Naringin ameliorates H2O2-induced oxidative damage in cells and prolongs the lifespan of female Drosophila melanogaster via the insulin signaling pathway
Xiomei Du, Kexin Wng, Xioyn Sng, Xingxing Meng, Jio Xie,Tinxin Wng, Xiozhi Liu, Qun Hung,, Nn Zhng,, Ho Wng,
a State Key Laboratory of Food Nutrition and Safety, Tianjin University of Science and Technology, Tianjin 300457, China
b The key Laboratory of Environmental Pollution Monitoring and Disease Control, Ministry of Education, Guizhou Medical University, Guizhou 550025, China
c Department of neurosurgery, the Fifth Central Hospital of Tianjin, Tianjin 300450, China
d Tianjin Key Laboratory of Epigenetics for Organ Department in Preterm Infants, the Fifth Central Hospital of Tianjin, Tianjin 300450, China
Keywords: Drosophila melanogaster Insulin signaling (IIS) pathway Naringin PC12 cell HepG2 cell
ABSTRACT Naringin exists in a wide range of Chinese herbal medicine and has proven to possess several pharmacological properties. In this study, PC12, HepG2 cells, and female Drosophila melanogaster were used to investigate the antioxidative and anti-aging effects of naringin and explore the underlying mechanisms. The results showed that naringin inhibited H2O2-induced decline in cell viability and decreased the content of reactive oxygen species in cells. Meanwhile, naringin prolonged the lifespan of f lies, enhanced the abilities of climbing and the resistance to stress, improved the activities of antioxidant enzymes, and decreased malondialdehyde content. Naringin also improved intestinal barrier dysfunction and reduced abnormal proliferation of intestinal stem cells. Moreover, naringin down-regulated the mRNA expressions of inr, chico, pi3k, and akt-1, and up-regulated the mRNA expressions of dilp2, dilp3, dilp5, and foxo, thereby activating autophagy-related genes and increasing the number of lysosomes. Furthermore, the mutant stocks assays and computer molecular simulation results further indicated that naringin delayed aging by inhibiting the insulin signaling (IIS) pathway and activating the autophagy pathway, which was consistent with the result of network pharmacological predictions.
1. Introduction
Aging is a multifactorial biological process (BP) of reducing physiological function that increases susceptibility to chronic diseases[1]. There are many pathways related to aging regulation,including antioxidative stress, insulin signaling (IIS), and autophagy pathways. Oxidative damage caused by reactive oxygen species(ROS) is considered to be a major mechanism of aging[2]. Endogenous antioxidant enzymes and exogenous antioxidants are the defenses system for eliminating ROS[3]. Meanwhile, the IIS pathway is an important pathway for regulating aging in organisms, from simple invertebrates to mammals, including humans[4].Foxo, the downstream gene of the IIS pathway, directly activates the expressions of autophagy genes to delay aging[5].
Naringin, a dihydro flavonoid, is the main active ingredient ofCitrus aurantium,Fructus aurantii, andPericarpium citri reticulatae.It has been proven to possess a variety of biological activities including antioxidant and anti-inf lammatory effects[6]. Adebiyi et al.[7]found that naringin reduced cardiac fibrosis in mice induced by hyperglycemia oxidative stress. Moreover, naringin inhibited colorectal cancer cell growth and induced apoptosis by repressing PI3K and AKT[8]. Zhang et al.[9]reported that naringin reduced the incidence of Tert-butyl hydroperoxide-induced oxidative stress apoptosis in nucleus pulposus cells and promoted the expression of autophagy markers LC3-II/I and beclin-1. Aging and related diseases are closely associated with impaired gut health. Mu et al.[10]found that naringin increased the beneficial flora and reduced the harmful flora in C57BL/6J mice induced by high-fat diets, which improved intestinal health and attenuated non-alcoholic fatty liver disease.
In this study, network pharmacology was used to predict the potential anti-aging and antioxidant mechanisms of naringin. The antioxidative effects of naringin on H2O2-induced PC12 and HepG2 cells were investigatedin vitro. Meanwhile, the anti-aging effects(including various intestinal indexes) and molecular mechanisms of naringin on natural agingD. melanogasterwere studiedin vivo.Moreover, computer molecular simulation and RNAi fly experiments were used to verify the mechanisms that naringin exerted anti-aging and antioxidant effects.
2. Materials and methods
2.1 Materials
Naringin and resveratrol (purity ≥ 98%) were purchased from Aladdin Chemical Co. (Shanghai, China). The chemicals and reagents were analytical grade.
2.2 Network pharmacology
The PharmMapper database (http://lilab-ecust.cn/pharmmapper/index.html) was used to search “naringin” to obtain complete target protein information, and the target proteins were imported into the UniProt database (http://www.uniprot.org) and converted into gene name. Then the GeneCards database (https://www.genecards.org) was used to search “Aging” to get the target genes of disease information.The VENNY 2.1 database (https://bioinfogp.cnb.csic.es/tools/venny/index.html) was used to download overlapped genes. The obtained gene names were imported into the STRING database (http://stringdb.org/) which was used to draw the protein-protein interaction (PPI)network. The enrichments of the Gene Ontology (GO) function and the Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were analyzed by the DAVID database (http://david.abcc.ncifcrf.gov/), and the main interaction and channels were analyzed.P< 0.05 and the first 20 enriched KEGG pathways were made into a bubble diagram.
2.3 Cell assays
2.3.1 Cell culture
PC12 and HepG2 cells were obtained from the Cell Resource Center of Shanghai Institutes for Biological Sciences (Shanghai,China) and maintained in a humidified 5% CO2incubator at 37.0 °C.PC12 cells were cultured in RPMI 1640 medium supplemented with 5% heat-inactivated fetal bovine serum (FBS), 10% horse serum,100 units/mL penicillin, and 0.1 mg/mL streptomycin. HepG2 cells were cultured in DMEM supplemented with 10% FBS, 100 units/mL penicillin, and 0.1 mg/mL streptomycin. After reaching 70%–80%confluency, the cells were used in the following experiments.
2.3.2 Cell viability
The assay was performed according to our previous method[11].Briefly, PC12 cells (1 × 104cells/well) were treated with H2O2(0,25, 50, 75, 100, 125, and 150 μmol/L) naringin (0, 20, 40, 80, and 160 μmol/L), and H2O2(150 μmol/L) with or without naringin at different concentrations (40 and 80 μmol/L) for 24 h. Similarly,HepG2 cells (1 × 104cells/well) were treated with H2O2(0, 100,200, 300, 400, 500, and 600 μmol/L), naringin (0, 20, 40, 80, and 160 μmol/L), and H2O2(600 μmol/L) with or without naringin at different concentrations (40 and 80 μmol/L) for 24 h. The cells were incubated in MTT solution (5 mg/mL) for 4 h, then the solutions were removed, and replaced with 150 μL dimethyl sulfoxide (DMSO). The absorbance was measured at 490 nm.
2.3.3 Measurements of intracellular ROS levels
Intracellular ROS production was measured with 2’,7’-dichlorofluorescein diacetate (DCFH-DA) (Aladdin, China).Briefly, PC12 and HepG2 cells (1 × 105cells/well) were treated with or without 150 μmol/L H2O2or naringin (40 and 80 μmol/L) and 600 μmol/L H2O2or naringin (40 and 80 μmol/L) for 24 h,respectively. After the old culture medium was removed, cells were washed three times with PBS and then treated with 10 μmol/L DCFHDA for 20 min at 37.0 °C in dark[12]. The cellular ROS fluorescence intensity was measured by an inverted fluorescence microscope (with excitation at 485 nm, emission at 520 nm, and magnification 200×).
2.4 Fly stocks and diet
The stocks were used in this study: wild-type Oregon-R-C (OR),CG11793 (sodRNAi), CG6871 (catRNAi), TB00044 (yw; Esg-Gal4 UAS-GFP/CyO), CG5216 (sir2RNAi), CG18402 (inrRNAi),CG5686 (chicoRNAi), CG4141 (pi3kRNAi), CG4006 (akt-1RNAi),CG3143 (foxoRNAi), CG10967 (atg1RNAi), CG1643 (atg5RNAi),CG32672 (atg8aRNAi), and CG12334 (atg8bRNAi). All RNAi flies were purchased from Tsinghua Fly Center. Only female flies were studied in this paper.
All flies were cultured at 25.0 °C and 65% humidity. The control diet was prepared according to our previous study[13]. DMSO was used to prepare the naringin stock, which was directly added to the control diet to obtain a final dose of 0.3% DMSO. The diets with different concentrations of naringin or control solvent (0.3% DMSO)were prepared.
2.5 Toxicity and lifespan assays
The safe dose range of DMSO was analyzed by a toxicity test.Different doses of DMSO (0, 0.1%, 0.3%, 0.5%, 0.7%, 0.9%, andn= 200 per group) were added to the control diet. The new diets were replaced every 3 days and survival rates were calculated. The experiments were repeated three times.
Resveratrol was used as a positive control in this study because of its significant antioxidant activity. Similarly, suitable naringin and resveratrol concentrations were also screened. Different concentrations of naringin and resveratrol (0, 0.35, 0.7, 1.4, 2.8,5.6 mmol/L, andn= 200 per group) were added to the control diet.The flies were moved into fresh diets every 3 days and the number of dead flies was recorded. The experiment was repeated three times.
The lifespan assay was consistent with the toxicity assay.Wild-type flies were randomly divided into 5 groups (control, DMSO,0.7 mmol/L naringin, 1.4 mmol/L naringin, 1.4 mmol/L resveratrol,andn= 200/group). Fresh diets were changed every 3 days and the survival rates were calculated. Each group had an average of 3 independent replicates.
2.6 Food intake and body weight assays
Food intake was determined by referring to the method of previous studies[14]. Briefly, wild-type flies (n= 100 per group) were cultured for 4 days in diets with or without naringin or resveratrol,then placed in empty tubes for 2 h. The flies were moved to diets including 0.2% rhodamine B sulfonate sodium salt (Sigma, USA) for 3 h. A subjective rating scale ranging from 0 to 5 (colorless abdomen to completely red) was used to reflect the food intake.
The methods referred to our previous study[15]. On the 15th,30th, and 45thday, wild-type flies were moved to empty pipes and anesthetized with CO2. The total body weight of every group of flies(n= 200 per group) was weighed. Each group had an average of three independent replicates.
2.7 Climbing assays
Aging is a process associated with reduced motor activity.Therefore, Yang et al.[16]method was used to measure climbing ability. Briefly, wild-type flies (n= 200 per group) were mediated with or without naringin or resveratrol. On the 15th, 30th, and 45thday,they were moved into the vacant pipes and climbed within 20 s. And the number of flies reaching a vertical height > 7 cm was counted.Each group had an average of three independent replicates.
2.8 Stress tests
2.8.1 Starvation stress
Wild-type flies (n= 200 per group) were mediated with or without naringin for 20 days. The flies were moved into tubes containing only 1.5% agar ensuring water supply[17]. The dead number of flies was counted every day.
2.8.2 Heat and cold stresses
Wild-type flies (n= 200 per group) were mediated with or without naringin for 20 days. Heat stress: the flies were cultured at 37.0 °C and 65% humidity. The number of dead flies was counted per 30 min.Cold shock: the flies (n= 200/group) were exposed to 4.0 °C until all were stunned. The time of flies reactivating was recorded.
2.8.3 Paraquat and H2O2 stresses
Wild-type flies (n= 200 per group) were mediated with or without naringin. On the 20thday, the flies were moved to tubes including filter papers soaked with 0.5 mL 20 mmol/L paraquat solution or 30%H2O2solution containing 6% glucose. The number of dead flies was counted every 2 h.
2.9 Antioxidant enzyme activities and MDA content
Wild-type flies (n= 200 per group) were mediated with or without naringin for 45 days, then placed in empty pipes, and anesthetized with CO2. The flies were treated according to our previous method to get a diluted supernatant[13]. Superoxide dismutase (SOD),catalase (CAT) activities, and malondialdehyde (MDA) content were measured according to the assay kits (Nanjing Jiancheng, China). Per group had an average of 3 independent replicates.
2.10 The intestinal analysis
2.10.1 “Smurf” assays
Wild-type flies (n= 200 per group) were mediated with or without naringin. On the 10th, 30th, 40th, and 50thdays, the flies were treated with a diet containing 2.5% FD&C Blue No. 1 (Sigma, USA)[18]. The“Smurf” flies were captured under a microscope and the number of them was counted.
2.10.2 Intestinal microflorae
The experiment was performed according to previous research[19].Briefly, wild-type flies were mediated with or without naringin.On the 5thand 40thday, 10 midguts of each group were dissected,homogenized, and diluted. Diluent of pre-group (0.1 mL) was coated on the Enterobacteriaceae (ENT), Lactobacilli MRS (LMRS), and Acetobacteraceae (ACE) selective mediums and nutrient-rich (NR)medium. Each group had an average of three independent replicates.
2.10.3 Determination of intestinal progenitor cells and lysosomal staining assays
Esg-Gal4 UAS-GFP flies (n= 200 per group) were mediated with or without naringin. The midguts were dissected, fixed, and sealed on the 20thand 50thdays. The intestinal Esg fluorescence intensity was measured by an inverted fluorescence microscope.
Wild-type flies (n= 100 per group) were mediated with or without naringin for 10 days. The midguts were taken and stained with Lyso-Tracker Red (Sigma, USA) for 3 min. The midguts were fixed and sealed according to the above method. The inverted fluorescence microscope was used to measure the intestinal lysosome fluorescence intensity.
2.11 Quantitative real-time PCR
Wild-type flies (n= 200 per group) were mediated with or without naringin for 45 days. The flies were treated according to our previous method[13]. Total RNA was extracted with TRIzol reagent (Takara,China), and reversely transcribed into cDNA. The primer sequences were listed in Table S1. The result was analyzed with 2-ΔΔCt.
2.12 Reverse validation
2.12.1 In cells
PC12 and HepG2 cells were pretreated with 30 μmol/L 740Y-P (an activator of PI3K) (Aladdin, China) for 1 h[20]. PC12 cells were treated with H2O2(150 μmol/L) and naringin (80 μmol/L) for 24 h. HepG2 cells were treated with H2O2(600 μmol/L) and naringin (80 μmol/L)for 24 h. Cell viability and ROS content were determined according to the above experimental methods.
2.12.2 In flies
The molecular mechanism of naringin was validated using mutant stocks.Inr,chico,pi3k,akt-1,foxo,sir2,atg1,atg5,atg8a, andatg8bRNAi flies (n= 200 per group) were used to conduct lifespan assays.SodandcatRNAi flies (n= 200 per group) were used to conduct oxidative stresses.Atg1,atg5,atg8a, andatg8bRNAi flies(n= 100 per group) were used to perform lysosomal staining assays.The experimental methods were followed by the above as 2.5, 2.8.3,and 2.10.3 sections, respectively.
2.13 Computer molecular simulation
2.13.1 Molecular docking
The structures of naringin and PI3K protein (PDB: 2X6H) were downloaded from PubChem (https://pubchem.ncbi.nlm.nih.gov/)and the Protein Data Bank (https://www.rcsb.org), respectively.The amino acid sequences of AKT1 (UniProtKB-Q8INB9) were downloaded from the UniProt database (https://www.uniprot.org/),and the homologous AKT1 protein structure modeling was performed by using SWISS-MODEL (https://swissmodel.expasy.org/).Discovery Studio 3.5 (Accelrys, CA, USA) was used to dock naringin with proteins[13].
2.13.2 Molecular dynamics (MD) simulation
To analyze the stability of naringin-PI3K and naringin-AKT1 complexes in a relatively real solvent environment and the interaction between naringin and PI3K or AKT1[21]. MD simulation was performed based on the AMBER 99SB-ILDN force field of the GROMACS 2021[22]. The optimal conformations of naringin docking with PI3K and AKT1 proteins were selected, and the steepest descent algorithm was used to minimize the initial energy and eliminate the interatomic contact. Dodecahedral boxes 1 nm from the edge of the complex were filled with water, and counterions were used to make the total charge in the system zero[23]. The water tank volumes of PI3K and AKT1 proteins were 1 020 and 600 nm3, respectively, covering the docking structure. The temperature was set to 310.15 K and the atmospheric pressure was set to 101 325 Pa. MD simulation was performed in the range of 20 ns with a 2 fs time step to obtain the radius of gyration (Rg), root-mean-square deviation (RMSD), root mean square fluctuation (RMSF), and solvent accessible surface area (SASA).
2.14 Statistical analyses
The Origin 2021 software was used for visualization of the data shown as mean ± standard deviation (SD). The fluorescence intensity was calculated by Image J software. The one-way analysis of variance(ANOVA) was performed using SPSS software to analyze differences between groups.P< 0.05 was considered statistically significant, andP< 0.01 was considered extremely significant.
3. Results
3.1 Network pharmacology prediction revealed crucial targets and pathways related to naringin
A total of 45 anti-aging targets of naringin were screened. The PPI results showed that AKT1 and PI3K3CA were the key target proteins of naringin, and these proteins had strong interactions(Fig. 1A). To reveal the potential anti-aging mechanisms of naringin,GO enrichment analysis was performed on the target proteins of naringin. The results showed that the top 10 results of enrichment in BP, cell component (CC), and molecular function (MF) were selected according toPvalue (Fig. 1B). The top 20 pathways were selected from KEGG pathway enrichment analysis, among them,IIS pathway, longevity regulating pathway-multiple species, and PI3K-Akt signaling pathway were the critical pathways (Fig. 1C). The“naringin-targets-pathways” association was constructed as a network diagram (Fig. 1D). The association between the critical pathways of naringin and common targets indicated that naringin might exert antiaging effects through AKT1, PI3K3CA, and the IIS pathway.
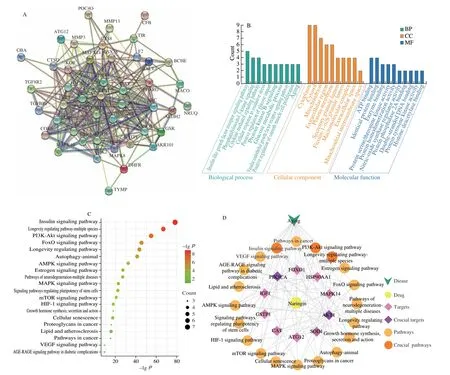
Fig. 1 Possible targets and pathways of the interaction between naringin and “Aging” were predicted using network pharmacology. (A) PPI interaction plot;(B) function enrichment analysis for GO: BP, CC, and MF; (C) the top 20 KEGG pathways associated with naringin; (D) the relationship between naringin and the top 20 KEGG pathways, targets, and disease.
3.2 Naringin ameliorated cells damage induced by H2O2
H2O2(150 and 600 μmol/L) intervention significantly reduced PC12 and HepG2 cells viability by 32.1% (P< 0.01) and 33.8%(P< 0.01), respectively (Figs. S1A and C). There were no significant changes in cell viability in the naringin (20, 40, and 80 μmol/L) groups(P> 0.05) (Figs. S1B and D), while 160 μmol/L naringin treatment reduced cell viability (P< 0.05). Naringin (40 and 80 μmol/L)reversed the H2O2-induced decrease in the viability of PC12 and HepG2 cells by 10.3% (P< 0.05), 27.8% (P< 0.01), 8.9% (P< 0.05),and 25.2% (P< 0.01), respectively (Figs. 2A and B).
However, when co-treatment with 740Y-P, the protective effect of naringin (80 μmol/L) on H2O2-induced PC12 and HepG2 cells was abolished (Figs. 2A and B), indicating that PI3K was a critical regulatory factor in naringin protecting cells from H2O2damage.
3.3 Naringin reduced the ROS content of H2O2-induced cells
In PC12 cell, H2O2(150 μmol/L) increased the ROS content by 68.4% (P< 0.01) compared with the control group, while naringin (40 and 80 μmol/L) reduced the ROS content by 13.7% (P< 0.05) and 28.5% (P< 0.01), respectively, in comparison with the H2O2treatment group (Figs. 2C and E). Meanwhile, in HepG2 cell, H2O2(600 μmol/L)increased the ROS content by 88.1% (P< 0.01) compared with the control group, while naringin (40 and 80 μmol/L) reduced the ROS content by 12.2% (P< 0.05) and 30.4% (P< 0.01), respectively, in comparison with the H2O2treatment group (Figs. 2D and F).
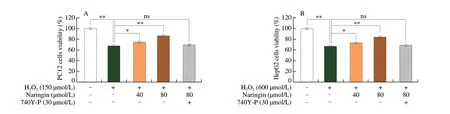
Fig. 2 Actions of naringin on cell viability and ROS content of PC12 and HepG2 cells. (A) and (B) naringin protected PC12 and HepG2 cells against H2O2-induced damage, respectively; (C) and (D) the fluorescence intensity ratio of ROS in PC12 and HepG2 cells, respectively; (E) and (F) naringin (40 and 80 μmol/L)reduced ROS content in PC12 and HepG2 cells damaged by H2O2, respectively, and the cellular ROS fluorescence intensity was observed under an inverted fluorescence microscope (with excitation at 485 nm emission at 520 nm, and magnification 200×). *P < 0.05; **P < 0.01; ns, P > 0.05. The same below.
However, when co-treatment with 740Y-P, the reduced ROS content by naringin (80 μmol/L) on H2O2-induced PC12 and HepG2 cells was abolished (Figs. 2C and D), indicating that PI3K was a critical regulatory factor in naringin protecting cells from ROS accumulation.
3.4 Naringin prolonged the lifespan in wild-type flies
DMSO (0.5%, 0.7%, and 0.9%) shortened the lifespan of flies(Fig. S1E and Table S2). The difference between the control and DMSO(0.1% and 0.3%) groups was not significant (P> 0.05) (Fig. S1E).Therefore, to achieve the best solubility of naringin, 0.3% DMSO was selected for subsequent experiments. The study by Nazir et al.[24]also showed that 0.3% DMSO did not damage the lifespan of flies. There was no significant difference between the naringin and resveratrol groups (Figs. S1F and G). Exposure to 2.8 and 5.6 mmol/L naringin shortened the lifespan of flies(Fig. S1F and Table S3). The difference between the control and 0.35 mmol/L naringin groups was not significant (P> 0.05). Therefore, 0.7 and 1.4 mmol/L naringin were used in subsequent experiments.
The difference in the average and maximum life expectancies between the control and 0.35 mmol/L naringin groups was not significant. Compared with the average life expectancy of the control group ((66.23 ± 3.26) day), the average life expectancy of naringin(0.7 and 1.4 mmol/L) and resveratrol (1.4 mmol/L) groups were(69.28 ± 1.18), (72.49 ± 1.48), and (72.32 ± 2.05) day, respectively, and increased by 4.6% (P< 0.05), 9.5% (P< 0.05), and 9.2% (P< 0.05),respectively (Figs. 3A, S2A, and Table S4). Moreover, compared with the maximum lifespan of the control group ((85.52 ± 2.64) day), the maximum lifespan of naringin (0.7 and 1.4 mmol/L) and resveratrol(1.4 mmol/L) groups were (88.33 ± 1.38), (90.17 ± 2.73), and(91.28 ± 1.47) day, respectively, and increased by 3.3%, 5.5% (P< 0.05),and 6.7% (P< 0.05), respectively (Figs. 3A, S2A, and Table S4).
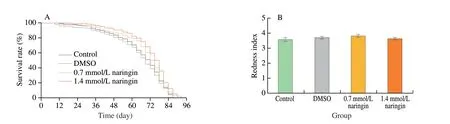
Fig. 3 Actions of naringin on the lifespan, food intake, average body weight, and climbing ability of flies. (A) The lifetime of flies (n = 200 per group); (B) the food consumption of flies (n = 100 per group); (C) the average body weight of flies (n = 200 per group); (D) the climbing ability of flies (n = 200 per group).
3.5 Naringin did not influence the food consumption and body weight in wild-type flies
To investigate whether naringin prolonged the lifespan of flies through the dietary restriction (DR) pathway, the food intake of flies treated with naringin was tested. Compared with the control group (3.57 ± 0.12), the abdomen redness index of naringin (0.7 and 1.4 mmol/L) and resveratrol (1.4 mmol/L) groups were 3.70 ± 0.11,3.63 ± 0.08, and 3.21 ± 0.12, respectively, and there was no significant difference among all groups (P> 0.05) (Figs. 3B and S2B).The results showed that the food intake of flies was not influenced by naringin and resveratrol. The average weights at 15, 30, and 45 days were about (1.20 ± 0.05), (1.19 ± 0.02), and (1.20 ± 0.06) mg,respectively, and there was no significant difference (P> 0.05)(Figs. 3C and S2C), indicating that the body weight of flies was not influenced by naringin and resveratrol.
3.6 Naringin enhanced the crawling ability in wild-type flies
Crawling ability is an indicator of the physiological state ofDrosophilaand decreases with aging. There was no significant difference among all groups on the 15thday, but on the 30thday, the crawling ability of naringin (0.7 and 1.4 mmol/L) and resveratrol(1.4 mmol/L) groups were increased by 8.7% (P< 0.05), 13.7%(P< 0.01), and 10.6% (P< 0.01), respectively (Figs. 3D and S2D).Similarly, on the 45thday, the crawling ability of naringin (0.7 and 1.4 mmol/L) and resveratrol (1.4 mmol/L) groups were increased by 5.6% (P< 0.05), 9.1% (P< 0.05), and 13.8% (P< 0.01), respectively(Figs. 3D and S2D).
Resveratrol and naringin extended the lifespan, did not alter food intake and average body weight, and improved the climbing ability ofD. melanogaster. Zhou et al.[25]reported that resveratrol prolonged the lifespan and enhanced the antioxidant enzyme activities of flies by stimulating Nrf2 activity and activating Sirt1. However, the antiaging mechanisms of naringin were not clear, thus, only naringin was analyzed in the subsequent experiments to study the anti-aging mechanisms of naringin.
3.7 Naringin enhanced the resistance to stresses in flies
3.7.1 Naringin enhanced the resistance to starvation, heat,and cold stresses in flies
DMSO did not influence the average and maximum lifespan under starvation, hot, and cold stresses. Naringin (0.7 and 1.4 mmol/L)extended the average lifespan under starvation stress by 10.4%(P< 0.05) and 18.3% (P< 0.05), respectively (Fig. 4A and Table S5).Moreover, the average survival time under the hot shock of the 0.7 and 1.4 mmol/L naringin groups was extended by 11.5% (P< 0.05)and 13.2% (P< 0.05), respectively (Fig. 4B and Table S5). Similarly,the average recovery time under the cold shock of the 0.7 and 1.4 mmol/L naringin groups was shortened by 6.6% and 13.9%(P< 0.05), respectively (Fig. 4C).
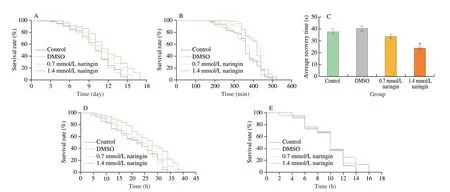
Fig. 4 Anti-stress actions of naringin in flies. (A) The lifetime of wild-type flies (n = 200 per group) on starvation stress; (B) the lifetime of wild-type flies(n = 200 per group) on heat stress; (C) the average recovery time of flies (n = 200 per group) after cold shock; (D) the lifetime of wild-type flies(n = 200 per group) on paraquat stress; (E) the lifetime of sod RNAi flies (n = 200 per group) on paraquat stress; (F) the lifetime of wild-type flies(n = 200 per group) on H2O2 stress; (G) the lifetime of cat RNAi flies (n = 200 per group) on H2O2 stress.
3.7.2 Naringin enhanced the resistance to oxidative stresses in flies
DMSO did not influence the average and maximum lifespan of wild-type flies under the paraquat challenge. The average lifespan of 0.7 and 1.4 mmol/L naringin groups were (24.88 ± 2.89) and (26.13 ± 1.36) h,respectively, and prolonged by 12.6% (P< 0.05) and 18.9%(P< 0.01), respectively (Fig. 4D and Table S5). However, naringin did not prolong the survival time ofsodRNAi flies (P> 0.05) (Fig. 4E).
A similar tendency was observed in experiments with H2O2stress.The average lifespan of the control group was (15.25 ± 2.73) h, and that of the two naringin groups was (17.12 ± 4.26) and (18.13 ± 1.63) h,respectively, which were extended by 13.7% (P< 0.05) and 18.9%(P< 0.01), respectively (Fig. 4F and Table S5). However, naringin did not prolong the lifespan ofcatRNAi flies (P> 0.05) (Fig. 4G).
3.8 Naringin enhanced the SOD1, SOD2, and CAT activities and decreased MDA content in wild-type flies
The activities of SOD1 in 0.7 and 1.4 mmol/L naringin groups were increased by 29.0% (P< 0.01) and 42.9% (P< 0.01),respectively (Fig. 5A). Meanwhile, the activities of SOD2 in naringin groups were improved by 16.6% (P< 0.05) and 56.8% (P< 0.01),respectively (Fig. 5A). CAT activity in naringin groups were increased by 12.7% and 28.5% (P< 0.05), respectively (Fig. 5B). MDA content infliesweresignificantly decreased by 28.4% (P< 0.05) and 43.2%(P< 0.01) after naringin supplement, respectively (Fig. 5C).
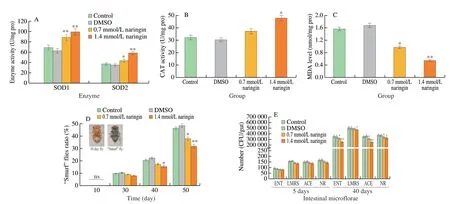
Fig. 5 Actions of naringin on antioxidant enzyme activities, MDA content, and intestinal barrier function of flies (n = 200 per group). (A) SOD1 and SOD2 activities; (B) CAT activity; (C) MDA content; (D) 10 day flies, “Smurf” flies, and the proportion of “Smurf” flies; (E) the number of intestinal microflorae in flies.
3.9 Naringin improved the intestinal barrier dysfunction in wild-type flies
The dye was in sight in the proboscis and digestive tract of flies at young flies, and the whole body of flies gradually turned blue with aging (Fig. 5D). After the naringin supplement, there was no dramatical difference in the proportion of “Smurf” flies among all groups on the 10thday, while the percentage of “Smurf” flies of naringin groups were reduced by 19.2% (P< 0.05) and 31.3%(P< 0.01) on the 50thday, respectively (Fig. 5D).
With aging, the number of intestinal microflorae is abnormal hyperplasia, and intestinal flora structure imbalance causes metabolic imbalance, accelerating aging[26]. The number of intestinal microflorae was about 100−200 CFU/gut (P> 0.05) on the 5thday (Fig. 5E),while it increased dramatically with aging. The number of intestinal microflorae of ENT, LMRS, ACE, and NR in the 0.7 mmol/L naringin group was decreased by 10.6% (P< 0.05), 3.4%, 11.3%(P< 0.05) and 10.1% (P< 0.05) on the 40thday, respectively (Fig. 5E).The total number of intestinal microflorae in 1.4 mmol/L naringin treatment group were decreased by 28.3% (P< 0.01), 6.6%(P< 0.05), 28.5% (P< 0.01) and 13.3% (P< 0.05), respectively(Fig. 5E). Results showed that naringin decreased the abnormal proliferation of intestinal microflorae.
3.10 Naringin decreased the over-proliferation of ISCs of wild-type flies
Age-related over-proliferation of intestinal stem cells (ISCs)causes loss of intestinal function and increased mortality in elderly flies[27]. Esg-Gal4 UAS-GFP flies were used to observe the changes in ISCs. The results showed that on the 20thday, there was no significant difference in the number of ISCs (Figs. 6A and D), while the overproliferation of ISCs dramatically multiplied on the 50thday (P< 0.01)(Fig. 6D), and the number of ISCs in naringin treatment groups were significantly decreased by 12.1% (P< 0.05) and 30.3% (P< 0.01) on the 50thday, respectively (Figs. 6B and D).
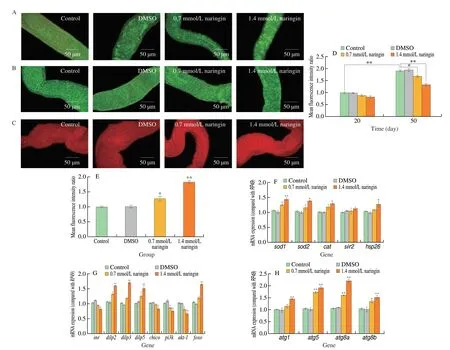
Fig. 6 Actions of naringin on the ISCs proliferation, the number of lysosomes, and the mRNA expression levels of flies. (A) ISCs of 20 day flies (n = 100/group);(B) ISCs of 50 day flies (n = 100/group); (C) lysosomes of 10 day flies (n = 100/group); (D) the fluorescence intensity ratio of ISCs in flies; (E) the fluorescence intensity ratio of lysosomes in flies; (F) sod1, sod2, cat, sir2, and hsp26 mRNA expression levels; (G) inr, dilp2, dilp3, dilp5, chico, pi3k, akt-1, and foxo mRNA expression levels; (H) atg1, atg5, atg8a, and atg8b mRNA expression levels.
3.11 Naringin increased the number of lysosomes in wildtype flies intestinal
Autophagy is a pathway of intracellular delivery of cytoplasmic substrates to lysosomes for degradation[28]. The red lysosome tracker was used to label lysosomes. After naringin treatment, intestinal lysosomes were improved by 26.4% (P< 0.05) and 83.2% (P< 0.01),respectively (Figs. 6C and E).
3.12 Anti-aging mechanisms of naringin in flies
3.12.1 Naringin regulated the mRNA expressions in wild-type flies
Naringin (1.4 mmol/L) significantly increased the expression levels ofsod1,sod2,cat, andhsp26by 36.9% (P< 0.01), 29.3%(P< 0.05), 24.3% (P< 0.05), and 23.1% (P< 0.05), respectively(Fig. 6F). However, the change ofsir2mRNA expression was not significant after naringin treatment (P< 0.05) (Fig. 6F).
Naringin (1.4 mmol/L) dramatically improved the expression levels ofdilp2((1.60 ± 0.01)-fold,P< 0.01),dilp3((1.70 ± 0.02)-fold,P< 0.01),dilp5((1.51 ± 0.09)-fold,P< 0.01), andfoxo((1.64 ± 0.03)-fold,P< 0.01), respectively (Fig. 6G), but significantly downregulated the expression levels ofinr((0.81 ± 0.01)-fold,P< 0.05),chico((0.83 ± 0.06)-fold,P< 0.05),pi3k((0.75 ±0.04)-fold,P< 0.01), andakt-1((0.66 ± 0.05)-fold,P< 0.01),respectively (Fig. 6G).
Similarly, 1.4 mmol/L naringin dramatically improved the expression levels ofatg1((1.44 ± 0.16)-fold,P< 0.01),atg5((1.90 ± 0.06)-fold,P< 0.01),atg8a((2.19 ± 0.07)-fold,P< 0.01),andatg8b((1.51 ± 0.08)-fold,P< 0.01), respectively (Fig. 6H).
3.12.2 Naringin did not alter the lifespan in relative RNAi flies
The anti-aging effect of naringin was tested with several mutant stocks with functional deficiency. The average lifespan ofinr,chico,pi3k, andakt-1RNAi flies treated with 1.4 mmol/L naringin was not significantly decreased (P> 0.05) (Figs. 7A-D). The average survival time offoxo,atg1,atg5,atg8a, andatg8bRNAi flies supplemented with 1.4 mmol/L naringin was not significantly increased (P> 0.05)(Figs. 7E and G-J), while the average lifespan ofsir2RNAiD. melanogastersupplement with 1.4 mmol/L naringin was dramatically improved by 8.6% (P< 0.05) (Fig. 7F). These findings suggested that naringin-mediated longevity was associated withinr,chico,pi3k,akt-1,foxo,atg1,atg5,atg8a, andatg8b, but not withsir2.

Fig. 7 Actions of naringin on the lifetime of mutant flies. The lifetime of (A) inr, (B) chico, (C) pi3k, (D) akt-1, (E) foxo, (F) sir2, (G) atg1, (H) atg5, (I) atg8a,(J) atg8b mutant flies (n = 200 per group).
3.12.3 Naringin did not change the number of intestinal lysosomes in atg1, atg5, atg8a, and atg8b RNAi flies
1.4 mmol/L naringin did not significantly increase the number of intestinal lysosomes 4.8% (P> 0.05), 7.5% (P> 0.05), 6.2%(P> 0.05), and 4.2% (P> 0.05) ofatg1,atg5,atg8a, andatg8bRNAi fly, respectively (Fig. 8). These results suggested that naringinmediated lysosomal formation was associated withatg1,atg5,atg8a,andatg8b, which indicated that the association was related to antiaging effects.

Fig. 8 Actions of naringin on the formation of lysosomes in RNAi flies. The lysosomes of 10 day (A) atg1, (C) atg5, (E) atg8a, (G) atg8b RNAi flies(n = 100 per group); the fluorescence intensity ratio of lysosomes in (B) atg1, (D) atg5, (F) atg8a, (H) atg8b RNAi flies.
3.13 Molecular docking revealed the interaction between naringin and anti-aging-related proteins
In molecular docking, the binding energy is commonly used to evaluate the stability of the binding between small and protein molecules. The lower the numerical values of binding energy between small and protein molecules, the stronger the stability of their binding[29]. The interaction residues and binding energies were described in Fig. 9 and Table S6. The binding energy of naringin to PI3K protein was −75.691 2 kJ/mol and that of naringin to AKT1 protein was −148.86 kJ/mol. The findings suggested that naringin was bound to PI3K and AKT1 proteins, which were consistent with network pharmacology prediction and real-time PCR results.
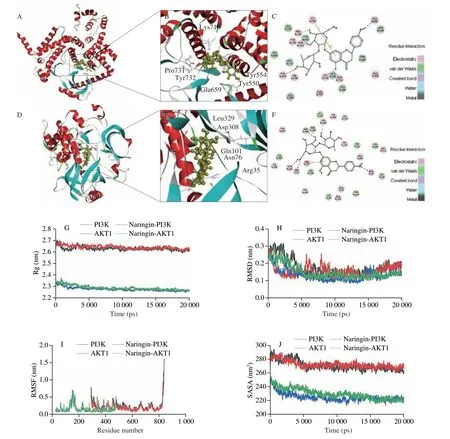
Fig. 9 Actions of naringin bind to PI3K and AKT1 proteins. (A) and (D) 3D diagram of naringin combined with PI3K and AKT1 proteins, respectively;(B) and (E) 3D local diagram of naringin combined with PI3K and AKT1 proteins, respectively; (C) and (F) 2D diagram of naringin combined with PI3K and AKT1 proteins, respectively; (G) the Rg plots of PI3K, AKT1, naringin-PI3K, and naringin-AKT1 complexes; (H) The RMSD plots of PI3K, AKT1,naringin-PI3K, and naringin-AKT1 complexes; (I) The RMSF plots of PI3K, AKT1, naringin-PI3K, and naringin-AKT1 complexes; (J) The SASA plots of PI3K,AKT1, naringin-PI3K, and naringin-AKT1 complexes.
3.14 MD simulations revealed stable binding of naringin to anti-aging-related proteins
Rg value reflects the tightness of protein structure. The Rg values of naringin-PI3K and naringin-AKT1 were stable at about 2.64 and 2.29 nm in the first 15 000 ps, respectively, while the Rg values of free PI3K and AKT1 fluctuated greatly in the first 15 000 ps, and then were stable at about 2.64 and 2.29 nm after 15 000 ps, respectively(Fig. 9G). These results suggested that the binding of naringin with PI3K and AKT1 led to tighter protein structures. RMSD value indicates complex conformational stability changes. The RMSD values of naringin-PI3K and free PI3K continued to decrease before 15 000 ps and then stabilized to 0.18 nm after 15 000 ps. Similarly, the RMSD values of naringin-AKT1 and free AKT1 were also observed in the first 15 000 ps and then stabilized with an average RMSD of 0.14 nm after 15 000 ps. The results showed that naringin binding to protein did not cause large fluctuations (Fig. 9H). Moreover, the RMSF value of flexibility of expressed amino acid residues indicated that complexes and free PI3K and AKT1 amino acid fluctuations were relatively stable (Fig. 9I). The SASA values of naringin-PI3K and free PI3K fluctuated within 15 000 ps, but stabilized after 15 000 ps. The same tendency was also observed for the SASA values of naringin-AKT1 and free AKT1 (Fig. 9J). These results reflected that the binding of naringin enhanced the stable ability of PI3K and AKT1 proteins.
4. Discussion
In this study, naringin alleviated the decline of cell viability induced by H2O2and decreased ROS content in PC12 and HepG2 cells. Naringin also improved physiological status, enhanced antioxidant enzyme and anti-stress abilities, improved intestinal microbial homeostasis, inhibited abnormal ISCs proliferation,and prolonged the lifespan of flies. Moreover, naringin inhibited the IIS pathway through key factorspi3kandakt-1and activated autophagy-related genes to prolong the lifespan, and the underlying mechanisms were verified by RNAi fly stocks and computer molecular simulation.
Physiological condition is the most basic index to measure the health of organisms[30]. The results showed that naringin and resveratrol prolonged the survival time of wild-type flies,enhanced the crawling ability, and increased resistance to hunger,heat, and cold abilities. It was in line with Wu et al.[30]finding that resveratrol (1 mmol/L) extended the lifespan and improved the motor dysfunction of flies. A previous study reported that the anti-aging mechanisms of resveratrol were through stimulating Nrf2 and activating Sirt1 to promote the activities of antioxidant enzymes and prolong the lifespan of flies[25]. However, in this study, the anti-aging mechanisms of naringin predicted by network pharmacology might repress oxidative stress and prolong lifespan by inhibiting the IIS pathway and activating the autophagy pathway. Therefore, only naringin was analyzed in subsequent experiments to study the anti-aging mechanisms of naringin.Hsp26is one of the most upregulated heat shock proteins after stress and maintains protein homeostasis[31]. The mRNA expression ofhsp26increased after 1.4 mmol/L naringin supplementation.Chattopadhyay et al.[17]studied that rutin (200 μmol/L)extended the lifespan and increased environmental stress tolerance inD. melanogaster. Lashmanova et al.[32]reported that naringin extended the lifespan and improved the ability of climbing and anti-starvation stress of flies. Furthermore, this study also comprehensively explored the molecular mechanisms of naringin prolonging the lifespan ofD. melanogastercompared with the above study.
The DR pathway is considered a way of extending the lifespans of many organisms, including yeast,Caenorhabditis elegans, andD. melanogaster[33]. The results showed that food intake and average body weight in flies were not changed significantly after adding naringin. Moreover,sir2exerts crucial effects on lifespan longevity through the DR pathway[34]. Real-time PCR results indicated that naringin did not change the expression level ofsir2, but the lifespan of naringin-mediatedsir2RNAi flies was significantly increased.These results suggested that naringin-mediated life extension was independent ofsir2and the DR pathway. This result was in line with our previous study, the naringenin treatment groups did not change the pharyngeal aspiration times ofC. elegans, and the lifespan ofeat-2mutant worms was significantly prolonged[35].
The intestinal barrier function and intestinal integrity of aging flies decreased, the permeability increased, and the intestinal microflorae hyperplasia abnormally caused intestinal dysfunction[36].In this study, the proportion of 1.4 mmol/L naringin-mediated “Smurf”flies was effectively decreased on the 50thday (Fig. 5D). It was proved that naringin effectively alleviated the increase of intestinal permeability during aging. Moreover, the total number of intestinal microflorae was significantly reduced after naringin treatment on the 40thday (Fig. 5E). These results were in line with the study of Fan et al.[19], dihydromyricetin (40 μmol/L) effectively reduced the proportion of “Smurf” and intestinal permeability, inhibited intestinal microflorae proliferation, and improved intestinal homeostasis. In the aging gut, excessive proliferation and abnormal differentiation of ISCs cause tissue dysplasia and promote cancer or degenerative diseases, accelerating aging[27]. The level of Esg reflects the degree of proliferation of ISCs. In this paper, KEGG pathway enrichment analysis indicated that naringin was related to the signaling pathway regulating stem cell pluripotency. The results indicated that the content of Esg was dramatically decreased in the 50-day-old flies treated with 1.4 mmol/L naringin, which showed the abnormal proliferation decrease of ISCs. This was consistent with our previous research, flavonoid-rich hawthorn extract (3.0 mg/mL) significantly reduced the content of Esg and inhibited the abnormal proliferation of ISCs in aging flies[15]. Similarly, Fan et al.[27]found that rapamycin reduced the proportion of “Smurf” flies, delayed the onset of the agerelated “Smurf” phenotype, reduced the intestinal microflorae of old flies, and improved the intestinal integrity of the old flies. Rapamycin slowed the proliferation of ISCs in the intestines of aging flies, thus helping to prolong their lifespan.
Paraquat and H2O2can stimulate the body to produce excessive ROS, which impairs cell function and accelerates aging[37]. The results showed that naringin increased H2O2-damage cell viability and reduced the ROS content of H2O2-induced cells in PC12 and HepG2 cells. It was in line with the following studies, in which naringin inhibited mitochondrial ROS production and mitochondrial dysfunction in fructose-exposed cardiomyocytes[38]. Naringin also prolonged the survival time of wild-type flies exposed to paraquat or H2O2. Endogenous antioxidant enzymes (SOD and CAT) play a natural defense against ROS at the cellular level[39]. Therefore,improving the SOD and CAT activities dramatically decreased oxidative damage, thus delaying aging[40]. MDA is the final product of lipid peroxidation, reflecting the destruction of tissue by oxidative stress[41]. This study showed that the activities of SOD1, SOD2, and CAT of flies were significantly increased after naringin treatment, and 1.4 mmol/L naringin decreased the MDA content. Meanwhile, the expression levels ofsod1,sod2, andcatwere increased after naringin treatment. Moreover, naringin did not increase the survival time ofsodorcatRNAi flies exposed to paraquat or H2O2, supporting the hypothesis that the anti-aging activity of naringin was mediated in part by interactions withsodandcatgenes. This result was consistent with the study reported by Kumar et al.[42]that naringin treatment reduced oxidative damage inD-galactose-induced aging mice and extended the lifespan of the mouse. Our previous study showed that phlorizin ameliorated theD-galactose-induced decline of PC12 cells vitalityin vitro, improved the activities and mRNA expressions ofsodandcat, and reduced MDA content in brain, liver, and serum ofD-galactose-induced mice[11].
Network pharmacology prediction results showed that the antiaging effects of naringin was dramatically related to the regulation of crucial genesPI3K3CAandAKT1in the IIS pathway. PI3K activator(740Y-P) abolished the protective effect of naringin on H2O2-induced cells, verifying the fact that the antioxidant effect of naringin was mediated bypi3kin the IIS pathway in cells. Similarly, naringin increased the apoptosis rate, which was partially eliminated by the PI3K activator (740Y-P)[43]. Inhibiting the IIS pathway prolongs the lifespans ofD. melanogaster,C. elegans, and mice[44]. DILPs act endocrinologically by binding toinron peripheral tissue target membranes, triggering phosphorylation of substrates containing insulin receptorschico,pi3k, andakt. Phosphorylation and activation ofaktsubsequently lead to phosphorylation of various downstream effector proteins. One of them is the transcription factorfoxo, which is regarded as a longevity factor[44]. Naringin up-regulated the mRNA expression levels ofdilp2,dilp3,dilp5, andfoxo, and down-regulated the mRNA levels ofinr,chico,pi3k, andakt-1. Furthermore, the lifeextending effects of naringin oninr,chico,pi3k,akt-1, andfoxoRNAi flies almost disappeared, proving that the anti-aging effect exerted by naringin was mediated byinr,chico,pi3k,akt-1, andfoxoin the IIS pathway. Similarly, a previous study showed that 50 μmol/L naringin extended the lifespan of Alzheimer’s disease and Parkinson’s diseaseC. elegansvia the IIS pathway[45].
KEGG pathway enrichment results showed that naringin was related to the autophagy pathway. Autophagy-related geneswere activated byfoxo, downstream of the IIS pathway, and the autophagy pathway is regulated by growth and associated with cell death.Atg1is the first identified autophagy-related gene that encodes a serine/threonine protein kinase[46].Atg5has been characterized as a protein required for autophagy[47]. Losses ofatg8aandatg8bcause complex delayed phenotypic development, reduce growth, phagocytosis,and cell viability, increase ubiquitinated proteins, and reduce proteasome activity[48]. In this paper, naringin dramatically up-regulated the mRNA levels ofatg1,atg5,atg8a, andatg8b. Meanwhile, intestinal lysosome staining confirmed that the formation of lysosomes was increased under naringin treatment (Fig. 6E), and the number of lysosomes was also improved accordingly. Moreover, the lifespan and the number of intestinal lysosomes ofatg1,atg5,atg8a, andatg8bRNAi flies hardly improved after naringin treatment, suggesting thatatg1,atg5,atg8a, andatg8bexerted crucial effects in the naringin-mediated anti-aging study. This result was consistent with the results reported by Chattopadhyay et al.[49]that rutin (400 μmol/L)up-regulated the mRNA levels ofatg1andatg5, activated the autophagy pathway, and exerted the effect of aging delay in flies.Similarly, Kharat et al.[50]found that ellagic acid (200 μmol/L)up-regulated the mRNA expression level ofatg1and increased the level of autophagy, indicating that it extended the lifespan of flies through the autophagy pathway.
Molecular docking is a process of ligands docking with receptors,and binding energies gained are used to verify the molecular mechanism[13]. MD simulation is a technology for deciphering the interaction of proteins with drugs to reveal the functional mechanisms of drugs[51]. Naringin was bound to key proteins PI3K and AKT1 in the IIS pathway. The RMSD, RMSF, and SASA values of complexes and corresponding free proteins had little change. The Rg value confirmed the tightness and stability of the proteins in the active site environment in the presence of naringin. These results indicated that naringin with PI3K and AKT1 proteins bound stably during MD simulation. It was mutually verified with the key targets and pathways of naringin predicted by network pharmacology and the results of cell and fly tests. Therefore, it was suggested that naringin exerted antioxidant and anti-aging effects via the IIS and autophagy pathways.
5. Conclusion
Naringin protected cells from oxidative damage and reduced ROS content in PC12 and HepG2 cells. Moreover, naringin extended the lifespan ofD. melanogasterand improved the crawling ability. Naringin also increased resistance to environmental and oxidative stress. Meanwhile, naringin improved intestinal barrier dysfunction and reduced the abnormal proliferation of ISCs. Naringin activated the autophagy pathway and inhibited the IIS pathway to prolong the lifespan. These results provided a theoretical basis for the development of naringin as an antioxidant in foods and drugs.
Conflicts of interest
Hao Wang is an editorial board member forFood Science and Human Wellnessand was not involved in the editorial review or the decision to publish this article. The authors declared that no conflicts of interest to this work.
Acknowledgments
This work was supported by the open project of the Key Laboratory of Environmental Pollution Monitoring and Disease Control, Ministry of Education, Guizhou Medical University, China(GMU-2022-HJZ-06).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.26599/FSHW.2022.9250103.
- 食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango

