Data-driven analysis of chemicals, proteins and pathways associated with peanut allergy: from molecular networking to biological interpretation
Emmanuel Kemmler, Julian Braun, Florent Fauchère, Sabine Dölle-Bierke,Kirsten Beyer, Robert Preissner, Margitta Worm, Priyanka Banerjee,
a Institute for Physiology, Charité – University Medicine Berlin, Berlin 10115, Germany
b Member of the KFO339, FOOD@, Berlin 10115, Germany
c Si-M / “Der Simulierte Mensch” a science framework of Technische Universität Berlin and Charité-Universitätsmedizin Berlin, Berlin 10115, Germany
d Charité -Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin 10115, Germany
e Division of Allergy and Immunology, Department of Dermatology, Venerology and Allergy, Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin 10115, Germany
f Pediatrics, Campus Virchow, Charité – University Medicine Berlin, Berlin 10115, Germany
Keywords: Allergy informatics Knowledge-graph Data analysis Food allergy Peroxisome proliferator-activated receptors Fatty acids
ABSTRACT Peanut allergy is majorly related to severe food induced allergic reactions. Several food including cow’s milk,hen’s eggs, soy, wheat, peanuts, tree nuts (walnuts, hazelnuts, almonds, cashews, pecans and pistachios), f ish and shellf ish are responsible for more than 90% of food allergies. Here, we provide promising insights using a large-scale data-driven analysis, comparing the mechanistic feature and biological relevance of different ingredients presents in peanuts, tree nuts (walnuts, almonds, cashews, pecans and pistachios) and soybean.Additionally, we have analysed the chemical compositions of peanuts in different processed form raw,boiled and dry-roasted. Using the data-driven approach we are able to generate new hypotheses to explain why nuclear receptors like the peroxisome proliferator-activated receptors (PPARs) and its isoform and their interaction with dietary lipids may have signif icant effect on allergic response. The results obtained from this study will direct future experimental and clinical studies to understand the role of dietary lipids and PPARisoforms to exert pro-inflammatory or anti-inflammatory functions on cells of the innate immunity and inf luence antigen presentation to the cells of the adaptive immunity.
1. Introduction
Diseases related to allergies are considered as one of the major world health problems due to their increasing prevalence. According to the published data, approximately 5% of adults and 8% of children have a food allergy[1]. Peanut is one of the most common triggers of food allergy. It is caused by a specific immune response against peanut proteins. According to the current literature, the prevalence of peanut allergy in Europe is 2.2%[2]. Peanut allergy contributes to approximately 59% of deaths caused by food allergies[3]. It is known that ingestion of even a little amount can cause allergic reactions such as gastrointestinal discomfort, allergic dermatitis and in some cases severe allergic shock and anaphylactic death[1]. Why peanuts exhibit this high allergenicity is not yet understood, but it may be related to the constituents of the peanut. On the other hand, health aspects of peanuts as an outcome of its chemical composition are well-known[4].Peanuts are consumed worldwide due to their high nutritional value and pleasant or unique f lavour after roasting or boiling.
Food allergy is an area that has traditionally tended towards a hazard or risk-based approach for safety assessment by the regulatory agencies, at least for most of the major food allergens[3]. Furthermore,the only currently feasible strategy for consumers with food allergies is to avoid the relevant allergenic food or food products, due to the absence of preventive therapies or treatment[5]. Conceptually, any protein in the human food supply can be a potential allergen, and thus allergen-free food for all is impractical. The immune system of different individuals sensitive to the same allergen may respond differentially, a mechanism, which has not been well understood. The minimum amount (threshold) needed to elicit an allergic response from an individual also depends on the sensitivity of the individual[1].
Fatty acids (FAs) are known to impact and influence the immune system on multiple levels[6]. Peanuts and many other allergens are known to contain significant amounts of triglycerides,which affects the absorption of antigens but have unknown effects on sensitization and anaphylaxis[7]. It is also known that a direct interaction between allergenic proteins and lipids can occurimpacting allergenicity. On the other hand, the influence of the mucosal-associated microbiota on the innate and adaptive immune systems has been well studied, suggesting changes in microbiota composition and/or metabolism affecting the development of asthma,food allergy and atopic dermatitis[8]. The composition and activity of the microbiota is influenced by many factors-hygiene practices,antibiotics, medications, infections, and most importantly, food and diet[9]. Polyunsaturated fatty acids (PUFAs) are known to have important roles in pathological and physiological processes, a proper understanding is needed regarding the contribution of these fatty acids to the coinciding increases in inflammatory diseases seen with the disruption of the balance in the ratio ofω-6:ω-3 associated with the diet[10]. Studies investigating the role of fatty acids in allergy seldom evaluate food allergy as an outcome. Typically, studies make use of sensitization data as a proxy for potential food allergies-cow’s milk,hen’s egg and peanut[11].
Meanwhile, advancements in genomics, proteomics,metabolomics, and analytical techniques have resulted in considerable contributions in the field of allergy research, which has also led to the accumulation of a huge amount of data. Additionally, databases like the food composition database by the European Food Safety Authority (EFSA)[12], the Food and Nutrient Database for Dietary Studies (FNDDS) by the U.S. Department of Agriculture[13], and many others provide information on the nutrient values of food and the amount of vitamins, fatty acids, minerals contained in different groups. Computational analyses associated with allergenicity of food ingredients require access to reliable and current sources of information on the structure, molecular targets and pathways associated with these ingredients.
In this study, we have curated data from peanuts, tree nuts and soybean related allergy data from several dietary intervention studies and dietary databases, to provide new scientific knowledge for novel research directions for the assessment of peanuts allergenicity. Ourin silicoresults suggest that PUFAs (intrinsic factors of the peanuts compositions) have the potential to promote the sensitization and elicitation of the allergic response by down-regulating important regulatory pathways. Furthermore, this study provided a novel understanding of the non-protein components of peanuts play an important role in peanut allergy, and allergenicity assessment of peanuts should consider the chemical composition of its fatty acids(dietray lipids) and their role in the immune response.
2. Materials and methods
2.1 Data curation
In this study, the data was obtained using an in-house text-mining pipeline using the PubMed query search[14]and manual curation by domain experts. Additionally, datasets were obtained and integrated from published literature sources[15-19](Fig. 1).

Fig. 1 Overview of the system architecture. The critical methodological components of the knowledge graph database consist of scientific, structural,procedural, and quality/rigour assurance components.
The data curation was done for 3 different data sets:
Dataset 1 consists of information about the amount of a number of different ingredients (vitamins, carbohydrates, fatty acids) present in almond, cashew, peanut, pecan, pistachio, soybean and walnut.
Dataset 2 consists also of information about the amount of different ingredients but in different forms of peanut e.g., raw and processed ones.
Dataset 3 is the most extended one and includes information about 204 different ingredients present in peanuts and their interaction with 316 different human proteins and 1 864 distinct pathways. The full Dataset 3 consists of 828 distinct compound-protein and 4 157 distinct protein-pathway interactions (Fig. 2).
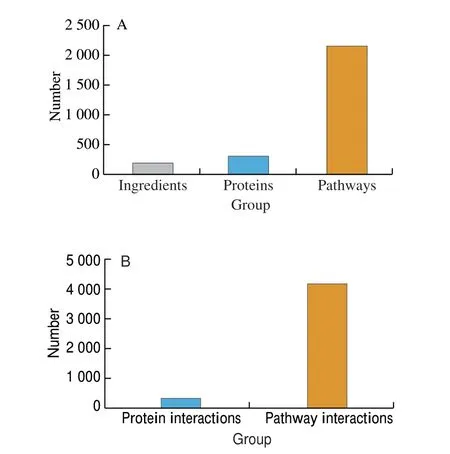
Fig. 2 (A) Numbers of distinct ingredients, proteins and pathways and(B) numbers of distinct protein and pathway interactions in the data set.
The data for Dataset 1 was collected by different literature search[4]and Dataset 2 from the FoodData Central (https://fdc.nal.usda.gov/), a platform maintained by the U.S. Department of Agriculture[15]. The ingredients included in Datasets 1 and 2 were selected on the basis of the availability of relevant literature and the various forms of thermal and pre-processing of peanut, to which they are normally subjected to.
Dataset 3 was derived in a semi-automated manner using multiple R-scripts. Database-specific identifiers were obtained using the cts_convert function of the R package Webchem[20]. The information on the chemical data included in the nut composition was collected from different platforms: FoodData Central[15],fooDB[21], phenole explorer[22], Duke’s database[23]and an in-house resource, superTCM[18]. The data was then filtered for duplications by the InChI key and incomplete entries were removed. The protein interaction data were derived from BindingDB[24]and ChEMBL[16]by matching the compound InChI keys to database-specific IDs and using web scraping techniques. Since ChEMBL and BindingDB contains also data about binding experiments including non-binders,strong, moderate and weak binders, the data was then filtered for well-recorded binding entries, to receive a reliable dataset based on recommendations described in reference[10]. The datasets were refined with the following criteria: 1) only experimental data from human proteins and assigned UniProt ID were selected; 2) a conclusive activity comment of the supplier like “active” or “inactive” or with unambiguous IC50, EC50,Ki,Kd, activity or inhibition values were extracted. Additionally, entries with inconclusive activity comments or comments indicating no binding were filtered out as well. The entries from the UniProt[17]database were matched, and the protein data was restricted to reviewed proteins only. Furthermore, to identify pathways-protein interactions, the extracted protein based on their UniProt ID was used to screen through different databases:Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway[25],Reactome[26]and the Small Molecule Pathway Database (SMPDB)[27].An informatics framework has been developed to capture above mentioned dataset into a pipeline of quality control, data exploration,and subsequent integration into the database. Establishing this knowledge framework (Fig. 1) has been integral to the development of the analysis reported in this study.
2.2 Prediction models and statistical analysis
Prediction of additional molecular targets and off-target activities of the chemicals present in peanuts, were computed using two different published platforms-SuperPred[28]and ProTox-II[29]. Both the platforms are open-sourced and available via https://prediction.charite.de/ and https://tox-new.charite.de/protox_II/ respectively.
SuperPred3.0 prediction server provides machine-learning-based molecular target predictions for user-provided molecular structures as input as well as reports information on known binders[30]. The server further allows comparing the input compounds to the known compounds binding to an individual’s known and predicted targets,including scores.
Adverse Outcomes Pathways (AOPs) for the chemicals were predicted using the ProTox-II platform. The platform includes 33 models to assess chemical-based toxicity endpoints, which include acute toxicity, hepatotoxicity, cytotoxicity, mutagenicity,carcinogenicity, immunotoxicity, AOPs and off-targets. The predictive models are built on data from bothin vitroassays(e.g. Tox21 assays, Ames bacterial mutation assays, HepG2 cytotoxicity assays, immunotoxicity assays) andin vivocases (e.g.carcinogenicity, hepatotoxicity). All the models are validated both on cross-validation and external validation set and achieved higher predictive performance. ProTox-II predicts chemical compounds active in toxicological pathways assays against a panel of 12 different biological target-based pathways, that involves two-major groups of adverse outcomes pathways: nuclear receptor signalling pathways and stress response pathways[31].
The analysis of the nut composition and the protein and pathway interaction data was performed using exploratory data analysis (EDA)techniques with the R programming language[32]. Statistical plotting was implemented using the ggplot package[33]and the force-directed graph was built using the network3D library. For data handling,cleaning, filtering and basic statistics like median and mean value calculation, the meta-package tidyverse[34]was utilized.
3. Results
3.1 Chemical data-driven analysis of peanuts, tree nuts and soybean
3.1.1 Fatty acids
To identify which fatty acids are present in peanuts in major quantities compared to similar food, the composition of peanut, five different tree nuts and of soybeans in their raw form were compared.
With 53.7%, monounsaturated fatty acids (MUFAs) account for most of the fat content in peanuts. Compared to tree nuts, peanuts ranked above the mean MUFAs content of 43.7%. For PUFAs,peanuts have a below-average PUFAs content of 27% compared to the mean content of 43.9% in other nuts. When it comes to total saturated fatty acids (SFAs), peanuts (19.3%) ranked top together with cashew nuts (20%). The investigation of concentrations of single fatty acids revealed a unique characteristic of peanuts, namely the presence of a high relative amount of docosanoic and paullinic acid compared to the other considered nuts (Fig. 3).
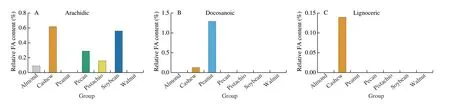
Fig. 3 Relative content of different FAs in almond, cashew, peanut, pecan, pistachio, soybean and walnut.
Further analysis of the pathways and molecular targets revealed that paullinic acid is known to be inhibitory to peroxisome proliferator-activated receptor (PPAR) α with an IC50value of 600 nmol/L[35]. Prediction results (Table 1) using the in-house target prediction tool “SuperPred”[30]confirmed the interactions of paullinic acid and docosanoic acid in allergy through the Toll-like receptor 4(TLR4), in asthma by interacting with cysteinyl leukotriene receptor 2(CYSLTR2), in atopic dermatitis with cytochrome P450 3A4 (CYP 3A4)and in inflammation in general by the formyl peptide receptor 1 (FPR1).
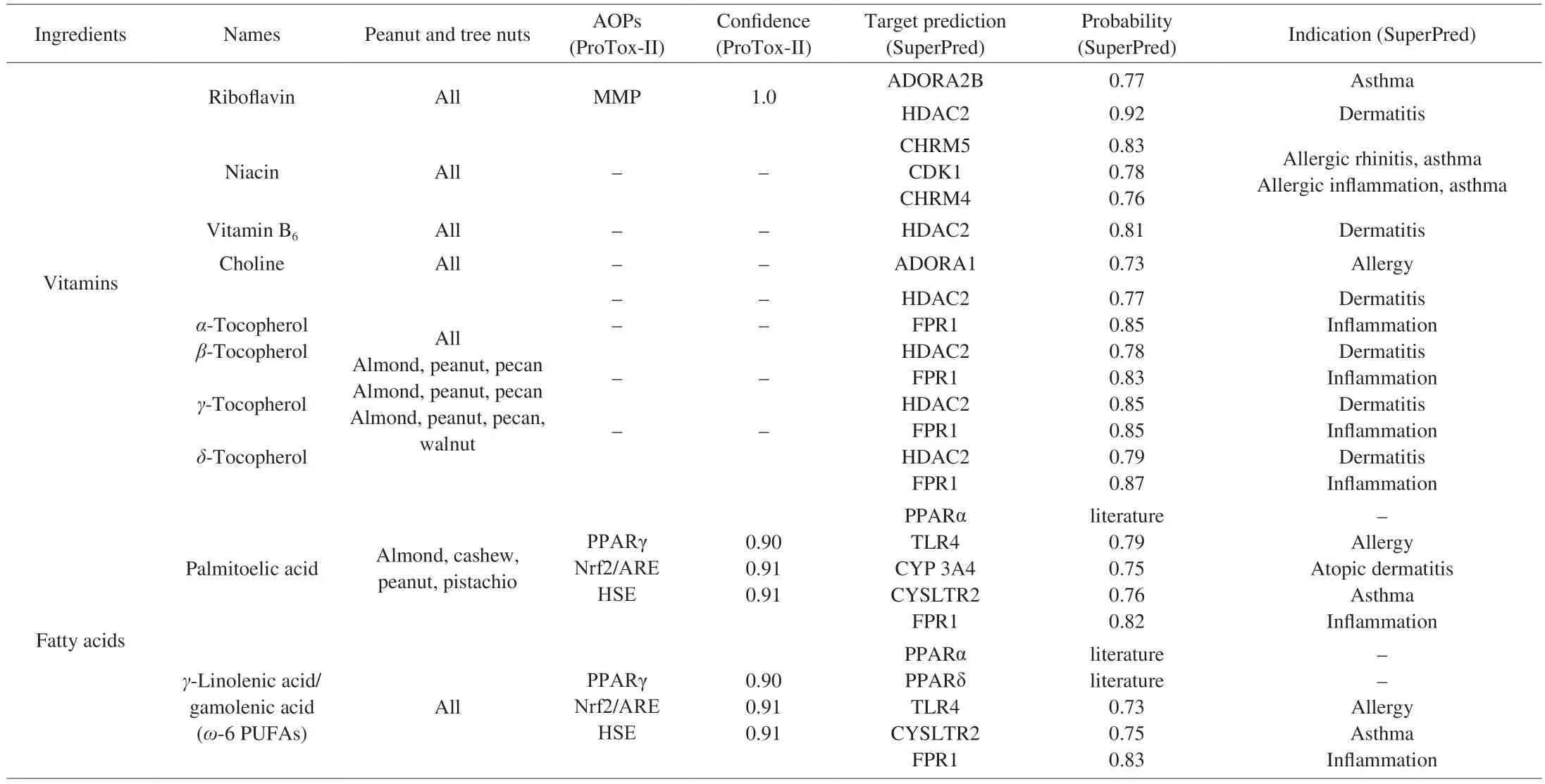
Table 1 Computational prediction of AOPs and molecular targets associated with the chemicals present in nuts.
Additionally, we analysed and compared the ratio ofω-6 andω-3 fatty acids present in the peanuts and other nuts. Overall, walnuts have the best ratio ofω-6 andω-3 fatty acids, and peanuts have the worst (Table 2).
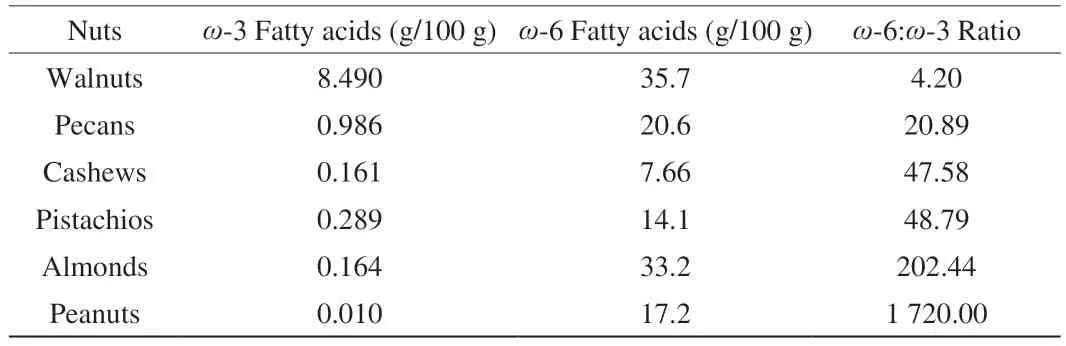
Table 2 Fatty acids content of each nut[53-54].
3.1.2 Vitamins
Considering the vitamins-based analysis, peanuts contain especially high amounts of choline and niacin (Fig. 4). Peanuts also contain high amounts of total tocopherol, mainly due to high contents ofα- andγ-tocopherol.
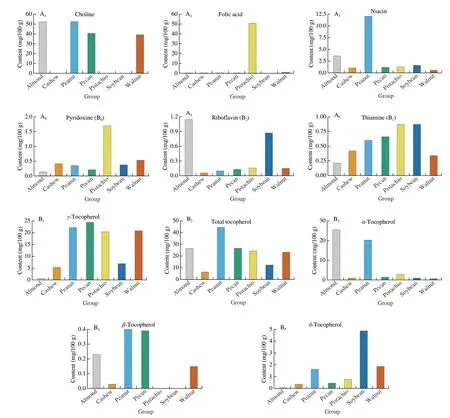
Fig. 4 (A) Vitamin and (B) tocopherol content of the considered in peanut, tree nuts and soybean.
The prediction using ProTox II indicates the potential of niacin for hepatotoxicity but with low acute toxicity (toxicity class 5, LD50:
3 720 mg/kg). Using SuperPred (Table 1), niacin was predicted to be involved in allergic rhinitis through interaction with the muscarinic acetylcholine receptor M5 (CHRM5) and in allergic inflammation through the Cyclin-dependent kinase 1 (CDK1)/cyclin B. The CHRM5 and CHRM4 have also been linked to asthma through the enhancement of the expression of interleukin (IL)-6, IL-8, cyclooxygenase (COX) 1 and 2 and urokinase-type-plasminogen-activator(PLAU)[36]. The different forms of tocopherol (α-,β-,δ-,γ-) are all predicted to have a certain impact in dermatitis through histone deacetylase 2 (HDAC2) and in inflammation by interacting with the FPR1 and estrogen receptor β.
3.2 Chemical composition of processed peanuts
Several studies have been conducted to understand the factors inducing peanut allergenicity including boiling and roasted treatments[37]. Since roasted peanuts are known to be more likely to trigger allergic reactions than raw or boiled peanuts[38], we compared the chemical compositions of peanuts in different processed forms.Therefore, Dataset 2 was used to investigate the differences between raw, boiled, and dry-roasted peanuts. Besides the boiled peanuts,which were salted, pure peanuts were analyzed (Fig. 5).

Fig. 5 Composition of peanuts in different forms: boiled, dry-roasted and raw (unroasted). (A) General values; (B) fatty acids; (C) vitamins; (D) dietary minerals.
Based on the chemical composition analysis in this study, boiled peanuts showed a decrease in energy, protein and fat (total, saturated,monounsaturated, polyunsaturated) content compared to all other differently processed peanut forms. Our initial analysis suggested that the dry-roasted peanuts have a higher amount of SFAs, but a decreased PUFAs content compared to raw ones (Fig. 5).
Further analysis of individual compound/fatty acids of dry-roasted peanuts clearly showed some main differences from raw peanut form.Our data show that the amount of myristic acid, palmitic acid and linoleic acid is decreased by half and a third respectively, while the amount of palmitoleic acid is increased by a factor of around three.Additionally, the amount ofγ-linolenic acid is even increased by a factor of around 10. Besides this, arachidonic acid and erucic acid are two compounds which are present in dry-roasted peanuts but not in the raw ones (Fig. 5). This “shift” in fatty acids content was already observed and confirmed in similar studies published on coffeeroasting[39]. By contrast, boiled peanuts show a decreased (by over a half) amount of all considered fatty acids compared to raw nuts.
The amount of thiamin (VB1), folate and vitamin E are lower in the processed forms of peanuts. In contrast to this the amounts of niacin, vitamin B6, choline and especially riboflavin are increased in the dry-roasted form. Again, boiled peanuts have the lowest amounts of all considered vitamins.
In terms of dietary materials, processed peanuts have lower amounts of calcium, iron, zinc, copper and potassium. In contrast to this, the amount of selenium is higher in dry-roasted than in raw peanuts. The phosphorus and magnesium contents were almost the same in both, raw and dry-roasted peanuts. The boiled peanuts showed again the lowest amount of all considered dietary minerals,with the only exception being sodium, due to the added salt (Fig. 5).
3.3 Protein interactions of peanut compounds
Using Dataset 3, considering all compounds (chemical composition) of peanuts, their interactions with proteins (molecular target) and with corresponding pathways (target-pathway) analysis was performed (Fig. 6). The figure shows the top human proteins interacting with peanut compounds identified using the network graph. The PPARs and their isoforms were identified to have the highest number of interactions. These receptors are, with respect to our data, exclusively interacting with a group consisting of nine fatty acids. Five fatty acids are also interacting with tyrosine-protein phosphatase non-receptor type 1. Our analysis also reveals another protein of interest – the aldo-keto reductase family 1 member B1,since there are interactions with five different peanut ingredients,mainly aromatic acids. These identified clusters from the network,suggest that especially the association of PPARs and certain fatty acids may provide deeper insights into the mechanism related to the allergenicity of peanuts.
3.4 Influence of fatty acids on allergy
Even so the research on allergy focuses mainly on proteins as a driver for allergic responses, there are also a few studies available that provide some insights into the possible link between allergic reactions and different fatty acids[40]. Few recent research also suggests a possible influence of fatty acids in the development and severity of the allergy. Within the PUFAs,ω-3 may reduce inflammation and improve allergic symptoms[41]and whereasω-6 PUFAs are generally considered to have a pro-inflammatory effect as they can favour T helper cell 2 (Th2) immune response and allergy development[42].Toll-like receptor 2 (TLR2) and TLR4 dependent mechanisms are discussed in the context of allergenicity via an enhancement of allergen uptake, and protection of allergens against proteolysis through fatty acids[43]. There is already evidence from the literature that saturated fatty acids can contribute to the allergenicity of hen’s eggs and cow’s milk[6]. Other studies suggest an impact of MUFAs[44]or PUFAs like linolenic acid[45]. However, there is evidence for a strong role of lipids allergy in general[46]and for peanut lipids in particular[47]. This indicates that a more differentiated view of single fatty acids in a given food allergen is needed.
Palmitoleic acid is especially abundant in roasted peanuts,according to our analysis, and previous studies suggested a positive association with sensitization in atopic disease and hay fever in female adults[44].γ-Linolenic acid (ω-6 PUFAs), which content is also increased due to the roasting process, is known to be partly converted to arachidonic acid (ω-6 PUFAs)[48], which is only present in roasted peanuts. As it is a precursor of prostaglandin E2 (PGE2), it may in turn promote allergic sensitization by inhibition of interferon (IFN) γ formation[49]. For behenic acid (docosanoic acid) and paullinic acid,which are may more abundant in peanuts, compared to other nuts and erucic acid, which is only present in roasted peanuts and not in raw ones, is, to the best of our knowledge, no research concerning their connection to inflammation or allergy available. Therefore, it would be of especially high interest to uncover their involvement in food allergy, if there is any.
3.5 Interaction of fatty acids with PPARs
Since our data shows that a high number of fatty acids in peanuts are interacting with the different isoforms of PPARs and to reveal their possible involvement in the immune system and allergic reactions; the experimental IC50values were investigated.
As depicted in Fig. 6, fatty acids in peanuts have the highest inhibitory potential for PPARα. However, a more nuanced view can be observed by looking at the IC50values for the fatty acids individually (Figs. 7 and 8). Especially arachidonic acid (ω-6 PUFAs)andγ-linolenic acid (ω-6 PUFAs) have low median IC50values on all three PPARs isoforms. The interactions of the arachidonic acid with the PPARs isoforms are 1 200 (PPARα), 3 100 (PPARδ) and 1 600 nmol/L (PPARγ), and the interactions ofγ-linolenic acid are 270 (PPARα), 750 (PPARδ) and 2 200 nmol/L (PPARγ). Besides this, PPARα interactions with other fatty acids with comparable low median IC50values are linoleic acid (ω-6 PUFAs, 1 100 nmol/L),linolenic acid (ω-3 PUFAs, 1 200 nmol/L), oleic acid (600 nmol/L),palmitic acid (1 500 nmol/L), palmitoleic acid (1 700 nmol/L) and stearic acid (1 100 nmol/L). For PPARδ and γ, the IC50values were generally higher for fatty acids besides arachidonic acid andγ-linolenic acid. Noteworthy (median IC50≤ 10 000 nmol/L),γ-linoleic acid,linolenic acid, oleic acid, and palmitoleic acid are 6 200, 6 000, 4 100 and 6 400 nmol/L, respectively for PPARγ, and for PPARδ, linoleic acid, oleic acid, palmitic acid, palmitoleic acid and stearic acid are 10 000, 5 300, 7 400, 9 600 and 6 000 nmol/L respectively. There is good evidence that fatty acids in peanuts have an inhibitory influence,especially on PPARα, but also, to a certain degree, on PPARδ and γ.

Fig. 6 Network plot of the human proteins (orange) with the highest number of interactions with ingredients of peanuts by IC50 value and the corresponding peanut compounds (blue). ω-6 Fatty acids are coloured green. The thickness relates to the IC50 values: as thicker as lower the corresponding IC50. The colour of the target nodes corresponds to the number of interacting molecules: as darker/more reddish as more interactions with peanut molecules. Filtered for targets with at least 3 interactions.
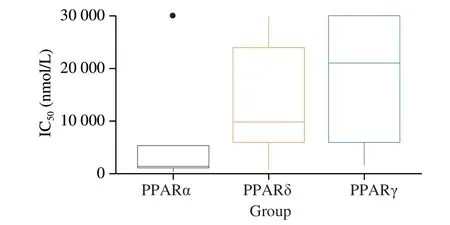
Fig. 7 Boxplot of the IC50 values for PPARα, δ and γ of all considered fatty acids combined present in peanut.
3.6 Inhibition of PPARs isoform through fatty acids might enhance allergic reactions to peanuts
PPARγ has been found to promote the functions of T helper cell 2(Th2 cells), type 2 innate lymphoid cells, M2 macrophages and dendritic cells. Therefore, it is hypothesised that the availability of environmental ligands for PPARγ might be a major reason for the rise of allergic diseases worldwide[50]. As shown in the sections above,there is good evidence that certain fatty acids in peanuts are capable to inhibit PPARγ signalling, resulting in a down-regulation of the PPARγ pathway. Besides that, there is an even stronger association between certain fatty acids and PPARα. By analysing the data for the involvement of PPARα in pathways in the human body, the tolllike receptor pathway 1 (https://smpdb.ca/view/SMP0063899) was identified to be highly connected to the considered fatty acids with PPAR isoform and, to a certain degree, also through the TLR2.The toll-like receptor pathway 1 is part of the innate immune system. Through the different TLRs, pathogen-associated molecular patterns from different microbes are recognised, which results in the recruitment of MyD88, TIR-domain-containing adapter-inducing interferon β (TRIF), toll-interleukin 1 receptor domain containing adaptor protein (TIRAP), or translocating chain-associated membrane protein (TRAM), leading to the release of proinflammatory cytokines (IL-12, IL-1, TNF-α) and thus leading to the activation of transcription factors NF-κB causing innate immune response.
The hypothesis of a possible role of fatty acids in peanuts allergenicity via this pathway is that the PPARα or other sub-types receptors act anti-inflammatory via nuclear factor NF-κB p105 subunit(NFKB1) inhibition, resulting in an enhanced allergic response. Fig. 9 shows the systematic demonstration of the fatty acids’ interactions with the PPAR receptor (α) and immune regulation.γ-Linolenic(gamolenic) acid (ω-6) and palmitoleic acid are highly increased in roasted peanuts compared to raw ones. They are known to inhibit PPARα with low IC50values. Palmitic acid and myristic acid seem to play an ambiguous role in this pathway since they are known to inhibit the TLR2 receptor (IC50of 5 000 nmol/L), which could lead to a down-regulation of the immune response. These finding matches the observation of the analysis above, as both fatty acids are less abundant in roasted peanuts than in raw, thus reducing their potentially positive immune regulating effect.
4. Discussion
The application of a comprehensive analysis of chemicals and molecular targets datasets in the food sciences has increased considerably during the last years. In this study, we have used several data mining and extractions methods followed by a data processing pipeline to extract novel useful information on the nut composition and their possible impact on allergenicity-from large amounts of collected data. By application of data analytics strategies, a complete interpretation of data and exploitation of the information contained therein is possible. Using statistical methods and knowledge graphs,data were converted into useful information such as the identification of novel pathways, which can be analysed further experimentally.
Herewith we analysed several chemical compositions of the peanuts and tree nuts. The information on the molecular targets’were extracted and used for further analysis by applying published prediction methods. Different fatty acid compositions present in peanuts and other nuts were investigated in a comparative manner(Fig. 4). Furthermore, the fatty acid profile in different processed forms of peanuts (raw, boiled and dry-roasted) was analysed (Fig. 5).Based on the deep chemical composition analysis, we detected that boiled peanut showed a decrease in energy, protein and fat (total,saturated, monosaturated, polysaturated) content compared to all other differently processed peanut forms. In general,ω-6 fatty acids are known to be associated with pro-inflammatory responses, whileω-3 fatty acids are associated with anti-inflammatory responses[10].Interference with fatty acids synthesis pathways may exert profound effects on the metabolic programming of T cells. Many of the lipid mediators that regulate inflammation are metabolites derived fromω-6 orω-3 fatty acids, including arachidonic acid (20:4n-6), linoleic acid (18:2n-6),α-linolenic acid (18:3n-3), and docosahexaenoic acid(22:6n-3)[51]. Hence, it is important that theω-6:ω-3 ratio in the food is important in influencing host immunological activity[6,52].
Since PUFAs have important roles in pathological and physiological processes, more data are needed regarding the biological contribution of these fatty acids to the coinciding increases in inflammatory diseases seen with the disruption of the balance in the ratio ofω-6:ω-3 associated with the diet[10]. Peanuts have shown the worst and walnuts have the bestω-6:ω-3 (Table 2). Excessive amounts ofω-6 PUFA and a very highω-6 toω-3 ratio are known to promote the pathogenesis of many diseases, including cardiovascular disease, cancer, and inflammatory and autoimmune diseases, and can also interfere with normal brain development[53]. It is suggested that a ratio of 1:1 to 2:1ω-6/ω-3 fatty acids promotes health. A diet predominantly rich inω-6 PUFAs has been suggested as a possible cause of the high incidence of allergic diseases in the industrialised world[54]. A high concentration of dietaryω-6 PUFAs has been proposed to promote Th2 differentiation of the immune system[54].Erucic acid and arachidonic acids are only present in the dry roasted form of peanuts (Fig. 6). On the other hand, palmitoleic acid andγ-linolenic acids are present in higher quantities in dry roasted form than raw or boiled. Our data-driven analysis shows that an increased number of fatty acids in peanuts interact with the different isoforms of PPARs (Figs. 7 and 8). PPARs are known to regulate the transcription of a large variety of genes involved in metabolism, inflammation,proliferation, and differentiation in different cell types[35]. PPARs may be important cellular targets mediating the modulation of immune responses by fatty acids. The fact that fatty acids and their metabolites are endogenous ligands for the PPARs underlines the potential importance of the PPARs as molecular targets through which fatty acids can modulate immune and inflammatory responses. Each sub-types of PPARs regulates the transcription of different types of genes. Although it is well documented that PUFAs are activators of each subtype of PPARs, there appears to be a lack of consistent patterns in the relative specificity of individual PUFAs in activating each subtype of PPARs. This may be due to differences in assay methods assessing PPAR binding or activation. Therefore, the relative potency and specificity of individual PUFA in activating or suppressing the expression of the target genes that regulate PPARs need to be determined by quantifying levels of expression of endogenous target genes involved in the immune regulation.
Additionally, PPARγ emerged as an important regulator of multiple cell types involved in the inflammatory response to allergens,from airway epithelial cells to T helper cells[50]. Preclinical models of allergy in gene-targeted mice have increasingly implicated PPARγ in driving allergic inflammation[50]. The importance of specific ligands(agonists) of the PPARγ in inhibiting the allergic response has been well-studied[55]. The activation of PPARα and γ are known to have anti-inflammatory effects through the decrease of antigen-induced airway hyperresponsiveness, lung inflammation, eosinophilia,cytokine production, and GATA-3 expression, as well as serum levels of antigen-specific immunoglobulin E (IgE)[35]. There have been studies supporting the contrasting role of PPAR-isoforms in allergic inflammation. Mechanistically, PPARγ appeared to promote the expression of the IL-33 receptor on the surface of Th2 cells[56].However, there are almost no studies conducted on the antagonism of the PPAR-gamma and adverse outcome pathways in peanut allergy.Studies have shown that PPAR-gamma antagonists exacerbate neural specific-antigen Th1 and experimental allergic encephalomyelitis[57].A down-regulation of the PPARγ has been linked to allergic airway inflammation in an allergen challenge model[57]. PPARγ is also expressed in various immune cells, and it not only regulates genes involved in lipid metabolism but can also regulate immune and inflammation-related genes. It has been reported that the expression of PPARγ decreased in the high-fat diet group[58]. Therefore, the antagonism of the PPARγ may attenuate the anti-inflammatory effects of PPARγ by down-regulating its expression in the cells.
Elucidating the mechanism of actions of fatty acids on receptormediated signaling pathways in immuno-competent cells will provide a new insight for understanding the immunomodulatory roles of dietary fatty acids. To get deeper insights into the molecular mechanism behind such a response, anin-vitroassay will be developed to investigate how APC polarization is affected by allergens and different signalling molecules. Furthermore, the effects of these different APC polarizations on T cell priming will be characterized. If these processes are disturbed by the different fatty acids, different outcome should be observable.
5. Conclusions
The purpose of this data-driven study was to investigate and highlight the chemical constituents present in peanuts, tree nuts and soybean, to elucidate their potential role in the development and management of allergic responses. In addition to the nutritional value, fatty acids have significant immunoregulatory functions.Despite gaps in our current knowledge, it is increasingly apparent that fatty acids may influence the development of inflammatory and tolerogenic immune responses. However, the lack of standardised formats (i.e., food versus supplement) and standardised doses, and a lack of pre-study serum fatty acid level assessments in clinical studies significantly limit the ability to compare allergy outcomes across studies and to provide a clear understanding of the mechanistic role associated with such immune responses. Polymorphisms in genes associated with fatty acid synthesis, catabolism, and utilisation may also influence the host fatty acid requirements and function. Prevention and intervention studies led by Genome-Wide Association Studies (GWAS), including the functional microbiome,immunological, metabolomics and lipidomic assessments, are required and will provide deeper insights into the understanding of the importance of fatty acids in allergies. We investigated the composition of fatty acids and the ratio ofω-6 andω-3 in peanuts and other nuts.The chemical composition of vitamins, fatty acids and other nutrients in different forms of peanuts-boiled, raw and dry-roasted were also compared and analysed. The computational analysis suggests thatω-6 fatty acids likeγ-linolenic acid, which is present in high quantities in the dry roasted peanuts, might act as an antagonist of PPAR-gamma and other sub-types resulting in the down-regulation of the pathways and other associated endogenous genes. This inhibition of the PPARγ can play an active role in exacerbating the allergic response.
To better understand food allergies an extrapolation of data as performed in this study can support unraveling novel pathways and interactions. As this is a data-driven approach further experimental and or clinical confirmation is necessary.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
- 食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango

