diabetes mellitus (T2DM) possess an enormous burden on patients and the health care system and is one of the leading causes of death worldwide[1]. According to the data from the International Diabetes Federation, 415 million people are currently suffering from diabetes, and this number is expected to continuously rise to more than 700 million by 2045[2]. T2DM is characterized by insulin resistance, and is thought to make up 90%–95% of the total diabetes mellitus cases[3]. An accumulating body of evidence indicated that insulin resistance can strengthen the pathological progression of multi-organ impairments, including kidney and liver in patients.In the past, studies have been mainly focused on nerve, vascular and renal damages in diabetes. The liver is critical to regulate blood glucose homeostasis. In recent years, diabetic liver injury has received increasing attention with more and more cases of diabetes[4]. As an important insulin-sensitive organ, the liver maintains glucose homeostasis via its ability to store and release glucose[5].Inf lammation, together with disturbance of systemic and hepatic fat metabolism, is mainly regarded as causative factor in diabetic liver injury[6]. Hyperglycemia increases the production of free radicals,and induces oxidative stress, thus leading to liver injuries[7]. Thus, the effective control of inflammation, oxidative stress, and hyperglycemia is very important for curbing the diabetic liver injury.
Gentianaceae plants are widely used in medicine or tea for curative purposes. For instance, the consumption of bitter gentian teas gains in popularity among the nomadic people of Siberia.These teas have been extensively used as a remedy to treat diverse digestive disorders involved in the food consumptions[8]. Gentian root extract, a bitter food additive registered and used in Japan, contains sweroside and gentiopicroside with the later one as one of two major components.VeratrillabailloniiFranch, a member of Gentianaceae family, is used widely as a folk dietary supplement for its diversity health-improving qualities, including anti-toxic and liver protection properties (Fig. 1A)[9].V.bailloniihas been widely used in the treatment of hepatitis-induced jaundice and drug-induced hepatitis for decades[8]. Gentiopicroside, sweroside and swertiamarin are the characteristic components of the medicinal plants of Gentianaceae used in the practice of traditional Chinese Medicine[10]. Our previous work has confirmed that the water extracts ofV.baillonii(WVBF),which are also rich in sweroside, swertiamarin and gentiopicroside[11],can exert anti-hepatotoxic effect induced by aconitum plants[11]and possess insulin-mimicking effects by regulating glucose metabolism and the insulin resistance[12]. However, the protective mechanism on diabetic liver injury remains to be discussed more thoroughly.
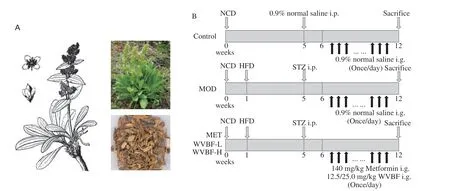
Fig. 1 The plant of V. baillonii Franch (A) and the schematic diagram of animal treatment (B).
In this study we evaluated the antidiabetic effect of WVBF on T2DM rats induced by a high-fat and high-sugar diet (HFD)combined with streptozotocin (STZ) injection. Further, the underlying mechanism of the WVBF on improving multiple organ injuries induced by diabetes was elucidated. The results could give a systematic explanation of the protective effect of WVBF on T2DM.
2. Materials and methods
2.1 Materials
Streptozotocin (STZ) was obtained from Saiguo Biotechnology Co., Ltd. (Guangzhou, China). Metformin hydrochloride was obtained from Aladdin Biotechnology Co., Ltd. (Shanghai, China). Blood glucose test paper and blood glucometer purchased from Sinocare Biosensor co. (Changsha, China), Rat Insulin ELISA kit was obtained from Beyotime Biological Technology Co., Ltd. (Shanghai, China).Tumor necrosis factor alpha (TNF-α) ELISA kit and interleukin 6(IL-6) ELISA kit were obtained from Raybiotech Co., Ltd.(Guangzhou, China). Superoxide dismutase (SOD), malondialdehyde(MDA) and glutathione peroxidase (GSH-Px) assay kits were obtained from Nanjing Jiancheng Bioengineering Research Institute Co., Ltd. (Nanjing, China).
2.2 Pharmaceutical preparation
V.bailloniiFranch (Dali Herb Co., Ltd., Yunnan Province, China,Lot: S20140710) was identified by Dr. Xinqiao Liu at the University of Central South University for Nationalities, and the voucher specimen (specimen no. HSN13098) was stored in the herbarium of the University (Fig. 1A). WVBF was prepared according to the method described in our previous work[11]. The extracted powder was stored in a desiccator and diluted with distilled water to the specified concentrations before use.
2.3 Animals and model establishment
Sixty male Sprague Dawley (SD) rats weighing 160–180 g were purchased from the experimental animal center of Huazhong Agricultural University. The production license number of experimental animals is SCXK - (E) 2020-0019, and the use license number of experimental units is SCXK (E) 2021-0089. The animals were kept in the SPF animal room of the experimental animal center of the Central-South University for Nationalities in strict accordance with the regulations of the animal ethics committee of Central-South University for Nationalities and the international NIH guidelines. The feeding room was kept under the normal temperature ((22 ± 2) °C)and air humidity ((60 ± 10)%), and the dark/light cycle was 12/12 h.
The animal treatments and experimental design were shown in Fig. 1B. After one week of acclimatization, 60 rats were randomly divided into cages. Eight rats were included in the normal control(NC) group and given normal chow diet. The remaining 52 rats were fed the HFD for 4 weeks, and then received a single intraperitoneal injection of STZ. Immediately prior to injection, dissolve the STZ in 50 mmol/L sodium citrate buffer (pH 4.5) to a final concentration of 3.5 mg/mL, and inject the STZ solution at 35 mg/kg to produce a T2DM model. Fasting blood glucose (FBG) was measured after 1 week. The animals, whose FBG over 11.1 mmol/L were considered diabetic and finally 32 diabetes rats were confirmed and used in this study. The T2DM rats were randomly divided into 4 groups:
1) a model group, T2DM rats with saline orally, refer as MOD;
2) a positive control group, administrated via oral gavage with 140.0 mg/kg metformin dissolved in 0.9% saline, referred as “MET”;
3) the WVBF low dose group, administrated via oral gavage with 12.5 mg/kg WVBF dissolved in 0.9% saline, refer as “WVBF-L”;
4) the WVBF high dose group, administrated via oral gavage with 25.0 mg/kg WVBF dissolved in 0.9% saline, refer as “WVBF-H”.
The dosage of WVBF abided by our previous investigation[11]and affirmed by pilot study. NC group was given an equal volume of saline. Eight rats in each group were administered the respective reagents once a day for 6 weeks.
2.4 Body weight, FBG and organ index measurement
During the experiment period, the rats were observed weekly for general status, including mental status, reactivity, activity, diet,urine volume and hair color, etc. The FBG of each rat was measured weekly after fasting from food for 12 h after drug intervention. At the end of the experiment, rats were euthanized for tissue collection. The organs such as liver and kidney were dissected and rinsed with saline,the surface water was blotted out with filter paper, and organs were weighed and the organ coefficient was calculated as below:
2.5 Oral glucose tolerance test (OGTT)
At the end of 5thweek of WVBF treatment, an OGTT was performed as described in the literature[13]. Rats were fasted for 12 h with free access to water. Glucose solution at a concentration of 30% (m/V) was given by gavage at 2 g/kg 30 min after WVBF administration. The tail tip whole blood glucose levels were measured at 0, 30, 60, 90, and 120 min using a glucometer, and the area of the curve (AUC) was calculated according to the following equation.
2.6 Homeostatic model assessment of insulin resistance(HOMA-IR) and HOMA-β cell function index (HOMA-β)
At the end of drug treatment, rats of all groups were fasted overnight without water and then whole blood was collected from the rat abdominal aorta under general anesthesia. The serum was aspirated, divided, and stored at -80 °C for detection of blood biochemical parameters. Fasting serum insulin was determined using an ELISA kit (Beyotime Biological Technology Co., Ltd., Shanghai,China) following the manufacturer’s instructions. HOMA-IR and HOMA-β calculated from FBG and FINS.
2.7 Biochemical analysis
2.7.1 Serum biochemical analysis
The serum biochemical analysis was conducted using fully automatic biochemical analyzer (Myriad Technology Co., Shenzhen,China). Liver function indexes including alanine aminotransferase(ALT), aspartate transferase (AST), alkaline phosphatase (ALP) andγ-glutamyltranspeptidase (γ-GT), the lipid function indexes including triglyceride (TG) and total cholesterol (TC), and the pancreatic function indexes includingα-amylase (α-AMY) and serum lipase(LIP) were measured.
2.7.2 Evaluation of oxidative stress indices and inflammatory cytokines in the liver
After blood was removed from the rat abdominal aorta and executed, intact rat liver tissues were removed and transferred to a-80 °C refrigerator for storage. SOD activity, GSH-Px and MDA contents in liver tissues were determined using commercial kits(Nanjing Jiancheng Biological Engineering Research Institute Co.,Ltd., Nanjing, China). IL-6 and TNF-α levels of liver tissues were determined using ELISA kits (Riboao Biotechnology Co., Ltd.,Guangzhou, China). Concentration of tissue protein was determined using bicinchoninic acid (BCA) protein quantitative assay kit(Kangwei century Biotechnology Co., Ltd., Beijing, China) and used to normalize the indicated indices.
2.8 Histopathological analysis
Histopathological analysis of tissue was conducted by Seville Biotechnology Co., Ltd., Wuhan, China. The liver, kidney and pancreas were removed and immersed in 4% paraformaldehyde fixative for fixation, and the epididymal adipose was fixed in fatspecific fixative. Tissues fixed for 24 h were paraffin embedded and hematoxylin and eosin (H&E), oil red O staining, Sirius red,Periodic Acid-Schiff (PAS) and Masson staining were conducted respectively to observe pathological changes. Liver paraffin was cut for Sirius scarlet andα-SMA immunohistochemical analysis.As for diaminobezidin (DAB) staining, the nuclei of the cells were re-stained, dehydrated and sealed, and examined microscopically. All sections were analyzed after digital full-field scanning and imaging.
2.9 Western blot analysis
Western blot detection of IRS1/PI3K/AKT proteins in the liver and IRS1/PI3K/AKT/AMPK/GLUT4 proteins in the soleus muscle was performed as described in the literature[14]. Tissues were homogenized with RIPA buffer (containing protease inhibitor mixture) and incubated on ice for 30 min. Total proteins were extracted by differential centrifugation (13 000 ×g,15 min at 4 °C) and protein concentrations were determined by BCA assay kit.Equal amounts of protein (40 μg) were loaded for electrophoresis, and then electrophoretically transferred to nitrocellulose membranes (Pall Corporation, Pensacola, FL, USA). After blocking with 5% (m/V)milk, antigens including p-PKC-θ, PKC, p-IRS1, IRS, p-PI3K,p-AMPK, AMPK, p-AKT, AKT, p-GSK3, GLUT4 were detected with primary antibodies at (1:1000), followed by secondary antibodies(horseradish peroxidase-coupled anti-goat IgG) at 1:10 000.Immunoreactive bands were observed using an ECL assay kit (ABP Biosciences, USA). After processing the strips, the grayscale of the strips was detected by Image J software.
2.10 Statistical analysis
Data were expressed as mean ± standard error (SEM). All data between groups were statistically analyzed using SPSS 26.0 software,and statistical differences between groups were analyzed using one-way analysis of variance (ANOVA) and the least significant difference (LSD) test comparing normally distributed data with a post hoc test. Statistical significance was set whenP< 0.05.
3. Results
3.1 WVBF can improve the general condition and body weight in T2DM rats
The results of clinical observation were shown in Fig. 2A.The rats in the NC group had smooth and lustrous fur. The rats in the MOD group gradually showed coarse and yellow fur, while the rats in the WVBF-L and WVBF-H groups both showed obvious improvement compared with the MOD group to some degrees.
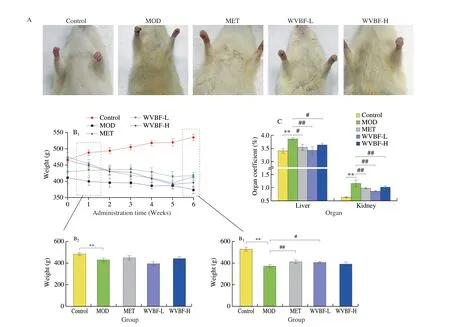
Fig. 2 Antidiabetic effects of WVBF on T2DM rats. (A) The physical appearances; (B) the body weight gain; (C) the organ coefficients of the liver and kidney;(D) the FBG levels; (E) the results of OGTT; (F-I) thee insulin resistance indexes of AUC, serum insulin, HOMA-IR, HOMA-β cell. Mean ± SME, *P < 0.05,**P < 0.01 v.s. NC group; #P < 0.05, ##P < 0.01 v.s. MOD group.
As shown in Fig. 2B, after 6 weeks of administration, the body weight gain of rats in the MOD group was significantly lower than that in the NC group (P< 0.05), which could be attenuated by both L-WVBF and H-WVBF treatments (P< 0.05). The organ coefficients of liver and kidney were shown in Fig. 2C. Compared with that of the NC group, both the liver and kidney coefficients of the MOD group were significantly increased (P< 0.05), which were decreased after the treatment with WVBF (12.5 mg/kg) for 6 weeks (P< 0.05).
3.2 WVBF treatment improved glucose metabolism in T2DM rats
The FBG levels of rats in each group were continuously monitored for 6 weeks. As shown in Fig. 2D, the FBG level of the MOD group continued to increase and reached a peak at the 4thweek.After 3 weeks of treatment, the FBG in the MET and WVBF groups began to drop. At the 6thweek of treatment, the FBG levels in the WVBF-L and WVBF-H groups were significantly lower than that in the MOD group (P< 0.05). This indicated that WVBF intervention had a significant hypoglycemic effect in rats with T2DM.
Since a feature of T2DM is glucose intolerance, we conducted the OGTT. The OGTT results were shown in Figs. 2E-F. The WVBF treatment at concentrations of both 12.5 and 25.0 mg/kg improved the glucose tolerance, which was impaired in the MOD group. These results suggest that the WVBF treatment can significantly improve the glucose control in T2DM rats.
3.3 WVBF treatment enhanced insulin sensitivity in T2DM rats
The effects of WVBF on insulin resistance in rats with T2DM model are shown in Figs. 2G-I. After 6 weeks of treatments, the FINS levels of rats in the MOD group were significantly decreased compared with that in the NC group (P< 0.05), which could be attenuated in both WVBF-L and WVBF-H groups. Compared with NC group, the level of HOMA-IR index was markedly enhanced,but the levels of serum insulin and HOMA-β were reduced in T2DM rats. These were reversed after 6 weeks of WVBF administration at concentrations of 12.5 and 25.0 mg/kg. The aforementioned result indicates that WVBF (12.5–25.0 mg/kg) can reduce insulin resistance and improve pancreatic β-cell functions.
3.4 WVBF treatment attenuated pathological damage in the liver, kidney, pancreas and epididymal adipose and improved liver function in T2DM rats
As shown in Fig. 3A, in the liver tissues, focal necrosis,inflammatory cell infiltration and steatosis edema were observed in the MOD group. Compared with that in the MOD group, the hepatocyte steatosis edema and inflammatory cell infiltration were significantly attenuated in the WVBF administration groups. In addition, Sirius red staining showed that many collagen fibers were stained red in the MOD group, and the collagen fibrosis in the liver was significantly reduced in the WVBF administration groups (Fig. 3B).
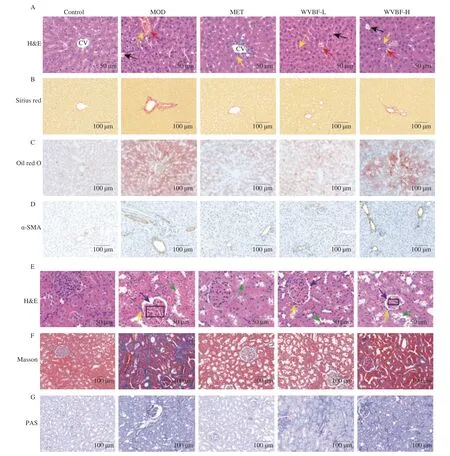
Fig. 3 Effects of WVBF on pathmorphism of liver and kidney tissues and liver function in T2DM rats. (A) H&E staining of the livers; red arrows represent hemorrhage; yellow arrows, inflammatory cells infiltration; black arrows, Steatosis; (B) Sirius red staining; (C) Oil red O staining and (D) α-SMA staining of liver;(E) H&E staining of the pancreas; green arrows represent edema and swelling of renal cortical cells; yellow arrows, inflammatory cells infiltration; black arrows,Baumann space; rectangle, glomerular morphology; (F) Masson staining and (G) PAS staining of the kidney. (H-K) ALT, AST, ALP and γ-GT levels.Mean ± SME, *P < 0.05, **P < 0.01 v.s. NC group; #P < 0.05, ##P < 0.01 v.s. MOD group.
As shown in Fig. 3C, the oil red O staining of the liver tissue showed that extensive neutral fat was stained red in the MOD group, and the hepatocyte neutral fat was significantly reduced in the WVBF administration groups, compared with that in the MOD group. The results of liver immunohistochemistry are shown in Fig. 3D. Compared with that of the NC group, the liver tissues of MOD group showed increased expression ofα-SMA with brownishyellow coloration and darker coloration, this especially at the confluent zone and fibrous septum, indicating that hepatic stellate cells were activated. After WVBF (12.5, 25.0 mg/kg) administration,α-SMA expression in the liver was significantly reduced, suggesting that WVBF can reduce hepaticα-SMA expression and improve liver fibrosis in T2DM rats. We also carried out liver function tests.As shown in Figs. 3H-K, compared with that of the NC group, the serum levels of AST, ALT, ALP andγ-GT in the MOD group were significantly increased (P< 0.05); these were significantly reversed by the WVBF administration (12.5 and 25.0 mg/kg).
As shown in Fig. 3E, in the kidneys, MOD group rats showed severe renal cortical cell damage, edematous degeneration and swelling, glomerular atrophy, widened Bowman space, and increased inflammatory cell infiltration, which could be ameliorated by WVBF(12.5 and 25.0 mg/kg) administration. Masson staining of renal tissues(Fig. 3F) showed that a large number of collagen fibers were stained blue in the MOD group. Compared with that in the MOD group, renal fibrosis was significantly improved in both WVBF-L and WVBF-H groups. In PAS staining of renal tissues, a large amount of glycogen was stained purple in the MOD group (Fig. 3G). Compared with the MOD group, the renal glycogen content was significantly improved by the WVBF (12.5 and 25.0 mg/kg) administration.
The pancreas is also one of the main target organs of T2DM damages. As shown in Fig. 4A, compared with that in the NC group,the islets in the MOD group had abnormal shapes and blurred borders,and the pancreas showed degenerative atrophy, dilated lobular ducts and inflammatory cell infiltration. Compared with that in the MOD group, the structures of the islets in the WVBF administration groups were restored, and the degree of dilatation of the lobular ducts and inflammatory infiltration were also alleviated. Consistent with these pathological results, biochemical tests indicated that WVBF treatment could significantly improve the decreased theα-AMY level and increased the LIP levels in the serum of the T2DM rats (Figs. 4E-F).

Fig. 4 Effects of WVBF on the pathomorphismof adopose and pancreas tissues in T2DM rats. (A) H&E staining of epididymal adipose tissue; (B-C) the TC and TG levels in serum; (D) H&E staining of pancreas; red arrows represent the lobular ducts; yellow arrows, inflammatory cells infiltration; black arrows, islet morphology; (E-F) the α-AMY and LIP levels in serum. Mean ± SME, *P < 0.05, **P < 0.01 v.s. NC group; #P < 0.05, ##P < 0.01 v.s. MOD group.

Fig. 5 Effects of WVBF on liver oxidative stress and inflammatory markers. (A-C) Oxidative stress indexes and (D-E) inflammatory indexes in the liver of T2DM rats. Mean ± SME, *P < 0.05, **P < 0.01 v.s. NC group; #P < 0.05, ##P < 0.01 v.s. MOD group.
Impaired homeostasis of lipid metabolism is one of the characteristics of T2DM. H&E staining of epididymal adipose tissue showed that the size of adipocytes increased and the density of adipocytes decreased in MOD group compared with NC group(Fig. 4D). However, the adipocytes became smaller after the WVBF administration, which indicated the increased insulin sensitivity.Figs. 4B-C depicted the effect of WVBF on serum lipid profiles. It was shown that the WVBF treatment inhibited the rise in the levels of serum TC, TG in T2DM rats.
The above results suggest that WVBF has protective effects on the liver, kidney, pancreas injuries and dyslipidemia in T2DM model rats.
3.5 WVBF treatments attenuated oxidative stress and inflammation in the liver of T2DM rats
As shown in Figs. 5A-C, the SOD and GSH-Px activities in the liver were significantly decreased and MDA levels were significantly increased in the MOD group, compared with that in the NC group.However, treatment with WVBF significantly reversed the imbalance of SOD activity, GSH-Px and MDA levels (P< 0.05). The results of inflammatory indexes are shown in Figs. 5D and E. The increased proinflammatory markers, the TNF-α and IL-6 were reduced in the WVBF treated T2DM rats.
These results showed that WVBF treatments alleviated the oxidative stress and the inflammation in the liver of T2DM rats.
3.6 WVBF regulates protein expression of IRS1/PI3K/AKT pathway in the liver of T2DM rats
The expression levels and activation states of components in the IRS/PI3K/AKT signaling pathway in the liver were assessed using Western blot. As shown in Fig. 6, the WVBF administration for 6 weeks reversed the decreased expression levels of IRS1, and phosphorylated p85-subunit of PI3K and AKT in the liver tissue,which were significantly decreased in the liver of the rats in the MOD group compared with that in the NC group (P< 0.05), indicating that WVBF can improve glucose metabolism in T2DM rats through modulating the IRS1/PI3K/AKT pathway.

Fig. 6 The effects of WVBF treatments on the protein expression levels of IRS1/PI3K/AKT pathway in the liver of T2DM rats. (A) Representative bands of Western blot; (B) densitometry analysis of IRS-1; (C) densitometry analysis of p-PI3K; (D) densitometry analysis of p-AKT. The experiments were independently repeated 3 times. *P < 0.05, **P < 0.01 v.s. NC group; #P < 0.05, ##P < 0.01 v.s. MOD group.
3.7 WVBF regulated protein expression of IRS1/PI3K/AKT/AMPK/GLUT4 in the skeletal muscle of T2DM rats
The skeletal muscle is one of the main targets of insulin resistance,so we chose the skeletal muscle tissue to explore the potential mechanism by which WVBF improved the insulin resistance in detail.As shown in Fig. 7, the expression levels of proteins in the IRS/PI3K/AMPK/AKT pathway in the skeletal muscle of the MOD group were impaired, as reflected by the increased expression levels of p-PKC-θ and p-IRS1 (S307), and decreased expression levels of p-PI3K,p-AMPK, and p-AKT compared with that of the NC group. For the glucose metabolism, one of the key targets of IRS/PI3K/AMPK/AKT signal pathway is GLUT4. Our data showed that the total GLUT4 expression was no changes, but the membrane associated GLUT4 was reduced in the T2DM rats. The WVBF treatment restored the reduced membrane GLUT4 due to the impaired IRS/PI3K/AMPK/AKT pathway activity. There was no significant difference in the total and phosphorylated GSK-3β expression levels among these groups.
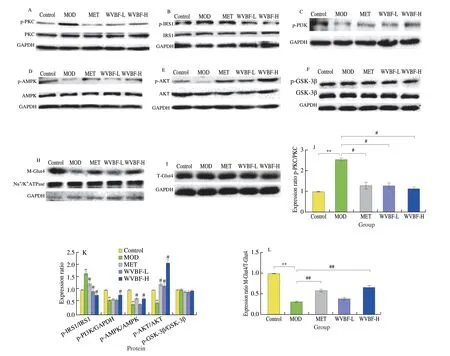
Fig. 7 Effects of WVBF on protein expression of PKC-θ/IRS1/PI3K/AKT/AMPK/GLUT4 in skeletal muscle of T2DM rats. (A-I) Representative bands of Western blot;(J-L) densitometry analysis. The experiments were 3 times repeated independently. *P < 0.05, **P < 0.01 v.s. NC group; #P < 0.05, ##P < 0.01 v.s. MOD group.
4. Discussion
T2DM is a chronic disorder characterized by impaired homeostasis of lipid and carbohydrate metabolism, which ultimately results in hyperglycemia, insulin resistance and multiple organ complications[15-16]. In the present study, we investigated the pharmacological effects of WVBF on T2DM rats. We found that WVBF treatment had a significant therapeutic action on T2DM by ameliorating insulin resistance and reducing multiple organ damages.The underlying molecular mechanisms were associated with the normalization of IRS/PI3K/AMPK/AKT/GLUT4 pathway in skeletal muscle and IRS1/PI3K/AKT pathway in the liver.
Compared with the NC rats, the T2DM rats induced by STZ injection and HFD-feeding developed hyperglycemia, and the WVBF treatments effectively decreased blood glucose. Furthermore, the WVBF treatment ameliorated dyslipidemia by correcting the serum TC and TG levels, as well as reversed the FINs, serum insulin level and the HOMA-IR index in the T2DM rats. These results indicated that WVBF improved dyslipidemia, glucose tolerance and insulin resistance. In addition, WVBF-treated T2DM rats exhibited smaller adipocytes, which have been reported to reflect enhanced insulin sensitivity[17]. These morphological observations also support the results that WVBF can alleviate insulin resistance.
Persistent insulin resistance result in multiple organ injuries[16,18-20].In the liver, the excessive blood lipid promotes its steatosis, which is tightly associated with the development of insulin resistance[18]. Our morphological results demonstrated that severe steatosis, fibrosis and inflammatory cell infiltration were observed in the liver of T2DM rats. The immunohistochemical experiment also proved thatα-SMA, a fibrosis marker, mediated further matrix increase around the hepatocytes in the T2DM rats. The biochemical test results further suggested that the liver functions of the rats in the MOD group were impaired as the abnormally increased levels of ALT, AST, ALP andγ-GT. However, the WVBF treatment significantly improved the hepatic steatosis and fibrosis, as well as the liver functions.
Oxidative stress and inflammation have been implicated as two important contributors to both the onset and the progression of diabetes and its associated complications[21-22]. In this study, the oxidative stress markers like SOD and GSH-Px were found to be lower, as well as MDA was higher in the liver of T2DM rats, which were all restored by the WVBF treatment. Meanwhile, in the WVBFtreated T2DM rats, IL-6 and TNF-α in the liver, the indicators of inflammation, were significantly decreased. There was a strong correlation between oxidative stress and insulin resistance[23],therefore, we explored whether insulin signal pathway modulation involved in the salutary effect of WVBF on T2DM rats. Normal insulin signaling occurs through the activation of the insulin receptor.The activated insulin receptor directly phosphorylates IRS on multiple tyrosine residues. Tyrosine phosphorylated IRS proteins then act as a binding site for signaling molecules such as PI3K[24]. PI3K is the main signal mediator of the metabolic and mitogenic actions of insulin[25].Following association of PI3K with IRS, this allows phosphorylation of its substrate to generate PtdIns(3,4,5)P3, which activates serine kinase AKT[26]. The activation of AKT results in insulin responses,including GLUT4 translocation to the membrane, increased glycogen synthesis after phosphorylation of GSK-3[27-29]. In the liver of T2DM rats, reduced IRS-1, p-PI3K and p-AKT expressions were found,which were significantly increased after the treatment with 12.5 mg/kg WVBF treatment. These results demonstrated that WVBF might restore the impaired insulin signaling through inhibiting oxidative stress and inflammation, which endowed it with anti-diabetic effect.Besides the liver damages, T2DM is also closely associated with the long-term damage of kidney and pancreas[21]. In addition, WVBF also effectively alleviated renal fibrosis and glycogen aggregation, as well as restored the damaged pancreas to near-normal islet cells and improved the pancreatic function of T2DM rats.
However, before the liver, pancreas and kidney events, there are pathologic alterations in the response of skeletal muscle to insulin[30].To address whether WVBF protected from insulin resistance in the skeletal muscle of T2DM rats, we assessed the role of WVBF on the activation of components of IRS1/PI3K/AKT/GLUT4 pathway and explore the potential upstream target. Consistent with the results in the liver, WVBF treatment activated the inhibited p-PI3K and p-AKT in skeletal muscle of T2DM rats. The activation of AKT resulted in the GLUT4 translocation to the membrane as the membrane GLUT4 expression was increased by WVBF treatment. These results indicated that the WVBF treatment enhanced the glucose disposal by increasing the insulin sensitivity in the skeletal muscle of T2DM rats. However,the glycogen synthesis was not modulated by the WVBF treatment,since the phosphorylation of GSK-3β was not changed.
Insulin induces the GLUT4 translocation in skeletal muscle acts via the increased tyrosine phosphorylation of IRS1 that allows it to bind and activate PI3K, which in turn results in activation of GLUT4[31-32]. Previous studies demonstrated the HFD-induced insulin resistance in the skeletal muscle is mediated through increased serine phosphorylation of IRS-1[33]. In particular, the phosphorylation of IRS-1 on Ser307, which can inhibit the association between the insulin receptor and IRS-1 because Ser307exists near the phosphotyrosine binding domain[34]. Meanwhile, the phosphorylation of IRS-1on Ser307has been shown to promote protein degradation[33]. The data in the present study supported that the serine phosphorylation of IRS-1 inhibits the activation of insulin signaling pathway, since the increased phosphorylation of IRS-1 on Ser307was associated with decreased phosphorylation of PI3K, AMPK, and AKT, as well as decreased membrane GLUT4 expression in the skeletal muscle of T2DM rats[35]. The WVBF treatment significantly reversed these abnormal phosphorylation levels of IRS-1 on Ser307, PI3K, AMPK and AKT, as well as the decreased membrane GLUT4. On the other hand,our data suggested that phosphorylation of IRS-1on Ser307did not lead to increased IRS-1 degradation, since the expression of IRS-1 protein was unchanged (Fig. 7B). Furthermore, we explored the upstream target of the phosphorylation of IRS-1on Ser307. Studies have further shown that hyperglycemia and abnormal lipid metabolites activate PKC-1d6ff and PKC-θ, which, via a serine-threonine kinase cascade,promotes serine phosphorylation of IRS1 and blunt its activity,ultimately leading to the impaired insulin signaling[30,36]. Consistent with these reports, our data indicated that the phosphorylation levels of PKC-θ in the skeletal muscle of T2DM rats were dramatically increased. The WVBF treatment protected against the increases,suggesting that the inhibition of the serine phosphorylation of IRS1 may be a potential pharmacological target in the treatment of insulin resistance associated with T2DM. Interestingly, the WVBF treatment also markedly activated AMPK by increasing its phosphorylation,implying that AMPK-mediated energy homeostasis was involved in the therapeutic action of WVBF (Fig. 8). However, it is noticeable that the therapeutic effect of WVBF was not dose-dependent between 12.5 and 25.0 mg/kg. More meticulous work should be conducted to further determine the dose-effect relationship in the future.
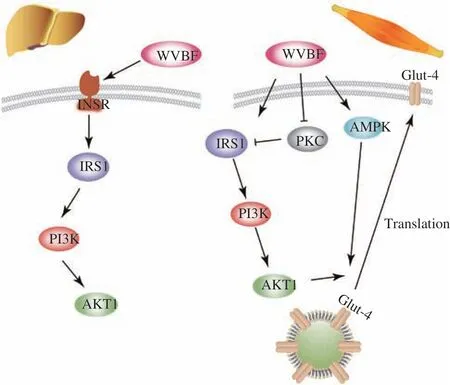
Fig. 8 The potential mechanism of the anti-diabetic action of WVBF. In the liver tissues of T2DM rats, WVBF treatment enhanced the expressions of IRS1/PI3K/AKT1 (left); in the skeletal muscles of T2DM rats, WVBF treatment activated both IRS1/PI3K/AKT1 and AMPK signaling pathways to enhance the translocation of GLUT4 (right).
In summary, in the present study, WVBF alleviated the pathological characteristics, including insulin resistance, hyperglycemia and dyslipidemia in T2DM rat. Promotion GLUT4 translocation by enhancing the PKC-θ/IRS1/PI3K/AKTl and AMPK signal pathways were involved in the pharmacological action of WVBF.
5. Conclusion
In conclusion, WVBF improved the insulin resistance,hyperglycemia and dyslipidemia, as well as alleviated multiple organ injuries in the T2DM rats. The regulations of insulin and AMPK signal pathways were involved in the functional mechanisms of the WVBF’s pharmacological actions in T2DM rats. The results of our study provide a theoretical basis for the therapeutic uses of WVBF in T2DM.
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by grants from the National Natural Science Foundation of China (81873090), Support Innovation and Development of Enterprise Technology Projects in Hubei Province(2021BLB174) and the modern transmission and innovation research team of Traditional Chinese Medicine, South Central Minzu University.
- 食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango

