Oral administration of Bacillus coagulans TQ-35 alleviates allergic responses inOVA-sensitive BALB/c mice
Yifn Wng, Shnjun Chen, Chong Wng, Yi Zhng, Hongling Zeng, Linglin Fu, Yno Wng,c,
a Food Safety Key Laboratory of Zhejiang Province, School of Food Science and Biotechnology, Zhejiang Gongshang University, Hangzhou 310018, China
b College of Food Science, Fujian Agriculture and Forestry University, Fuzhou 350002, China
c School of Food and Health, Beijing Technology and Business University, Beijing 100048, China
Keywords: Bacillus coagulans TQ-35 Food allergy Ovalbumin (OVA)Gut microbiota
ABSTRACT Bacillus coagulans has been extensively studied so far, but there has been a lack of research on its usage in allergy. In this study, we designed to assess the effect of different concentrations of B. coagulans on food allergy in a BALB/c mouse model of ovalbumin (OVA)-induced food allergy and its effect on gut microbes.The assessment of symptoms, specific immunoglobulin E (IgE), T-cell differentiation, and related gene expression levels in sensitized mice by assay indicated that high doses of oral B. coagulans could alleviate allergic symptoms. Treatment with B. coagulans, in the high-dose group, signif icantly reduced IgE and IgG1 levels and modulated the balance of T helper type 1 cell (Th1) and Th2 and the expression of relevant genes in the spleen. 16S rRNA analysis showed that probiotics improved the structure of the microbiota, in particular by boosting the percentage of Clostridia, Bacteroides vulgatus and Enterococcus faecium, and by increasing the abundance of microbial species, thereby modulating the immune system. Therefore, this study can provide insights into the practical application of B. coagulans doses to alleviate OVA allergy.
1. Introduction
Food allergy, which was usually harmless to healthy people, was an undesirable response to a specific food antigen[1]. At the onset of a food allergy, the body’s immune system reacted abnormally to certain food proteins, which could involve many systems in the body and lead to anaphylaxis and even life-threatening conditions[2]. The most serious symptoms of food allergy as known as allergic reactions can involve skin, respiratory and gastrointestinal disturbances as well as cardiovascular abnormalities and lead to life-threatening hypovolemic shock and respiratory compromise[3]. In the past few years, food allergy with the incidence of the disease increasing year by year worldwide has become a food safety and public health issue of global concern. Data indicated that the prevalence of allergy in Western countries remained high at 10% and rising, with the highest prevalence in young children, while at the same time, the prevalence of allergy in developing countries was increasing every year[4]. Protein in food could contribute nutrients to the body, but simultaneously it may also trigger food allergy[5-6]. The ovalbumin (OVA) in eggs,with a high research value, was the most abundant protein in egg whites, containing up to 54%[7]. OVA was the main protein causing egg allergy. Egg allergy was the second most common food allergy in infants and children apart from milk.
Nevertheless, the lack of robust treatment and prevention for food allergy was still a problem[8]. Accurate avoidance of food allergens remained the only effective method. However, egg avoidance can cause severe dietary restrictions. Eggs made up a large part of people’s food leading to frequent misuse. Therefore, alternative treatment options and effective preventive measures for OVA allergy were urgently needed to restore natural oral immune tolerance.
In addition to host factors, environmental factors, such as diet and microbiota, have been shown to influence allergy symptoms[9]. For this reason, new allergy treatments have been sought. Among them,probiotic therapy has gained increasing attention. The prevention of food allergy through probiotics has been highlighted as a promising goal[10]. Probiotic modulated the immune system of the intestinal mucosa and thus intervened in food allergy. Probiotics and their metabolites can reduce food allergy by interacting with immune cells and the intestinal microbiota[11]. On the other hand, both the duration and dosage of probiotic administration can influence the outcome of food allergy treatment. Therefore, there was still a need for further research into the clinical use of probiotics.
Probiotics, living microorganisms that benefited the wellness of the subject, provided that when consumed in sufficient quantities. By influencing the rich and diverse microbiota and immune system of the human gastrointestinal mucosa and skin surface, probiotics can improve health and reduce the disease risk[12]. As a result, probiotics received widespread attention. However, some traditional probiotics,such asLactobacillus,Bifidobacterium, andSaccharomyces[13], had great probiotic functions but were poorly resistant. Consequently,they can’t survive in the complex environment of the human gastrointestinal tract, and their survival rates were generally low[14].Therefore, spore-producing bacteria gained increasing attention from scholars.Bacillus coagulanswas a bacillus-producing probiotic whose acid-tolerant spores make it highly resistant to gastric juices and bile salts, so highly tolerant of the gastrointestinal environment[15].Studies have shown thatB. coagulansalters the T helper type 1 cell (Th1)/Th2/regulatory T cell (Treg) balance by inducing the production of CD4+, Foxp3+, Tregs and interleukin (IL)-10, thereby inhibiting sensitization in shrimp[16]. In past studies,B. coagulanscompared toBifidobacterium longumwas relatively less effective in alleviating shrimp promyosin allergy[17]. Even for the same probiotic,its effectiveness in treating allergy symptoms caused by different proteins may vary. It has been shown that lactic acid-producing bacteria were effective in relieving allergy caused by OVA, so we speculated thatB. coagulans, which had the same function, may be effective in alleviating OVA allergy[18].
We obtained a high colony countB. coagulansliquid by highdensity fermentation and preparedB. coagulanspowder by spray drying. This study aimed to compare the effectiveness of probiotic preparations at different concentrations while studying the effect ofB. coagulanspowder on OVA-allergic BALB/c female mice.On the other hand, to investigate the effect ofB. coagulanspowder administration on the mouse gut flora. The study can provide some concentration reference for the practical use ofB. coagulanspowder in allergy relief and provide theoretical support for the practical production of this probiotic preparation in the food industry, which had market application value.
2. Materials and methods
2.1 Probiotic preparation
B. coagulansTQ-35 was screened from milk by the laboratory.B. coagulansTQ-35 was conserved in the China Center for Type Culture Collection (China) under the conservation number CCTCC NO: M 2022298. The activatedB. coagulansTQ-35 was implanted in a liquid medium containing bran, soybean cake flour and yeast extract (Hongrunbaoshun, Beijing, China) at a rate of 4%, cultured at pH 7.0 and 37 °C for 36 h, filter impurities, and centrifuged at 14 000 r/min for 15 min to collect the bacteria (Beckman Coulter Avanti J-E Centrifuge, USA). Then the bacterial sludge was washed 3 times with phosphate buffered saline (PBS) and redissolved to a bacterial concentration of 5.2 × 1010CFU/mL. Probiotic preparations with a concentration of 4.5 × 1010CFU/g were obtained by spray drying the bacterial solution with algae, fructans (Huarui Biotechnology, Henan, China) and skimmed milk powder (NZMP,New Zealand). The survival rate of probiotics reached 98.685%. The probiotic powder was stored at 4 °C.
2.2 Animals
Forty-five female BALB/c mice at 6 weeks old were obtained from Zhejiang Academy of Medical Sciences. Mice were housed in a room at 23 °C with a 12-h light-dark cycle under specific pathogenfree conditions. Standard mouse food and water were provided to all mice. All experimental protocols were carried out in strict accordance with the recommendations of theNational Guidelines for the Care and Use of Laboratory Animals of China. All the animal procedures were approved by the Zhejiang Gongshang University Laboratory Animal Welfare Ethics Review Committee.
2.3 Experimental design
Thein vivoimmunization model was depicted in Fig. 1. The mice were randomly and equally divided into 9 different groups (n= 5):a control group (Ctrl), an adjuvant group (Ad), an ovalbumin group(OVA), a low-dose treatment group (L-BC), a medium-dose treatment group (M-BC), a high-dose treatment group (H-BC), a low-dose placebo group (L-PL), a medium-dose placebo group (M-PL), a high-dose placebo group (H-PL). The mice were pre-housed for 1 week to acclimatize to the environment. Then the mice were sensitized for a further 2 weeks. The mice in Ctrl group were not treated in any way. Mice in Ad group were treated daily with 2 nmol of MC903 in 20 μL of 100% EtOH on both ears (apical surface).Other mice were all treated daily with 2 nmol of MC903 in 20 μL of 100% EtOH and 100 μg OVA in 10 μL PBS on both ears. All groups of mice were treated orally from day 0 to day 21. The Ctrl and Ad received 200 μL sterile PBS, andB. coagulansTQ-35 was orally administered to 3 different treatment groups by dissolving in 200 μL PBS: L-BC (0.001% probiotic preparation), M-BC (0.1%probiotic preparation) and H-BC (10% probiotic preparation). And placebo (spray-dried protectant: algae, fructans and skimmed milk powder) was orally administered to 3 different treatment groups by dissolving in 200 μL PBS: L-PL (0.001% placebo), M-PL (0.1%placebo), H-PL (10% placebo). Mice were challenged by oral 200 μL OVA solution (250 mg/mL) on day 22 and clinical symptom scores were obtained by individual observation of the mice as indicated in Table 1[19]. The mice were bled from the retro-orbital plexus and fecal samples were collected after 30 min and the above samples were stored at −80 °C until subsequent analysis. And the rectal temperature of the mice before and after the challenge was measured. The mice have fasted overnight after the challenge, and blood, spleen, small intestines and large intestines were collected after humane sacrifice for immunological analysis.

Table 1 Scoring criteria for clinical allergic symptoms in mice.
2.4 Measurement of OVA-specific immunoglobulin E (IgE)and IgG1
Serum allergen-specific IgE and IgG1 were determined by enzyme-linked immunosorbent assay (ELISA). The 96-well plates were coated with 100 μL/well of 10 μg/mL OVA solution overnight at 4 °C. The plates were washed at least 5 times and blocked with 5% BSA (200 μL/well, 0.01 mol/L in PBS at pH 7.4) for 1 h at 37 °C. After washing again, the plate was incubated with mice serum(IgE diluted 1:6, 100 μL/well; IgG1 diluted 1:2 000, 100 μL/well)for 2 h at 37 °C. The plate was washed and incubated with goat antimouse IgE or IgG1 labeled with horseradish peroxidase (diluted 1:5 000, 100 μL/well) for 1 h at 37 °C. After washing, the plate was incubated with 3,3’,5,5’-tetramethylbenzidine in the dark for 20 min at 37 °C and the reaction was terminated with 2 mol/L H2SO4. At last,the optical density was measured at 450 nm by a microplate reader(Molecular Devices, USA)[20].
2.5 Flow cytometry analysis
Single-cell suspensions from the spleen were prepared and incubated for 5 h with the addition of Leukocyte Activation Cocktail(2 μL/mL) (BD, USA). After the collected cells were stained with FVS780-APC-A750, Fc receptor blocking solution (FC) block was added for blocking and incubation. Cells were surface-stained with CD4-fluorescein isothiocyanate (FITC) and then fixed with Fix/perm Working Solution. To detect Th1 and Th2, cells were stained with IFN-γ-APC and IL-4-PE (BD Biosciences, USA) for intracellular markers. The stained cells were measured by flow cytometry and data were analyzed by Cytexpert 2.4 software (Beckman Coulyer, USA).
2.6 Gene expression determination in spleen by real-time quantitative polymerase chain reaction (RT-qPCR)
To measure theA260nm/A280nmabsorbance ratio, the quantity and quality of the total RNA extracted using an E.Z.N.A.®Total RNA Kit II from mouse spleen were assessed with a NanoDrop 2000 spectrophotometer. Using HiScript®II Q RT SuperMix for qPCR +gDNA wiper, the total cDNA was acquired by reverse transcription PCR. The specific gene expressions were measured by RT-qPCR(Roche, Switzerland) with AceQ®qPCR SYBR®Green Master Mix.The housekeeping geneβ-actinwas used as a reference gene, and the data were analyzed by the 2−ΔΔCtmethod[21].
2.7 Analysis of the gut microbiota
16S rRNA sequencing of the intestinal bacterial flora of the Ad,OVA and H-BC groups, named MAd, MOVA and MBC. Sample DNA, which had been checked for purity and concentration by agarose gel electrophoresis, was diluted to 1 ng/μL, in sterile water.PCR was performed by adding specific primers with Barcode,Phusion®High Fidelity PCR Master Mix with GC Buffer andTaqplus DNA Polymerase, using genomic DNA as a template, as a way to improve the efficiency and accuracy of PCR amplification[22]. The PCR products that passed the electrophoresis test were purified by magnetic beads and quantified. The PCR products were mixed in equal amounts according to the concentration of the PCR products and then subjected to electrophoresis using a 2% agarose gel. Libraries that have been constructed by TruSeq®DNA PCR-Free sample preparation kit and sequenced by Qubit and qPCR were quantified using NovaSeq 6000[23].
2.8 Statistical analysis
Data were presented as means ± standard error of the mean(SEM). Statistical differences between the two groups were subjected to analysis with SPSS 25 (IBM, USA) using the Student’st-test and between three or more groups using one-way analysis of variance(ANOVA), withP< 0.05 being considered significant.
3. Results
3.1 Treatment with probiotics alleviated OVA-induced allergy
To investigate the effect of different concentrations ofB. coagulanspowder on OVA allergy, we have established a BALB/c female mouse model of OVA sensitization. As assessed by the allergic symptom score, no allergy sign witnessed in the Ctrl group of mice, the OVA group showed more significant allergy symptoms compared to the Ctrl and Ad groups, with higher allergy scores(Fig. 2A) and a greater drop in anal temperature (Fig. 2B). At the same time,only the Ctrl groups revealed weight gain, while the other groups showed a more pronounced weight loss with the sensitization process (Fig. 2C). These indicated the success of this OVA-sensitized BALB/c female mouse model.
We introduced different concentrations of probiotic preparations into the established mouse sensitization model to investigate the effect of different doses ofB. coagulanspowder in alleviating OVA allergy. Compared to the OVA group, the H-BC group exhibited significantly fewer allergic symptoms with the difference in anal temperature. At the same time, allergy symptoms in the M-BC group were also relieved but not significantly. The above results illustrated that different concentrations ofB. coagulanspowder produced vary effects, but only the high dose of the probiotic preparation significantly alleviated the allergy caused by OVA. The placebo group was not effective in relieving allergy symptoms, in contrast to theB. coagulansgroup, which was effective in alleviating OVA allergy.
3.2 Down-regulation of immunoglobulin isotype-associated antibody activity in serum
As one of the egg allergens, OVA is induced by binding to allergy-specific IgE, resulting in various food allergy symptoms[24].The serum IgE and IgG1 levels in the OVA group were obviously higher than the Ctrl and Ad groups. This result can prove that the mouse sensitization model of OVA sensitization was successfully established. Oral administration ofB. coagulanspowder effectively reduced OVA-specific IgE and IgG1 levels in mice serum in the H-BC group (Fig. 3). There was little change in IgE levels in the other treatment groups and the placebo group compared to the H-BC group. This result suggested that the high concentration ofB. coagulanswas effective in relieving OVA allergy and the other concentrations were less effective. IgE levels in the H-PL group decreased but not significantly. It excluded the effect of the placebo on the treatment group. As the concentration increased, serum IgE levels in mice in the placebo group gradually decreased, suggesting that the placebo also had some allergy-relieving function. Administration of high concentrations ofB. coagulanspreparations reduced the production of IgE and IgG1, thereby reducing OVA and antibody binding levels and ultimately alleviating allergic symptoms in mice[25].

Fig. 3 Oral administration of B. coagulans reduced OVA-specific IgE and IgG1 levels in mouse serum. (A) OD values of IgE in the serum of mice.(B) OD values of IgG1 in the serum of mice. The data were presented as the mean ± SEM. Statistical analysis was conducted by using one-way ANOVA. Different lowercase letters indicate significant differences at the 0.05 probability levels.
3.3 Treatment with probiotic induced CD4+ T cell differentiation in spleen
During food allergy, allergen-specific B cells were activated and developmentally directed by T cells, which acted as a core regulator,and the Th2/Th1 ratio was positively correlated with the allergic response[26]. Th1 and Th2 cells secreted the representative cytokines IL-4 and interferon gamma (IFN-γ), and the number of Th1 and Th2 cells could be determined by staining and detecting the corresponding antibodies on T cells[27]. The proportion of Th1 cells markedly downregulated in the L-PL and M-PL groups, with little change in the other groups relative to these two groups (Fig. 4C). The Th2 cell ratio was substantially lower in the Ctrl and M-BC groups compared to the OVA group (Fig. 4D). For the ratio of Th2 cells to Th1 cells, the Ctrl group was considerably below the OVA group, while the M-BC group downregulated. There was moderate growth in Th2/Th1 ratio levels in the placebo group, compared to a decrease in both the M-BC and H-BC groups (Fig. 4E). Representative flow cytometry images of each group of cells counted were shown in Figs. 4A, B. These results demonstrated that the administration ofB. coagulanspowder relieved allergy by reducing the differentiation of T cells to Th2 while Th1 levels remained unchanged. After administration of the placebo group to the mice, both Th1 and Th2 cells were reduced by differentiation of T cells, leading to little change in the ratio. Thus, oralB. coagulanspreparations could alleviate OVA-induced food allergy by affecting the differentiation of T cells in the spleen.

Fig. 4 Oral administration of B. coagulans powder induced CD4+ T cell differentiation in mice spleen. (A) The typical images of CD4+ and IL-4+ cells for every group and the percentage of cells. 1-9. Ctrl, Ad, OVA, L-BC, M-BC, H-BC, L-PL, M-PL, H-PL. (B) The typical images of CD4+ and IFN-γ+ cells for every group and the percentage of cells. 1-9. Ctrl, Ad, OVA, L-BC, M-BC, H-BC, L-PL, M-PL, H-PL. (C) The proportion of Th1 cells for every group. (D) The proportion of Th1 cells for each group. (E) The ratio of Th2 cells to Th1 cells. The data were presented as the mean ± SEM, * and *** indicated P < 0.05 and P < 0.001.
3.4 Oral administration of B. coagulans powder affected the expression of allergy-related genes in the spleen
As shown in Fig. 5, gene expression ofIFN-γin the spleen was low in the OVA group, compared to a substantial increase in both the treatment and placebo groups. There was a small upregulation in the treatment group relative to the placebo group, but no meaningful difference. TheIL-4gene expression in the Ctrl and H-BC groups was seriously inferior to that in the OVA group. In the treatment group, it demonstrated that the increase in the powder dose, the more it reduces the gene expression ofIL-4(Fig. 5B). The imbalance ofIL-4andIFN-γgene expression has been known to affect the immune system in mice, which in turn is responsible for the development of allergic symptoms[28]. Allergy symptoms were alleviated in the H-BC group treated with high concentrations of probiotics, with significant upregulation ofIFN-γgene expression and inhibition ofIL-4gene expression. As seen in Fig. 5C,T-betgene expression decreased in the mouse spleen, and the administration of probiotics could improve the expression level of the gene, but the change in concentration has little effect on it. The placebo had little effect onT-betgene expression in the spleen of mice.GATA3gene expression was elevated in the spleen of OVA-sensitized mice and was regulated to normal values after administration of the probiotic preparations and the placebo.GATA3andT-betwere konwn to have essential functions in the differentiation of Th2 and Th1 cells[29]. As a result, alterations inGATA3andT-betgene expression caused an imbalance in the Th1/Th2 response,leading to the activation of IL, which induced IgE production, leading to food allergy symptoms[30]. Oral administration ofB. coagulansupregulatedT-betgene expression levels and downregulatedGATA3gene expression levels.Foxp3gene expression in the spleen reduced after sensitization of BALB/c mice.Foxp3expression levels slightly increased in the M-BC and H-BC groups with respect to the OVA group, and the increase was proportional to the concentration of probiotics (Fig. 5E).Foxp3was a Treg cell-specific transcription factor that promotes and maintains the differentiation and normal physiological function of Treg cells[31]. Reduced numbers or impaired function of Tregs and aberrant expression ofFoxp3may lead to diminished suppression of other Th cells, mast cells, and other effector cells by Tregs, thereby contributing to the development and progression of food allergy[32-33]. HigherFoxp3gene expression in T cells of mice orally administeredB. coagulanscompared to the sensitized group. It promoted the differentiation of Treg cells and allergy alleviation.
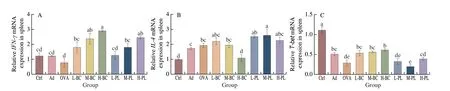
Fig. 5 Treatment with probiotics affects the expression of allergy-related genes in the spleen. The relative gene expressions of IFN-γ (A), IL-4 (B), T-bet (C),GATA3 (D) and Foxp3 (E) in the spleen were measured using RT-qPCR. The data were presented as the mean ± SEM. Statistical analysis was conducted by using one-way ANOVA.
3.5 Oral administration of B. coagulans modulated intestinal flora structure in OVA-allergic mice
To investigate phylogenetic relationships, we constructed a species evolutionary tree by combining base differences in operational taxonomic units (OTU) sequences at the phylum level with species annotation information for individual OTU sequences,as shown in Fig. 6A. According to Fig. 6B, among the top 10 phylum level abundances in the intestinal flora, the proportion of bacilli and Gammaproteobacteria decreased in the treatment group relative to the sensitized group. But the percentage ofClostridiaandBacteroidiaincreased. Cheng et al.[34]found that OVA-sensitized BALB/c mice treated orally withBifidobacterium bifidumshowed relief of allergic symptoms and a reduction in bacilli and an increase inClostridiaandBacteroidiawithin the intestinal flora.Clostridiumperfringens alleviated food allergy by affecting the upstream MyD88-dependent mechanism, which in turn induces the production of the transcription factor RORgt in nascent Treg cells and improves tolerance to dietary antigens[35]. At the same time, gene expression ofIL-10, a critical anti-inflammatory cytokine, was positively correlated with the amount of Gammaproteobacterial[36].

Fig. 6 (A) Species evolutionary trees at the phylum level; (B) relative abundance of the top 10 at phylum level.
A linear discriminant analysis effect size (LEfSe) analysis with LDA scores greater than 2.0 was performed to distinguish specific bacterial taxa between the Ad, OVA, and H-BC groups (Fig. 7).Fig. 7 illustrated the differences in species abundance between the MBC and MOVA groups and the magnitude of the differences.Bacteroides vulgatus,Verrucomicrobiaes, Pasteurellaceae andAlloprevotellawere significantly increased in the intestinal flora of mice orally administeredB. coagulanscompared to the sensitized group. de Filippis’[37]study found a lack ofB. vulgatusin the gut microbes of allergic children. On the other hand,Verrucomicrobia,Pasteurellaceae[38]andAlloprevotella[39]recovered somewhat in the gut flora of the treated mice, which in turn provided some relief from allergy.

Fig. 7 Species difference LEfSe diagram for MBC vs MOVA. (A) Cladogram, (B) LDA score.
Lachnoclostridiumdramatically increased in the gut flora of the mice treated with oralB. coagulanspreparations relative to the MAd group (Fig. 8A). Stark et al.[40]found a disruption of the microbiota and a reduction inLachnoclostridiumin allergic BALB/c mice. But more importantly, we found higher levels ofEnterococcus faeciumin the treatment group relative to the MAd and MOVA groups. In the treated group, the number ofE. faeciumin the intestinal flora, used as a probiotic to relieve allergy symptoms[41], increased, thus improving the intestinal environment and alleviating allergy.Raineyabelonged to the group ofBacteroidiawhose numbers were inversely proportional to allergy symptoms in the intestinal flora[34].Acinetobacter(Fig. 8B)exposure can relieve allergy symptoms[42].

Fig. 8 (A) T-test of relative abundance between genera of MAd and MBC; (B) T-test of relative abundance between genera of MOVA and MBC.
The rarefaction curve and the rank abundance curve were common curves that describe the diversity of samples within a group.The dilution curves (Fig. 9A) of the MAd and MBC groups tended to be flatter than those of the MOVA group, while the span on the horizontal axis of the hierarchical clustering curves (Fig. 9B) of both groups was also greater than that of the MOVA group, indicating that the species richness of the MAd and MBC groups was higher.According to Fig. 9C, we can see that the MAd and MBC groups have higher species richness compared to the MOVA group. According to Fig. 9C, we could observe that the MAd and MBC groups had higher species richness compared to the MOVA group.

Fig. 9 (A) Grade clustering curve chart; (B) Dilution curves; (C) Shannon plots of alpha species abundance.
To investigate the changes in microbial function caused by changes in the gut microbial community, a functional analysis of the microbiota was carried out. In the functional analysis of the intestinal flora, the celluar processes and organismal systems pathways were upregulated in the treatment group, while the human diseases pathway was reduced (Fig. 10A). Where infectious diseases: bacteria, other amino acid metabolism, immune diseases, immune system, energy metabolism, etc., have reduced function but increased amino acid metabolism, selective regulation of amino acid metabolism in balance(Fig. 10B). It was not surprising to find that immune diseases and the immune system were consistent with some relief of allergic symptoms in treated mice compared to sensitized mice. This phenomenon demonstrated that the administration ofB. coagulanspowder to OVA-sensitized mice resulted in an improved and balanced intestinal flora structure and a certain regulation of the flora associated with immune diseases and the immune system.
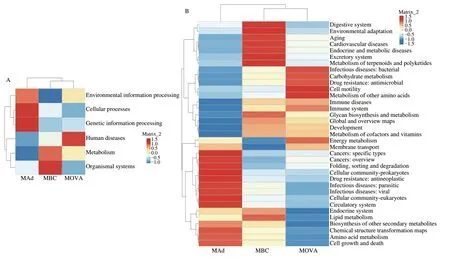
Fig. 10 Clustering heat map of differences in functional predictions between groups. (A) Heat map of the relative abundance of genes related to the function of the intestinal flora at the first level of clustering; (B) Heat map of relative abundance clustering of genes related to gut flora function at the secondary level.
4. Discussion
In recent years, food allergy, a serious food safety and public health issuse has become increasingly popular. As a result, food allergy was receiving more and more attention. People were devoting themselves to researching how to alleviate and treat food allergy.There are a great number of treatments that had attempted to treat food allergy, with the use of probiotics receiving some attention.Probiotics treat food allergy by promoting the secretion of regulatory cytokines by immune cells, regulating intestinal permeability and mucus thickness and modulating gut microbes[43]. The effectiveness of probiotics depends on the microbial species or strain, the number of probiotic cells and the type of probiotic carrier as well as its derived metabolites and by-products[44]. There were no recommendations for probiotic strains or dosages from the World Organisation for Metabolic Diseases[45]. Previous studies have shown thatB. coagulanshad some ability to alleviate food allergy[16-17]. However, there were still some research gaps in the number of bacteria and powder doses ofB. coagulansused to treat food allergy.B. coagulanswas generally effective in relieving shrimp promyelocyte allergy, but some studies have shown that lactic acid-producing bacteria can alleviate OVA allergy. Therefore, this study investigated the effect of oral administration of different concentrations ofB. coagulansTQ-35 on allergic symptoms in OVA-sensitized mice.
In this research, we constructed a BALB/c female mouse model of OVA sensitization, and had three treatment groups with various concentrations and a corresponding placebo group. The response to different treatments assessed by allergic clinical signs, OVAspecific antibodies (IgE and IgG1), T-cell differentiation levels in the spleen, and gene expression levels. Simultaneous 16S rRNA sequencing of mouse intestinal flora was used to explain the relief of allergic symptoms byB. coagulansand to explore the mechanism.Our results demonstrated that high concentrations ofB. coagulansTQ-35 alleviated the symptoms of OVA allergy, suggesting thatB. coagulansTQ-35 can be used to treat OVA allergy but at a certain concentration.
The main symptoms of OVA allergy in mice increased scratching frequency, tremors, respiratory distress and diarrhoea, accompanied by a drop in anal temperature[19]. The allergic symptoms and anal temperature difference in the OVA group were significantly greater than in the Ad group. They indicated the success of the OVA sensitization model. In contrast, the mice in the H-BC group showed reduced allergic symptoms and a lower anal temperature difference,indicating that the high dose ofB. coagulansrelieved OVA allergy,while the M-BC showed insignificant symptom relief, suggesting that the high dose ofB. coagulanswas more therapeutic. Food allergic reactions mediated by IgE induce the production of IgE antibodies by B cells after exposure to the allergen. On the other hand, food allergy promoted the differentiation of the Th2 subpopulation of cells,which mainly mediated humoral immunity, promoted the appreciation and differentiation of mast cells and eosinophils, and stimulates the differentiation of B cells to produce IgG1. Currently, serum levels of allergen-specific IgE were used as a method of diagnosing food allergy and for allergen screening[46]. OVA-specific IgE and IgG1 were significantly reduced in H-BC, suggesting that high concentrations ofB. coagulansalleviated allergy. IgG1 was also somewhat reduced in M-PL and H-PL, suggesting that the placebo group could reduce IgG1 production, possibly related to Th2 cell differentiation.
The results of T-cell differentiation suggested that placebo inhibited the differentiation of Th1 and Th2 cells. The findings of Th2 cell differentiation and IgG1 levels corroborated each other. IgG1 levels can respond to Th2 cell levels.B. coagulanscan down-regulate the levels of Th2 and thus lower the levels of IgG1. These two results were consistent with each other’s trends. Comparison of the treatment and placebo groups suggested thatB. coagulanspromotes Th1 cell differentiation and inhibits Th2 differentiation. Overall,oral administration of medium and high doses ofB. coagulanspowder reduced the ratio of Th2 to Th1 cells, demonstrating that it relieved allergy.B. coagulansinduced T cell differentiation, downregulated Th2 differentiation and up-regulated Th1 differentiation,thereby alleviating the Th1/Th2 immune imbalance in food-allergic mice. Zhao’s study[47]found thatB. coagulans13002 could regulate Th2/Th1 cell balance and improve immunity. The results of this experiment were consistent with our results.
At the level of gene expression,B. coagulanspowder and carrier alleviated allergy by up-regulating the expression of theIFN-γgene,while high doses ofB. coagulansalso significantly down-regulated the expression of theIL-4gene, contributing to a more efficient alleviation of allergy symptoms. Oral administration ofB. coagulanspowder also increased the expression of theT-betgene and decreased the expression of theGATA3gene. In this way,B. coagulanscan regulate the balance of allergy-related gene expression and thereby alleviate OVA-induced allergy. T-bet directly inhibited the expression of IL-4 and GATA3 functions. Meanwhile,GATA3inhibited Th1 differentiation, not only by inducing Th2 cytokine expression (e.g.IL-4) but also by inhibiting IFN-γ and T-bet[28,48-49]. The significant downregulation ofFoxp3gene levels in the Ad group was due to the principle of mouse ear coating sensitization which caused inflammation in mice. While the M-BC and H-BC groups could enhanceFoxp3gene expression. Current research suggested that theFoxp3gene played a crucial role in oral tolerance[50]. Antigen-specific Foxp3+Treg suppresses adverse responses to food in the lamina propria of the gut through the production of the cytokines TGF-β,IL-10 and IL-35[51].
In the immune system, the intestinal flora maintained homeostasis in the body and dysbiosis of the gut microbiota promoted the severity of food allergy[52]. There were significant differences in the composition and function of the intestinal flora in patients with food allergies compared to the healthy population. Our study found that high concentrations ofB. coagulansincreased the number of bacteria in the intestine that were beneficial in relieving food allergy and decreased the number of bacteria that were positively associated with food allergy. The presence ofClostridiainduced the production of Treg-inducing factors that may cooperate with dendritic cells to induce a general accumulation of Treg in the colon[53]. The significant increase inB. vulgatuswas consistent with the results of other related allergenicity studies[37,54]. In Sudo’s study[55],B. vulgatusalleviated antibiotic-induced overexpression of Th2-associated genes, thereby regulating the balance of Th1 and Th2-associated genes.E. faeciumreduced total IgE production in serum and enhanced IFN-γ production by T cells[56]. On the other hand, high doses ofB. coagulansalso increased the species abundance of the intestinal flora, which was important to maintain immune system homeostasis. The functional integrity and stability of the intestinal flora was ensured when the intestinal flora had a high level of species abundance. The type and abundance of intestinal flora affected the metabolic pathways of the intestinal flora, which in turn affected allergy symptoms,where the immune signaling pathways and molecular mechanisms needed further investigation. However,B. coagulanswas not found in the intestinal flora, which was consistent with other studies[17,57].B. coagulanswas a transient bacterium that did not colonize the gut but could influence the intestinal flora by producing lactic acid and short-chain fatty acids[58]. Short-chain fatty acids worked to stimulate the formation of the intestinal barrier, promoted the development and maturation of the immune system and acted on cells such as T and B cells or reduced the inflammatory response by acting on mast cells to induce food allergy reduction[52]. At the same time, the immune system and the microbiota associated with immune disease function were weakened.
5. Conclusion
In conclusion, the effect ofB. coagulanspowder in alleviating OVA-induced food allergy has been demonstrated byin vivoexperiments. The allergy symptom scores, cell differentiation and gene expression levels indicated that only high concentrations ofB. coagulanshad a significant allergy-relieving effect. In addition, the rationale ofB. coagulansto alleviate OVA allergy was analyzed in conjunction with intestinal flora. This study provided some implications for the industrial practical application ofB. coagulansand was a reference for the practical dosage ofB. coagulansfor allergy relief. The daily dose ofB. coagulansto mice can be controlled at around 9 × 108CFU when used to alleviate OVA-induced food allergy, contributing to the practical production and application ofB. coagulansand promoting the development of the probiotic industry.
Declaration of competing interest
Yanbo Wang and Linglin Fu are editorial board members forFood Science and Human Wellnessand was not involved in the editorial review or the decision to publish this article. The authors declare no conflict of interests.
Acknowledgements
This study was financially supported by the National Key R&D Program of China (2019YFC1605003), and the Zhejiang Provincial Natural Science Foundation of China (LGN21C200013).
- 食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango

