Calcium-fortif ied fresh milk ameliorates postmenopausal osteoporosis via regulation of bone metabolism and gut microbiota in ovariectomized rats
Qishn Wng, Bin Liu, Xinping Li, Junying Zho, Zongshen Zhng,Weicng Qio, Xinyue Wei, Lijun Chen,
a School of Bioengineering, Dalian Polytechnic University, Dalian 116034, China
b National Engineering Research Center of Dairy Health for Maternal and Child, Beijing Sanyuan Foods Co. Ltd., Beijing 100163, China
c Beijing Engineering Research Center of Dairy, Beijing Sanyuan Foods Co. Ltd., Beijing 100163, China
Keywords: Dairy products Calcium Vitamin D Bone turnover markers Gut microbiota Postmenopausal osteoporosis
ABSTRACT The aging of the global population has made postmenopausal osteoporosis prevention essential; however,pharmacological treatments are limited. Herein, we evaluate the effect of calcium-fortif ied fresh milk (FM)in ameliorating postmenopausal osteoporosis in a rat model established using bilateral ovariectomy. After 3 months of FM (containing vitamin D, and casein phosphopeptides, 1 000 mg Ca/100 g) or control milk(110 mg Ca/100 g milk) supplementation, bone changes were assessed using dual-energy X-ray absorptiometry, microcomputed tomography, and bone biomechanical testing. The results revealed that FM can regulate bone metabolism and gut microbiota composition, which act on bone metabolism through pathways associated with steroid hormone biosynthesis, relaxin signaling, serotonergic synapse, and unsaturated fatty acid biosynthesis. Furthermore, FM administration signif icantly increased bone mineral content and density in the lumbar spine and femur, as well as femoral compressive strength, while improving femoral trabecular bone parameters and microarchitecture. Mechanistically, we found that the effects may be due to increased levels of estrogen, bone formation marker osteocalcin, and procollagen type I N-propeptide, and decreased expression of the bone resorption marker C-telopeptide and tartrate-resistant acid phosphatase 5b. Overall, the f indings suggest that FM is a potential alternative therapeutic option for ameliorating postmenopausal osteoporosis.
1. Introduction
Osteoporosis is a chronic bone disease characterized by decreased bone mineral density (BMD)[1]. The current global prevalence of osteoporosis among the elderly (50–85 years of age) is 21.7% (35.3%in women and 12.5% in men), with postmenopausal osteoporosis caused by estrogen deficiency being reported as the most common type[2-3]. Current treatments for postmenopausal osteoporosis are primarily pharmacological; although most drugs are effective in slowing the rate of bone loss[4], they have certain limitations or adverse effects, which limit their long-term use[5]. Therefore,developing non-pharmacological methods for postmenopausal osteoporosis prevention and treatment is essential.
Calcium and vitamin D play major roles in skeletal development and have shown effectiveness in the treatment of postmenopausal osteoporosis[6]. Furthermore, vitamin D deficiency reduces BMD.Hence, considering that dietary sources of vitamin D are limited, food fortification with vitamin D has been suggested as a preventative measure against osteoporosis[7]. Moreover, calcium can only be obtained from dietary sources, and decrease in dietary calcium intake is associated with decreased bone mass[8]. Aging is also associated with irreversible and continuous loss of bone calcium via urine,sweat, and feces. Thus, calcium must be continuously absorbed from the diet to maintain its balance[9], a process that is promoted by casein phosphopeptides (CPPs)[10].
Milk is the most common food source of calcium and therefore used for calcium supplementation. Diets low in dairy products have been associated with reduced BMD in the lumbar spine and hip[11]and increased susceptibility to postmenopausal osteoporosis[12]. Although studies have assessed the effects of dairy products fermented with different probiotics, vitamin D- and calcium-fortified milk powder,and shelf-stable milk on bone health[13], health-conscious consumers tend to prefer fresh milk, which has a short shelf life. To the best of our knowledge, no studies have investigated the effect of fresh milk on postmenopausal osteoporosis. Compared with milk powder,fresh milk shows very little denaturation of whey protein and limited vitamin loss, thereby maximizing the retention of “fresh” components,such as immunoglobulins, lactoferrin, water-soluble vitamins, soluble calcium, phosphorus, and other functionally active ingredients[14].In addition, the nutritive value of fresh milk is more conducive to human digestion and absorption than that of powdered milk. The consumption of vitamin D- and calcium-fortified dairy products is a simple, nutritious, healthy, and cost-effective alternative to drug therapy[15], as minerals such as calcium, phosphorus, and magnesium are absorbed in the gut and contribute toward maintaining mineral balance and bone health.
The microbiota-gut-bone axis has attracted increasing attention in the field of bone health/disease, and the involvement of intestinal microbial disorders in a variety of bone diseases has been reported[16-17]. Scholars have found that the gut microbiota regulates bone mass by regulating hormone secretion, affecting immune response, promoting the absorption and metabolism of nutrients related to bone formation, and maintaining intestinal barrier function[18]. A study of 181 older adults found that decreased bone density in patients with low bone mass and osteoporosis was significantly associated with changes in gut flora[19]. In addition, the normal intestinal microflora has a bone immunoregulatory effect on osteoblast and osteoclast generation in healthy adult bones and affects the osteoprotegerin (OPG)/receptor activator of nuclear factor (NF)-κB ligand (RANKL) pathway in osteoclasts[20]. Moreover, the intestinal flora is the main regulator of bone mass in germ-free mice, and colonization of these mice by normal intestinal microflora can reduce the number of osteoclast precursor cells and increase bone mass[21].
Calcium influences intestinal regulation and inhibits the abundance ofDesulfovibrioand inflammatory cytokines, thereby attenuating osteoclast formation and bone resorption[22]. The gut microbiota enhances intestinal calcium absorption and regulates bone metabolism through calcium-dependent interactions with the Wnt/β-catenin signaling pathway[23]. Milk-calcium may act on Ca-sensing receptor (CaSR)in the epithelium that lines the intestine. In the ileum, approximately 70%–80% of ingested calcium is absorbed by vitamin D[24].CaSR activation increases the gene and protein expression levels of BMP-2 in fibroblasts and the phosphorylation ofβ-catenin in normal colonic mucosal epithelial cell lines. Thus, CaSR regulates the Wnt/β-catenin signaling pathway[25]. In addition, transplantation of probiotics, prebiotics, and fecal flora has shown early success in the prevention and treatment of postmenopausal osteoporosis[26]. Studies have also shown that dietary calcium can be beneficial to bone metabolism by exerting a prebiotic effect on potentially beneficial bacteria in the gut and by protecting colon permeability[27]. However,there are no relevant studies on the effects of calcium-fortified fresh milk (FM) intake on the gut microbiota. Moreover, the exact mechanism by which the intestinal microflora affects postmenopausal osteoporosis is rather complex and requires further study.
Thus, the present study investigated the therapeutic effects of FM on postmenopausal osteoporosis and explored the mechanisms underlying the effect of the gut microbiota on bone metabolism regulation in an ovariectomized (OVX) osteoporosis rat model.
2. Materials and methods
2.1 Preparation of FM
Raw milk was heated to 70 °C, and calcium lactate (purity ≥24%), vitamin D (100 000‒110 000 IU/g), and CPPs (purity 22%)(obtained from Sanyuan Beijing, China) were added and mixed for 5 min. The milk was homogenized, and then sterilized by heating to 85 °C for 15–30 s. The milk was aliquoted inside an ultra-clean bench and stored at −80 °C in an ultra-low temperature freezer. The nutritional content of the milk is presented in Table 1.
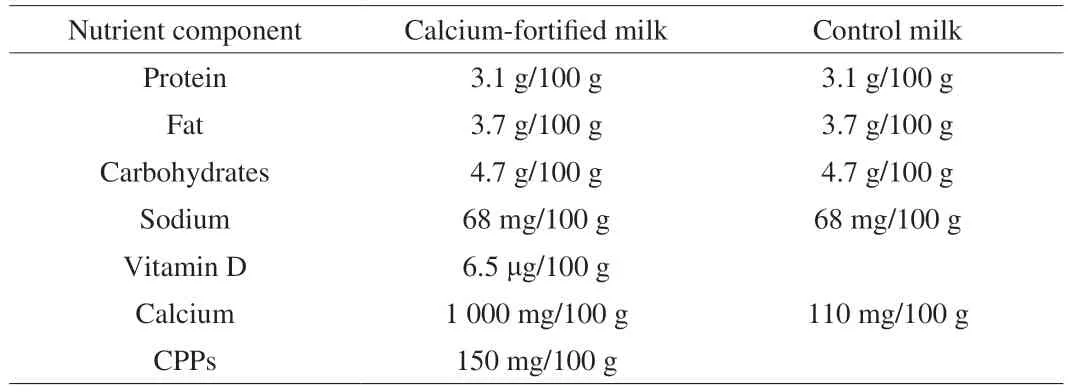
Table 1 Nutritional content of the milk used in the study.
2.2 Experimental animals and groups
Fifty-six 10-week-old female, non-pregnant specific pathogen-free Sprague-Dawley rats were purchased from SiPeiFu (Beijing, China;license number: SCXK (Beijing) 2019-0010; animal quality certificate number: 110324220100282383). Throughout the experiment, the rats hadad libitumaccess to water and food and were kept in a barrier environment with a temperature of 20–26 °C, humidity of 40%–60%,and an alternating light/dark cycle of 12 h/12 h.
To assess the effects of nutrient addition on bone mineralization at normal calcium intake, the recommended daily intake of 800 mg calcium in humans was converted to a dose of 16 mg per day in rats based on body weight (a rat weighing 200 g was administered 1.6 mL/day). The health status was monitored daily, and body weight was recorded weekly. At the end of the experiment, rats were euthanized, and their blood was collected and centrifuged at 1 445.5 ×gfor 10 min to extract supernatant and at 7 552 ×gfor 3 min to separate serum, which was stored at −80 °C until further analysis. Their fecal samples were collected after final milk gavage and stored at −80 °C for microbiome analysis.
The protocols were approved by the Animal Care and Use Committee of Sino Animal (Beijing) Science and Technology Development Co., Ltd. (20210118YZE-3R, Beijing, China). The experiments were performed following the Guide of Experimental Animals of the Ministry of Science and Technology (Beijing, China).
2.3 Ovariectomy procedure
Bilateral ovariectomy or sham surgery was performed on 12-week-old rats. After anesthesia with isoflurane inhalation, the abdominal surgical area was shaved and disinfected. An incision of 2–3 cm was made on either side of the abdominal midline, and the abdomen was cut open. The ovaries were exposed, and the blood vessels around the ovaries were clamped with hemostatic forceps, and the fat around the ovaries was ligated using sterile surgical suture. In the sham group, a small amount of fat was removed, whereas in the OVX group, the ovaries were ligated using sterile surgical sutures and subsequently removed.
2.4 Microcomputed tomography
Microcomputed tomography (micro-CT) scanning of rat femurs was performed using an MM CT (Inveon; London, England) at a resolution of 9.21 μm. Scanning parameters included a voltage of 80 kV, a current of 500 μA, and an exposure time of 1 000 ms. A single projection was taken every 1° throughout the scan (n= 360 total projections). The entire image contained 1 536 tomograms, with an effective pixel value of 18.42 μm. 2D images were reconstructed into 3D renderings using the Cobra EXXIM package. Scanned and reconstructed data were imported into the Inveon Research Workplace for analysis. The region of interest was analyzed in 1-mm steps, beginning downward from the lowest point of the meniscus.Accordingly, the analysis results included images and data related to the transverse, coronal, and sagittal planes of the femur, as well as the transverse and coronal stereograms of the region of interest.Conversions were performed to derive the BMD and the following trabecular histomorphometric parameters: bone volume/tissue volume(Bv./Tv.), trabecular number (Tb.N), trabecular thickness (Tb./Th.),and trabecular separation (Tb.Sp.).
2.5 Dual-energy X-ray absorptiometry (DEXA) measurement and bone biomechanical testing
The bone mineral content (BMC) and BMD of the femurs and lumbar vertebrae (L5 and L6) were measured using Discovery A DEXA instrument (Hologic, Marlborough, MA, USA) following the manufacturer’s protocol. A small-animal bone strength analyzer(YLS-16A; Yiyan, Jinan, China) was used for biomechanical testing.The rat femur was placed on the bracket, and a clamp was applied to turn on the instrument. The pressure head was advanced, and upon contacting the bone, the pressure on the femur was displayed. Pressure was increased continuously; when the femur received the maximum pressure for fracture, the pressure head was moved back, and the data displayed at the time point were recorded as the maximum lateral bearing pressure of the bone (i.e., the load-bearing strength). The ratio of the final load to the displacement of the femur at fracture was defined as bone stiffness.
2.6 Serum biochemical parameter testing
OPG, RANKL, 1,25-dihydroxyvitamin D3(1,25-(OH)2D3),procollagen type 1 N-propeptide (PINP), osteocalcin (OC), tartrateresistant acid phosphatase 5b (TRACP-5b), and collagen type 1 C-telopeptide (CTX-1) levels were measured using enzyme-linked immunosorbent assay kits (Feiya Biotechnology, Jiangsu, China).Alkaline phosphatase (ALP), blood calcium, and blood phosphorus levels were measured using a fully automated biochemical analyzer(AU5800, Beckman-Coulter, Brea, CA, USA) following the manufacturer’s protocol.
2.7 Gut microbiome analysis
Bacterial genomic DNA was extracted from 100 mg of homogenized fecal samples using a QIAamp Fast DNA Stool Mini Kit(Qiagen, GmbH, Hilden, Germany) according to the manufacturer’s protocol. The V4 region of 16S rRNA genes (primer 515F and 806R) was amplified by PCR using 10 ng of DNA as a template in a 30 μL reaction mixture containing 15 μL of Phusion High-Fidelity PCR Master Mix (New England Biolabs) and 0.2 μmol/L of forward and reverse primers. The PCR program was as follows:3 min at 95 °C, 25 cycles of 30 s at 95 °C and 30 s at 55 °C, and 30 s at 72 °C. The PCR products were electrophoresed on an agarose gel,and target bands were recovered using QIAquick Gel Extraction Kit according to the manufacturer’s instructions. Sequencing libraries were constructed using the NEBNext Ultra DNA Library Prep Kit,quantified using Qubit and quantitative PCR, and sequenced on the NovaSeq6000 platform after validation following the manufacturer’s recommendations.
2.8 Statistical analysis
Statistical analysis was performed using R (version 3.5.3)[28].Significant differences in body weight, femoral trabecular parameters,BMC, BMD, bone strength, and serum indicators among the 4 groups were determined using the Games-Howell test, whereas the Holm method was used forP-value correction. Gut microbiome data were filtered by disregarding taxa with a relative abundance < 1% of the sample population. Count data were normalized using total sum normalization by dividing feature read counts by the total number of reads in each sample. Relative abundances of gut microbial genera,α-diversity,β-diversity, and correlations were analyzed using the R package ‘microeco’ (version 0.5.1)[29].P< 0.05 was considered statistically significant.
3. Results
3.1 Effect of FM on the femoral microstructure of OVX rats
To evaluate the effects of FM on postmenopausal osteoporosis,we established an OVX rat model of osteoporosis. Thirty-eight Sprague-Dawley rats underwent bilateral ovariectomy, and 18 were sham-operated. After 2 months of recovery, OVX rats received no supplementation, control milk (CM, 110 mg Ca/100 g), or FM(including vitamin D, CPPs, 1000 mg Ca/100 g) for three months(n= 18 per group). At 2 and 5 months post ovariectomy, major indicators of bone health were assessed (Fig. 1).
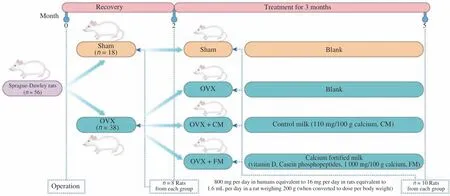
Fig. 1 Experimental flow chart. The rat model was established using bilateral ovariectomy (n = 38). Control rats were subjected to sham surgery (n = 18). After 2 months of recovery, eight rats per group were selected to detect the indices. The OVX group was divided into OVX (n = 10), OVX + CM (n = 10), and OVX + FM (n = 10). Rats were gavaged with the same amount of water, CM, or FM daily, according to their body weight index. After 3 months, 10 rats in each group were selected to detect the indices. OVX, ovariectomized; CM, control milk; FM, calcium-fortified fresh milk.
Ovariectomy led to rapid weight gain. At the end of the recovery period, compared with the weight of rats in the sham5M(5 months after sham operation) group, rat weight in the OVX + FM(P= 0.006), OVX + CM (P= 0.018), and OVX5M (5 months after ovariectomy) (P= 0.003) groups was significantly increased(Fig. 2A). After the 3-month intervention period, rat weight in the OVX group did not vary significantly (Fig. 2A). The effects of FM and CM on skeletal parameters were examined using micro-CT. Compared with those in the sham2M (2 months after sham operation) group,rats in the OVX2M (2 months after ovariectomy) group exhibited severe trabecular bone loss and cortical bone thinning (Fig. 2B).After the 3-month intervention period, rats in the OVX5M group showed severe trabecular bone loss when compared with those in the sham5M group. Although OVX + CM slightly protected against extensive bone loss, the trabecular bone of OVX + CM rats had large voids and a less dense structure. In contrast, rats in the OVX + FM group had a dense trabecular bone morphology comparable with that of sham rats (Fig. 2C), indicating that FM delayed trabecular bone loss in OVX rats. The trabecular parameters indicated that FM had a better protective effect than CM. At 2 months post ovariectomy,femur bone mineral density (FBMD) was significantly decreased(P= 0.002). After the 3-month intervention period, FBMDs in the OVX5M and OVX + CM groups remained significantly decreased compared with those in the sham5M group (P= 0.000 1), whereas the FBMD decrease in the OVX + FM group was not significant compared with that in the OVX5M group (Fig. 2D).
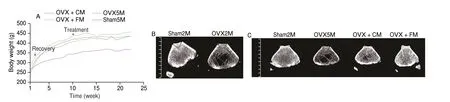
Fig. 2 Effect of FM on the femoral microstructure of OVX rats. (A) Mean body weight of rats. Twelve-week-old rats were subjected to ovariectomy, and intervention was initiated after 2 months of recovery from surgery (n = 10). (B, C) Micro-CT analysis of 3-dimensional (3D) images of vertical sections of the femur. Each interval of the ruler is 1 mm. (D–I) Quantification of femoral trabecular parameters based on micro-CT analysis. (D) FBMD. (E) Trabecular bone volume fraction (Bv./Tv.). (F) Trabecular thickness (Tb./Th.). (G) Trabecular number (Tb.N). (H) Bone surface area/bone volume (Bs./Bv.). (I) Trabecular spacing(Th./Sp.). *P < 0.05, **P < 0.01. Data are presented as the mean ± SEM (sham2M/OVX2M: n = 8, sham5M/OVX5M/OVX + CM: n = 10, OVX + FM: n = 9).CT, computed tomography; SEM, standard error of the mean.
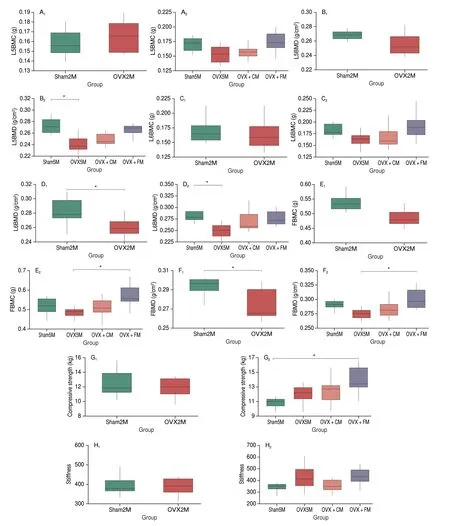
Fig. 3 Effects of FM on FBMC, FBMD, and bone strength in OVX rats. (A–F) DEXA analysis of BMC and BMD. (A, B) BMC and BMD of lumbar vertebra L5. (C, D) BMC and BMD of lumbar vertebra L6. (E, F) BMC and BMD of the femur. (G) Maximum horizontal pressure on the bone. (H) Femoral stiffness.*P < 0.05. Data are presented as the mean ± SEM (sham2M/OVX2M: n = 8; sham5M/OVX5M/OVX + CM/OVX + FM: n = 10). BMC, bone mineral content;BMD, bone mineral density.
At 2 months post bilateral ovariectomy, the Bv./Tv. (P= 0.000 8),Tb./Th. (P= 0.003), and Tb.N (P= 0.000 1) in the OVX2M group were significantly reduced (Figs. 2E–G). After the 3-month intervention period, these parameters were higher, although not significant in all cases, in the OVX + FM group than in the OVX5M and OVX + CM groups (Figs. 2E–G). The Bs./Bv.(P= 0.000 6) and Th./Sp. (P= 0.000 1) were significantly increased after ovariectomy, whereas after the 3-month intervention period, these parameters were lower, although not significant in both cases, in the OVX + FM group than in the OVX5M and OVX + CM groups (Figs. 2H, I).
3.2 Effects of FM on femur BMC (FBMC), FBMD, and bone strength in OVX rats
The BMD and BMC of two lumbar vertebrae (L5 and L6) and femurs in all groups are shown in Figs. 3A–F. At 2 months post ovariectomy, no significant change was observed in the BMC of the lumbar vertebrae and femur; however, the BMD of L6 (P= 0.022)and the femur (P= 0.016 3) was significantly decreased. After the 3-month FM intervention period, the BMC of L5 and L6 improved and was slightly, but not significantly, higher than that in the sham5M group. In contrast, FM significantly improved the FBMC (P= 0.031).Both FM and CM supplementation increased the BMD of the lumbar vertebrae and femur, which were reduced by OVX; however, the difference was only significant for the femur in the OVX + FM group(P= 0.022; thePvalue were 0.082 and 0.068 for L5 and L6 in the OVX + FM group, respectively).
Measurement of the biomechanical properties of the femur in the 4 groups revealed that at 2 months after ovariectomy, bone compressive strength and stiffness were slightly, but not significantly,reduced in the OVX2M compared with those in the sham2M group.After the 3-month intervention period, compressive strength was slightly increased in the OVX + CM group and significantly increased in the OVX + FM group (P= 0.033), whereas stiffness was slightly,but not significantly, improved in the OVX + FM group (Figs. 3G, H).
3.3 Effects of FM on regulators of calcium and phosphorus metabolism in OVX rats
Table 2 presents the changes in regulators of calcium and phosphorus metabolism in rats before and after intervention. At 2 months post ovariectomy, parathyroid hormone (PTH) and phosphorus levels were slightly increased, 1,25-(OH)2D3levels were relatively unchanged, and calcium levels significantly decreased(P= 0.007). After the 3-month intervention period, 1,25-(OH)2D3level was the highest in the OVX + FM group, although the increase was not significant. Compared with those in the OVX5M group,PTH and phosphorus levels in FM- and CM-supplemented groups exhibited increased, although not significantly. No significant changes in calcium levels were observed.
3.4 Effects of FM on bone turnover markers in OVX rats
Fig. 4 shows the changes in bone turnover markers in rats before and after intervention. At 2 months after ovariectomy, OC(P= 0.036) and OPG (P= 0.028) levels were significantly decreased in the OVX2M rats compared with those in the sham2M group. Additionally, estrogen (E2), ALP, and RANKL levels were increased, whereas PINP, TRACP-5b, and CTX-1 levels were decreased; however, the differences were not significant. After the 3-month intervention period, E2 levels tended to increase in the OVX + FM and OVX + CM groups compared with those in the OVX5M group, although not significantly. Furthermore, FM supplementation decreased ALP level similar to that in the sham5M group. Among the bone formation markers, OC and PINP levels were increased, although not significantly, in the OVX + FM group, whereas OPG level was significantly increased (P= 0.004).RANKL levels did not differ significantly among the four groups.The osteoclast markers CTX and TRACP-5b were also decreased(P= 0.000 05) in the OVX + FM group.
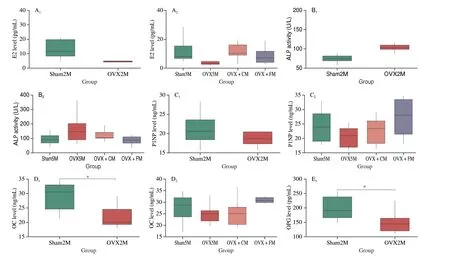
Fig. 4 Effects of FM on bone turnover markers. The levels of (A) E2, (B) ALP, (C) PINP, (D) OC, (E) OPG, (F) RANKL, (G) CTX-1, (H) TRACP-5.*P < 0.05, **P < 0.01. Data are presented as the mean ± SEM (sham2M/OVX2M: n = 8; sham5M/OVX5M/OVX + CM/OVX + FM: n = 10).
3.5 Effect of FM on the gut microbiota in OVX rats
3.5.1 Analysis of gut microbial composition and relative abundance
To determine the effects of FM and CM on the gut microbiota of OVX rats, the microbiome structure was compared at different phylogenetic levels using 16S rRNA sequencing analysis. At the phylum level, the gut microbiota of the Sprague-Dawley rats consisted of Proteobacteria, Actinobacteriota, Acidobacteriota, Bacteroidota,Verrucomicrobiota, and Firmicutes. At 2 months after ovariectomy,the relative abundances of Acidobacteriota and Verrucomicrobiota were reduced by 2%, while that of Actinobacteriota increased by 3%. After the 3-month intervention period, the relative abundances of Acidobacteriota and Actinobacteriota were reduced by 15% and 10%, respectively, in the OVX5M group compared with those in the sham5M group, whereas those of Bacteroidota and Firmicutes were increased by 13% and 14%, respectively. After FM intervention,compared with OVX5M group, the abundances of Acidobacteriota and Actinobacteriota were increased by 10% and 6%, respectively,whereas those of Bacteroidota and Firmicutes were decreased by 9%and 11%, respectively, resembling the phyletic abundance in the sham group. In the OVX + CM group, the abundance of Acidobacteriota was increased by 6%, whereas that of Actinobacteriota was decreased by 5%, with no significant differences in the abundance of Bacteroidota or Firmicutes, indicating that CM was not as effective as FM (Fig. 5A). To identify the specific microbial populations influenced by FM intervention, the relative gut microbiota abundances were also compared between OVX and sham rats at the genus level. The gut microbiota of the sham group was dominated by the genera Subgroup 2,Sphingomonas, SC-I-84, Ellin6067,Candidatusudaeobacter,Candidatussolibacter,Bryobacter, andBradyrhizobium(total abundance: sham2M, 67%; sham5M, 60%). In contrast, the relative abundances of these advantageous genera were reduced by 5% in the OVX2M group and by 55% in the OVX5M group, and the advantageous genera shifted toRhodanobacter(28%),Phos-He36(27%),Sinomonas(18%), andThiomonas(13%). After FM intervention, the relative abundances of these 4 advantageous genera decreased by 12%, 21%, 16%, and 10%, respectively, to proportions notably closer to those in the sham group (Fig. 5B). In the OVX + CM group, the abundances of PHOS-He36,Sinomonas,andThiomonaswere decreased, whereas that ofRhodanobacterwas increased to 41%.
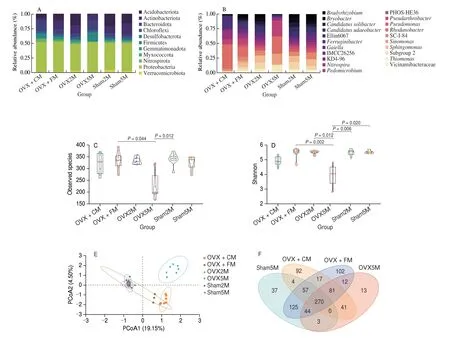
Fig. 5 Structural comparison of gut microbiota. (A) Relative abundance at the phylum level. (B) Relative abundance at the genus level. Changes in fecal microbial diversities upon different interventions measured by α-diversity. (C) Observed species index. (D) Shannon index. (E) Principal coordinates analysis of unweighted UniFrac distances was used to evaluate differences in the total gut microbiota abundance among the groups. (F) Venn diagram showing the numbers of unique and common bacterial genera in the gut microbiota of all groups.
3.5.2 Analysis of gut microbial diversity
The effects of ovariectomy and FM intervention on the gut microbiome structure were assessed by calculatingα- andβ-diversity indices. Theα-diversity index analysis showed that after ovariectomy,the observed species index (P= 0.012) and Shannon index(P= 0.006) decreased significantly in the OVX5M group. Following the 3-month intervention period, the observed species index(P= 0.044) and Shannon index (P= 0.002) were significantly improved in the OVX + FM group (Figs. 5C, D). The results of the principal coordinates analysis of unweighted UniFrac distances showed that samples collected after the three-month intervention period were clearly separated from the initial samples and more closely resembled the microbial community of normal rats (Fig. 5E).
We also created Venn diagrams to analyze common and unique bacterial genera among the 4 groups (Fig. 5F). With 102 unique genera, the OVX + FM group had the highest genus-level diversity,followed by the OVX + CM (92 genera), sham5M (37 genera), and OVX5M (13 genera) groups.
3.5.3 Analysis of the association among gut microbiota composition, gene functional signature, and BMD value/bone turnover markers
We evaluated the correlation among the relative abundance of GM composition, gene functional annotation, and BMD-values/bone turnover markers in the sham5M, OVX5M,OVX + FM, and OVX + CM groups (Fig. 6). Methyloligellaceae,Reyranellaceae, Mycobacteriaceae, Dongiaceae, Geminicoccaceae,and Phaselicystidaceaewere significantly positively correlated with serotonergic synapse, the relaxin signaling pathway, steroid hormone biosynthesis, and the biosynthesis of unsaturated fatty acids signaling pathway, whereas they were significantly negatively correlated with vitamin B6metabolism and antifolate resistance. In contrast,Desulfovibrionaceaeand Corynebacteriaceaewere significantly negatively correlated with serotonergic synapse, relaxin signaling pathway, steroid hormone biosynthesis, and the biosynthesis of unsaturated fatty acids signaling pathway, whereas they were significantly positively correlated with vitamin B6metabolism and antifolate resistance. Serotonergic synapse, relaxin signaling pathway,and biosynthesis of steroid hormones and unsaturated fatty acids were significantly positively correlated with OC, FBMD, and lumbar BMD(L5, L6), and significantly negatively correlated with TRACP-5b.Gene functional vitamin B6metabolism and antifolate resistance were significantly negatively correlated with FBMD and lumbar BMD (L5,L6) and significantly positively correlated with TRACP-5b.
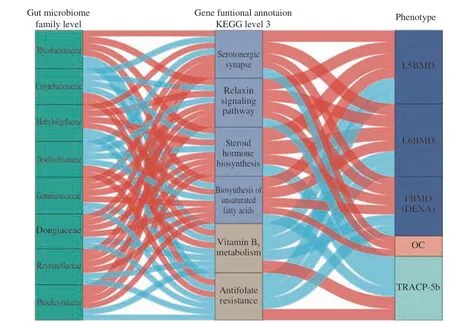
Fig. 6 Interrelationship between gut microbiota composition, gene functional signature, and BMD value/bone turnover markers. Visualization of the correlation network according to Spearman correlation test (P < 0.05 for all correlations); Red line indicates a positive correlation, whereas blue line represents a negative correlation. Kruskal-Wallis adjusted P-value test was used to screen 8 significantly different bacteria at the family level in the sham5M/OVX5M/OVX + CM/OVX + FM4 group (P < 0.05). At level 3 of the Kyoto Encyclopedia of Genes and Genomes (KEGG) functional annotation,6 significantly different functional pathways were screened (P < 0.05). The detailed coefficient values are listed in Table S1.
Collectively, our findings suggest that these 6 functional gene pathways may play important roles in maintaining normal FBMD by regulating metabolic pathways and that the gut microbiota may affect postmenopausal osteoporosis progression by regulating host metabolism.
4. Discussion
New therapies for postmenopausal osteoporosis are still required.Dairy products are representative dietary source rich in nutrients[13].Herein, we used low-temperature fresh milk as a vehicle and fortified it with dairy-derived calcium, vitamin D, and CPP as a potential intervention for postmenopausal osteoporosis. Two months after ovariectomy, the BMD of the lumbar spine (L6) and femur decreased significantly, demonstrating that the OVX model was successfully established. In addition, bone stiffness and compressive strength of the femur decreased two months after ovariectomy. After 3-month FM intervention, the compressive strength of the femur increased significantly. Notably, the biomechanical strength of rat femurs is affected by various factors, including the length, shape, and flexibility of the femur, which inherently differ among rats. Additionally, bone is not a homogeneous tissue, implying that biomechanical tests may be less accurate and less conclusive than DEXA or micro-CT analysis.
The regulatory effect of FM on bone health in OVX rats occurs following uptake of the vitamin D therein, which is converted into the biologically active form of 1,25-(OH)2D3[30], which regulates the transcription of insulin, transforming growth factor (TGF)-β1,Wnt, and various cytokines by binding to the vitamin D receptor and retinoid X receptor heterodimer[31]. This stimulates the production of bone and skeletal muscle-derived factors[32]. Additionally,1,25-(OH)2D3modulates the innate and acquired immune systems by regulating intracellular calcium ion concentration[33-34]and protects against inflammation[35]. Thus, 1,25-(OH)2D3may alleviate inflammation caused by estrogen loss and protect bone tissue. Since urinary and fecal calcium levels were not measured, based on the observed results, we speculate that the calcium in FM was converted into bone calcium in the rats. Furthermore, the acidic motif in CPP in FM provides binding sites for divalent cations, potentially enhancing its ability to bind and solubilize minerals, such as Ca2+.The binding of CPP to calcium yields a hydrophobic form, preventing the formation of insoluble casein phosphate; this form accumulates at the terminal ileum and promotes passive absorption of Ca2+and other trace elements while increasing calcium bioavailability in the ileum or distal small intestine. Passive vitamin D-independent calcium transport pathways in the ileum and distal small intestine are the primary mode of calcium absorption from the diet[36]. Serum ALP levels are elevated in OVX rats; research shows that vitamin D and Ca supplementation significantly reduce serum ALP levels[37].This corresponds to the results of this study, which showed that FM intervention can reduce ALP levels to near-normal values, thereby suggesting that FM intervention improves the dynamic balance of bone formation and facilitates the maintenance of bone health[38].Low calcium and phosphorus levels as well as vitamin D deficiency elevate PTH levels and impair muscle function while increasing bone resorption, cortical bone porosity, and fracture risk[39]. However, in the present study, blood calcium, phosphorus, and vitamin D levels were not significantly reduced. Furthermore, there was a tendency toward BMD improvement, suggesting that in OVX rats, FM increases the levels of PTH, by which transient stimulation of parathyroid hormone 1 receptor (PTHR1) by PTH activates the cAMP-dependent protein kinase A (PKA) and phospholipase C-protein kinase C (PKC)pathways, resulting in the transcription of genes required for bone formation[40], and promoting the proliferation and differentiation of osteoblasts and their precursor cells[41]. In addition, the positive regulatory effect on bone metabolism can be attributed to the bettermaintained biological activity after pasteurization of milk proteins present in FM and the biologically active peptides derived from them,such as the bovine milk basic protein lactoferrin. Lactoferrin regulates the proliferation and differentiation of osteoblasts by activating the transforming growth factor β (TGF-β) and mitogen-activated protein kinase (MAPK) signaling pathways by binding to low-density lipoprotein receptor-related protein-1, and acts as a survival factor that protects osteoblasts from apoptosis. Lactoferrin interferes with TNF receptor-associated factor polyubiquitination and inhibits MAPK pathway activation, thereby attenuating lipopolysaccharide-induced osteoclastogenesis[42]and inhibiting osteoclast proliferation[43].Lactoferrin has also been shown to be positively correlated with bone mass in OVX rats and mice[44].
FM acts on bone metabolism by regulating gut microbes.The present study differed from previous studies on the effects of fermented milk on gut microbiome composition in OVX rats, in that no additional probiotics were added to the FM. To confirm the regulatory effects of the gut-bone axis in OVX rats, the changes in the gut microbiota caused by FM intervention and chronic estrogen loss in OVX rats were investigated. At the phylum level, the Firmicutesto-Bacteroidetes ratio increased after ovariectomy. This is consistent with the significant increase in body weight observed in OVX rats. After a three-month intervention period, the Firmicutes-to-Bacteroidetes ratio in the sham5M, OVX5M, OVX + CM, and OVX+ FM groups (0.21, 0.87, 0.45, and 0.46, respectively) revealed that both FM and CM effectively inhibited the increase in the Firmicutesto-Bacteroidetes ratio in OVX rats, and FM effectively inhibited the increase in the abundances of disadvantageous genera in OVX rats.Regarding to phyla and genera structure and abundance, the OVX+ FM group was most similar to the sham5M group. To the best of our knowledge, this is the first study to monitor long-term changes in the intestinal microecology of OVX rats. In contrast to the findings of a previous short-term study[45], we found that genus richness and diversity of the gut microbiota of OVX rats decreased significantly 5 months post-surgery, and the relative gut microbiota abundance was more significantly altered than that in the OVX2M rats. This suggests that chronic estrogen deficiency (5 months after ovariectomy) may lead to significant changes in the gut microbiota and that FM intervention may reverse these changes by significantly increasing species richness and diversity. Furthermore, FM may alter the taxonomic structure in the gut,alleviating adverse effects of estrogen loss.
Methyloligellaceae, Reyranellaceae, Mycobacteriaceae,Dongiaceae, Geminicoccaceae, and Phaselicystidaceae may affect 5-hydroxytryptamine (5-HT) receptor (5-HTR1A), increase 5-HT release and core gene-activated protein kinase PKA, activate serotonergic synapse signaling pathways, affect the calcium signaling pathway, and increase 5-HT expression. Previous studies have shown that 5-HT may also participate in the regulation of bone formation by affecting low-density lipoprotein receptor-related protein 5 of the Wnt/β-catenin signaling pathway; moreover, it can regulate RANKL or activate NF-κB to regulate osteoclast differentiation[46]. The Wnt pathway upregulates OPG transcription[47], thus corresponding to the significant increase in OPG levels in the OVX+FM group.The RANKL/receptor activator of NF-κB (RANK)/OPG signaling pathway is an important indicator for evaluating bone resorption and bone reconstruction to modulate bone metabolism[48]. RANKL,a TNF family member, is located on the surface of certain myeloid and dendritic cells and is involved in the regulation of osteoclast activation, development, differentiation, and maintenance[49]. The binding of RANKL to its receptor leads to osteoclast production,activation, differentiation, and survival[50]. Thus, OPG and RANK compete for binding to RANKL, and when RANK and RANKL bind,OPG inhibits their effects on osteoclasts (e.g., osteoclastogenesis)[51-52],thereby providing protection against bone loss and osteoporosis.Methyloligellaceae, Reyranellaceae, Mycobacteriaceae, Dongiaceae,Geminicoccaceae, and Phaselicystidaceae are involved in the relaxin signaling pathway. Relaxin is a hormone structurally related to insulin and insulin-like growth factor. By binding to the relaxin family peptide receptor, it regulates osteoblast metabolism and proliferation through the TGF-β, PKA, and phosphatidylinositol 3-kinase (PI3K)-protein kinase B (Akt) pathways, increases the expression of osteoblast markers OC and PINP, and acts on the calcium signaling pathway to affect calcium absorption[53]. These families can also produce polyunsaturated fatty acids through biosynthesis of unsaturated fatty acids, thereby inhibiting RANKLmediated osteoclastogenesis[54]. Since biomarkers of collagen synthesis and degradation are among the “gold standard” for assessment of bone histomorphometry, the International Osteoporosis Foundation and the European Calcified Tissue Society recommend the use of CTX and PINP as reference markers for bone resorption and formation, respectively[55]. TRACP-5b, an enzyme expressed only by intracellularly activated macrophages in osteoclasts, can be used to monitor therapeutic response in osteoporosis, and is significantly negatively correlated with BMD[56]. In addition,estrogen can inhibit the activity of osteoclasts and promote osteoblast proliferation and differentiation. Methyloligellaceae,Reyranellaceae, Mycobacteriaceae, Dongiaceae, Geminicoccaceae,and Phaselicystidaceae may promote estrogen (estrone and estradiol)production through the steroid hormone biosynthesis pathway, thereby alleviating estrogen loss after ovariectomy. This corresponds to the elevated estrogen levels observed in this study. Desulfovibrionaceae and Corynebacteriaceae may activate the vitamin B6metabolic pathway, promote estrogen metabolism[57]and 5-HT production[58],and inhibit NF-κB signaling through the antifolate resistance signaling pathway, thereby blocking the release of proinflammatory cytokines.These results suggest that FM relies on calcium, vitamin D, proteins,and other nutrients to directly act on bone metabolism. However,analysis of the experimental results indicated that there was no significant change in calcium and vitamin D levels in ovariectomized rats and that FM significantly regulated the composition, abundance,and diversity of intestinal flora in ovariectomized rats, and the strong and significant correlation between intestinal flora and BMD indicates that FM mainly plays an indirect role in bone metabolism by regulating intestinal flora, thereby effectively promoting bone health (Fig. 7).

Fig. 7 Potential mechanisms of FM acting on bone metabolism by regulating gut microbiota in OVX rats. FM relies on calcium, vitamin D, proteins, and other nutrients to directly act on bone metabolism, and significantly improve the diversity and richness of intestinal flora and regulate intestinal flora. Through the serotonergic synapse, the relaxin signaling pathway approach applied to TGF-β, calcium signaling pathways, PKA, PI3K/Akt, promote osteogenesis. The markers OPG, PINP, OC, calcium, 1,25-(OH)2D3, and PTH also promote production of osteogenic markers. OPG competes with RANKL to inhibit osteoclast production.The gut microbiota can promote estrogen production and osteoblast function, but inhibit osteoclast function through steroid hormone biosynthesis and vitamin B6 metabolism pathways. In addition, the microbiota can inhibit the production of osteoclast markers CTX and TRACP-5b through antifolate resistance and unsaturated fatty acid biosynthesis pathways.
Our study has some limitations. First, we did not compare fresh milk with room temperature milk, nor did we add different ratios of vitamin D and CPP to optimize the formula. Second, although the 16S rRNA technology is powerful, it has limited resolution in assessing functional potential. In the future, the effects of FM on the intestinal microbiota composition in different parts of the gastrointestinal tract of OVX rats should be further investigated at the gene expression level. Furthermore, the absorption process of calcium-rich milk in the body should be dynamically evaluated and optimal levels of calcium and vitamin D fortification should be determined. The benefits of dairy products cannot be simply attributed to added nutrients alone(e.g., calcium and vitamin D). Third, the FM used in this study demonstrated an excellent anti-osteoporosis effect; however, the synergistic effects of the functional factors added to the FM and the underlying mechanisms remain to be explored.
5. Conclusion
FM directly affected bone metabolism in ovariectomized rats,significantly increased gut microbiota abundance and diversity in ovariectomized rats, and regulated the composition of the gut microbiota. It mostly acted indirectly on the microbe-gut-bone axis through steroid hormone biosynthesis, the relaxin signaling pathway,serotonergic synapse, and the biosynthesis of unsaturated fatty acids pathways. Thus, it delayed estrogen loss, decreased the levels of ALP and the bone resorption markers CTX and TRACP-5b, and stimulated osteoblast activity by increasing the abundance of the bone formation markers (OC and PINP) and OPG levels. Consequently,osteoclast function was inhibited via the RANKL/RANK/OPG signaling pathway, and bone resorption was attenuated, thus exerting positive effects on BMC, BMD, bone microarchitecture, trabecular parameters, and bone compressive strength in the lumbar spine and femurs of OVX rats. Our results suggest FM as an intervention for ameliorating primary postmenopausal osteoporosis.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (32072191), Daxing District Major Scientific and Technological Achievements Transformation Project (2020006),Beijing Innovation Team Project of Livestock Industry Technology System, and Beijing Science and Technology Special Project(Z201100002620005).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.26599/FSHW.2022.9250105.
- 食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango

