Blood glucose-lowering activity of protocatechuic acid is mediated by inhibiting α-glucosidase
Huafang Ding, Shouhe Huang, Chui Yiu Chook, Erika Kwek, Chi Yan,Kaying Ma, Jianhui Liu, Hanyue Zhu, Zhenyu Chen
Food & Nutritional Sciences Programme, School of Life Sciences, The Chinese University of Hong Kong, Hong Kong 999077, China
Keywords: Protocatechuic acid α-Glucosidase Postprandial hyperglycemia Inhibition mechanism
ABSTRACT α-Glucosidase inhibitors are effective in controlling postprandial hyperglycemia, which play crucial roles in the management of type 2 diabetes. Protocatechuic acid (PCA) is one of phenolic acids existing not only in various plant foods but also as a major microbial metabolite of dietary anthocyanins in the large colon.The present study investigated the inhibitory mechanism of PCA on α-glucosidase in vitro and examined its effect on postprandial blood glucose levels in vivo. Results from in vitro experiments demonstrated that PCA was a mix-type inhibitor of α-glucosidase. Driven by hydrogen bonds and van der Waals interactions, PCA reversibly bound with α-glucosidase to form a stable α-glucosidase-PCA complex in a spontaneous manner.The computational simulation found that PCA could insert into the active cavity of α-glucosidase and establish hydrogen bonds with catalytic amino acid residues. PCA binding aroused the steric hindrance for substrates to enter active sites and caused the structural changes of interacted catalytic amino acid residues. PCA also exhibited postprandial hypoglycemic capacity in diabetic mice. This study may provide the theoretical basis for the application of PCA as an active ingredient of functional foods in dietary management of diabetes.
1. Introduction
Diabetes is characterized by having fasting plasma glucose greater than 7 mmol/L. Its prevalence and mortality have risen signif icantly in the recent years[1]. Currently, there are 537 million individuals suffering from diabetes in the world and the figure is expected to rise to 643 million by 2030[1]. It is estimated that 6.7 million deaths are due to diabetes[1]. Recently, diabetic patients have been shown to have 1.6 times higher in the incidence of COVID-19-related hospitalization as those without diabetes[1]. Among all diabetes, type 2 diabetes accounts for over 90%. The sustained high blood glucose in untreated type 2 diabetes will elicit the damage of body’s organs,such as vascular, kidney, eyes, and foot, leading to the development of diabetic complications[2]. Therefore, the control of postprandial hyperglycemia is of great significance in the management of type 2 diabetes.α-Glucosidase inhibitors are recommended as the firstline medicines to treat type 2 diabetes because they are effective in the delay of hydrolysis of carbohydrates in the intestine[3]. However,the synthesizedα-glucosidase inhibitors such as acarbose (Fig. 1A)have some side effects, including vomiting, f latulence, cramping and abdominal pain[4-6].
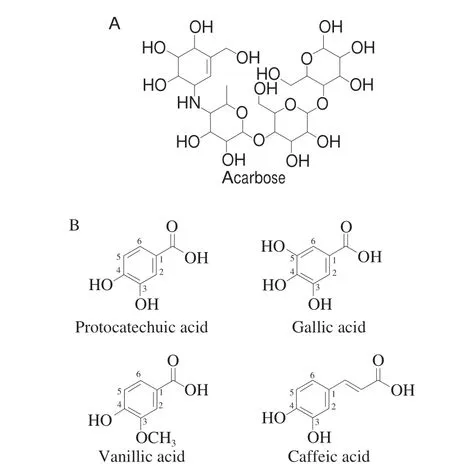
Fig. 1 Structures of (A) acarbose, (B) protocatechuic acid, gallic acid,vanillic acid and caffeic acid.
Protocatechuic acid (PCA) is one of phenolic acids, distributing in various food plants, such as olives, white wine grapes and tea(Fig. 1B). It is also the major microbial metabolite of dietary anthocyanins in the large colon[7-8]. PCA possesses a broad-spectrum of physiological activities, including cardiovascular-protection, antihyperglycemia, anti-inf lammation, and anti-oxidation[9-12]. It has been reported that PCA could inhibitα-glucosidase activity[13]. However,the inhibitory mechanism of PCA onα-glucosidase is lacking.
Multi-spectroscopic methods have been widely used to investigate the interaction between functional proteins and active compounds[14-15]. As a powerful tool to study the molecular interaction,computational simulation could provide the information including the binding sites, conformational alteration, stability and hydrophobicity of protein-ligand complex. This study aimed to: 1) investigate the inhibition kinetics and underlying inhibitory mechanism of PCA onα-glucosidase; 2) assess the effect of PCA on postprandial hyperglycemia in diabetic mice.
2. Materials and methods
2.1 Chemicals
PCA (purity ≥ 97%) was purchased from Aladdin Industrial Co.(Shanghai, China).α-Glucosidase (EC 3.2.1.20, G5003, 10.88 U/mg)fromSaccharomyces cerevisiaewas obtained by Sigma-Aldrich Co.(St. Louis, MO, USA). Theα-glucosidase solution was dissolved in sodium phosphate buffer (0.1 mol/L, pH 6.8). Acarbose hydrate(purity ≥ 98%) and 4-nitrophenylα-D-glucopyranoside (pNPG,purity > 99%) were obtained from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) and their respective solutions were prepared by dissolving in the same buffer asα-glucosidase.
2.2 α-Glucosidase activity assay
The inhibitory ability of PCA onα-glucosidase was evaluated as previously described with minor modification[16].α-Glucosidase(3.93 μg/mL) was incubated in the presence of varying concentrations of PCA or acarbose at 37 °C for 1 h. Then, the substratepNPG(0.14 mg/mL) was added into the pre-incubated reaction system and the absorbance was detected at 405 nm using an UV-1601 UV-visible spectrophotometer (Shimadzu, Japan). The relative activity ofα-glucosidase was counted according to the following equation:
WhereKandK0represent the slopes of the equation acquired by reactions with and without PCA. The half inhibitory concentration(IC50) means the concentration of PCA inducing a reduction of 50%α-glucosidase activity.
2.3 Inhibitory kinetic analysis
The inhibitory kinetics of PCA onα-glucosidase was ascertained using the same procedure asα-glucosidase activity assay. The reaction rates ofα-glucosidase were recorded in the presence of varying concentrations of PCA andpNPG. Then, the kinetic parameters and inhibitory type were probed by the following Lineweaver-Burk equations:
Whereνandvmaxdenote the reaction rate and the maximum reaction rate ofα-glucosidase (mmol/(L·min)), respectively.means the apparentvmax.Kmrepresents the Michaelis-Menten constant. The concentrations of inhibitor and substrate are expressed as [I] and [S], respectively.KiandKismean the equilibrium constants for PCA binding with freeα-glucosidase andα-glucosidase-substrate complex, respectively.
2.4 Fluorescence spectra
α-Glucosidase solution (3 mL, 0.09 mg/mL) was titrated by successively adding 5 μL of PCA. After the equilibrium for 5 min, the fluorescence spectra (310–450 nm) were monitored on a spectrofluorometer (model F-4500 Hitachi, Tokyo, Japan) at 292,298 and 304 K. The excitation wavelength is 280 nm and 2.5 nm was set for the widths of excitation and emission slits. According to the previous study, all fluorescence data were corrected to eliminate the re-absorption and inner filter effects of PCA.
2.5 Circular dichroism (CD) spectra
CD spectrometer (JASCO J-810) was used to record the CD spectra (190–250 nm) ofα-glucosidase in the presence and absence of PCA[17]. The concentration ofα-glucosidase and PCA were 0.27 and 0.06 g/L, respectively. The CD spectra was corrected using PBS as a background and analyzed by online SELCON3 program.
2.6 Molecular simulation
Discovery Studio 4.5 software (NeoTrident, Beijing, China) was used to conduct molecular docking between PCA andα-glucosidase.The 3-dimensional structure of PCA was available from the PubChem database, while the crystal structure ofα-glucosidase (PDB ID: 3AJ7)was obtained from the RCSB Protein Data Bank. After the water removal, hydrogenation and polarity addition, theα-glucosidase crystal was defined as the receptor to dock with PCA using the CDOCKER program. 100 and 0.25 Å were used for the running times and the docking tolerance. The optimal binding posture meant the docking posture with the lowest energy, which was chosen for further analysis.
2.7 Molecular dynamics (MD) simulation
GROMACS 4.5.6 was utilized to simulate the motion of freeα-glucosidase andα-glucosidase-PCA complex in solvent environment. PCA topology file was generated from Acpype server,which was combined with the dehydrated PDB file ofα-glucosidase to construct the topology file of the complex. The complex was immersed in a box filled with simple point charge water molecules,and the distance of the complex from the edge of the box was set at 1 nm. The surface charges of the complex were neutralized by adding Na+and Cl−. After energy minimization by steepest descent method,the system was equilibrated by NVT (300 K, 1 bar) for 100 ps. Then,the dynamics simulation of the equilibrated system was performed under the NPT ensemble for 30 ns.
2.8 Animals
Male C57BL/6J mice (n= 21, 7-week-old) were acquired from the Laboratory Animal Services Centre, The Chinese University of Hong Kong, Hong Kong, China. Mice were hold in an animal room at 23 °C with a 12 h/12 h light-dark cycle and given a one-week of acclimation. Then, diabetes was induced following a previous protocol with minor modification[18]. In brief, mice were fasted for 6 h and then injected with 40 mg/kg body weight (BW) streptozotocin(STZ) for 5 consecutive days. At day 9 after the last STZ injection,mice with the fasting blood glucose greater than 8.3 mmol/L were considered diabetic. All experiments were approved by the Animal Experimental Ethical Committee, The Chinese University of Hong Kong (Ref. No. 20-245-MIS).
2.9 Oral sucrose tolerance test (OSTT) in STZ-induced diabetic mice
After fasting for overnight, 21 mice were randomly assigned into three groups and administered an oral gavage of 4 g/kg BW sucrose together with 0.25% sodium carboxymethyl cellulose (CMC-Na,vehicle), 0.5 g/kg BW PCA or 1 g/kg BW PCA, respectively. Blood samples were collected from the tail vein at 0, 15, 30, 60, 90, 120 min and blood glucose concentration was measured with a glucometer[19].The area under the curve (AUC) was calculated.
2.10 Statistical analysis
All data were expressed as mean ± standard deviation (SD).One-way analysis of variance (ANOVA) and post hoc LSD were utilized to analyze the statistical difference in SPSS software (Chicago,USA).P< 0.05 was defined as statistical significance.
3. Results and discussion
3.1 α-Glucosidase inhibition by PCA in vitro
As depicted in Fig. 2A, both of PCA and acarbose exhibited inhibitory activities onα-glucosidase in a concentration-dependent pattern. The IC50value of PCA was 0.37 mg/mL, which was lower than that of acarbose (IC50= 0.56 mg/mL), suggesting that PCA was a potentialα-glucosidase inhibitor. However, the IC50value of PCA was higher than that in a previous report (IC50= 0.07 mg/mL), which might be owing to the differences in methods, reaction conditions and the sources of PCA and enzyme[20]. Previous studies found that other phenolic acids (Fig. 1B) with similar structures to PCA could also inhibitα-glucosidase activity. Gallic acid, which possesses one more phenolic hydroxyl group than PCA, exhibited a stronger inhibitory activity againstα-glucosidase than PCA (IC50= 0.22 mg/mL)[21].As reported, hydroxyl group is an electron-donating group, which elevates the electronegativity of molecules and contributes to the interaction of molecules with enzymes, resulting in a better inhibitory activity[22]. As a metabolite of PCA, vanillic acid displayed a weaker inhibition onα-glucosidase (IC50= 2.00 mg/mL), which might be attributable to the methylation in 3’-OH[23-24]. Moreover, caffeic acid,which has an extra unsaturated alkyl chain, had a lower IC50value(0.12 mg/mL) than PCA[25]. These results demonstrated that phenolic hydroxyl group and unsaturated alkyl chain are crucial for phenolic acids to exert their inhibitory activities againstα-glucosidase, whereas the methyl substitution of phenolic hydroxyl group was unfavorable for eliciting an inhibition onα-glucosidase activity.
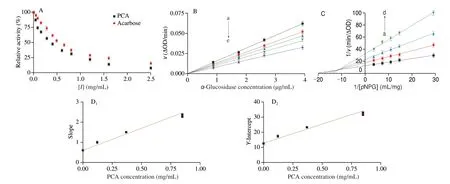
Fig. 2 Inhibitory characteristics of PCA on α-glucosidase. (A) Inhibition of α-glucosidase by PCA and acarbose (pH 6.8, T = 310 K). The concentrations of α-glucosidase and pNPG were 3.93 and 0.14 mg/mL, respectively. (B) Plots of ν versus the concentration of α-glucosidase. The concentration of pNPG was 0.14 mg/mL, and the concentrations of PCA were 0, 0.05, 0.08, 0.12, and 0.37 mg/mL for curves a−e, respectively. (C) Lineweaver-Burk plots. The concentration of α-glucosidase was 3.93 μg/mL, and the concentrations of PCA were 0, 0.12, 0.37, and 0.85 mg/mL for curves a−d, respectively. (D) The secondary plots of slope and Y-intercept vs. PCA.
3.2 Kinetics of α-glucosidase inhibition
The inhibitory kinetics assay was conducted to probe the reversibility and inhibitory type of PCA onα-glucosidase. As displayed in Fig. 2B, all straight lines intersect at the origin, and the slopes reduced with the rising concentration of PCA. These results revealed that PCA could non-covalently interact withα-glucosidase,which reversibly inhibitedα-glucosidase activity without reducing the amount of efficientα-glucosidase[26].
Lineweaver-Burk plots were constructed to further ascertain the inhibitory type. As depicted in Fig. 2C, all the straight lines crossed at the second quadrant. As the concentration of PCA increased, the value ofKmincreased and the value ofvmaxdecreased, manifesting that PCA mediated a mixed-type inhibition onα-glucosidase. In addition, the value ofKi(0.28 mg/mL) was lower than that ofKis(0.51 mg/mL), indicating that competitive inhibition dominated in PCA-induced inhibition onα-glucosidase. That’s to say, PCA tended to compete withpNPG to combine with freeα-glucosidase rather thanα-glucosidase-pNPG complex, which exhibited a similar inhibitory action of vanillin[27-28]. As shown in Fig. 2D, the slopes andY-intercepts of the lines in the Lineweaver-Burk plots were linearly correlated with PCA concentration, suggesting that there was one or a class of PCA binding sites present inα-glucosidase.
3.3 Fluorescence quenching of α-glucosidase
Due to the presence of aromatic amino acids,α-glucosidase could emit endogenous fluorescence. Fluorescence of the fluorophore can be quenched by various factors, including energy transfer, excited-state reactions, and interactions with small molecules[29-30]. Fig. 3A shows that there was an obvious fluorescence peak ofα-glucosidase observed at 350 nm (excitation wavelength at 280 nm). The fluorescence intensity ofα-glucosidase decreased gradually accompanied by adding PCA, but there was no obvious wavelength shift, which indicated that molecular interactions were present betweenα-glucosidase and PCA.
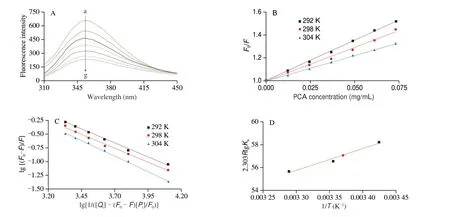
Fig. 3 Quenching characteristics of PCA on α-glucosidase. (A) Fluorescence spectra of α-glucosidase in the presence of PCA at different concentrations (pH 6.8,T = 298 K). The concentration of α-glucosidase was 0.09 mg/mL, and the concentrations of PCA were 0, 12.18, 24.35, 36.53, 48.55, 60.72 and 72.59 μg/mL for curves a−g, respectively. (B) Stern-Volmer plots for the fluorescence quenching of α-glucosidase. (C) Double-logarithm plots of α-glucosidase and PCA. (D) Plot of 2.303RlgKa vs. 1/T for α-glucosidase and PCA.
Based on the mechanism, fluorescence quenching could be classified into three forms: static quenching, dynamic quenching, and the combination of them. Static quenching was due to the combination of the fluorophore with quencher to form non-fluorescent groundstate complex while dynamic quenching was caused by the collision between the quencher and excited-state fluorophore[31]. These two kinds of quenching could be distinguished by their different responses to temperatures: higher temperatures lead to stronger dynamic quenching with increased quenching constants, whereas the opposite effect was observed for static quenching. The quenching mechanism was probed by the following Stern-Volmer equation:
WhereFandF0represent the fluorescence intensities ofα-glucosidase with and without PCA, separately. The Stern-Volmer quenching constant and the biomolecular quenching constant are expressed asKSVandKq, respectively. [Q] means the concentration of PCA.τ0denotes the average fluorescent life of the fluorophore (10–8s for biomacromolecules).
It could be observed in Fig. 3B that all lines in the Stern-Volmer plots were linearly fitted, indicating that PCA induced a single type of quenching. The quenching constants were represented in Table 1. There was a negative correlation betweenKSVvalues and temperatures, and calculatedKqvalues were higher than the value of the maximum scattering collision quenching constant(2.0 × 1010L/(mol·s))[32]. These results conjectured that PCA mediated a static fluorescence quenching ofα-glucosidase, which resulted from the combination ofα-glucosidase and PCA[33].

Table 1 Quenching constants (KSV), binding constants (Ka), number of binding sites (n), and thermodynamic parameters for α-glucosidase-PCA interaction at 292, 298 and 304 K.
The double-logarithm equation was utilized to calculate the binding constant (Ka) and the number of binding sites (n):
Where the concentrations of PCA andα-glucosidase mark as[Qt] and [Pt] (mg/mL), respectively. As shown in Fig. 3C, the doublelogarithm plots presented a good linearity at three temperatures (Table 1).The values ofKawere in the magnitude order of approximately 103L/mol, signifying that PCA possessed a relatively moderate affinity withα-glucosidase. Besides,nvalue was close to one, suggesting that there was only one site inα-glucosidase for PCA binding. Results sustained the conclusion of inhibitory kinetic analysis[34].
3.4 Thermodynamic analysis and binding forces
There are 4 kinds of non-covalent interactions between macromolecules and ligands, including electrostatic interactions,van der Waals interactions, multiple hydrogen bonds, and hydrophobic forces. As mentioned in kinetic analysis, PCA could non-covalently interact withα-glucosidase. To probe the binding forces between PCA andα-glucosidase, thermodynamic parameters were calculated by the Van’t Hoff equation:
WhereKastands for the binding constant acquired from equation (6),Ris the gas constant (8.314 J/(mol·K)),Tmeans the absolute temperature (K). ΔH, ΔSand ΔGrepresent the changes of enthalpy,entropy, and free energy, respectively. Table 1 concluded the thermodynamic parameters obtained from Fig. 3D. The negative ΔGmeant that PCA bound withα-glucosidase spontaneously[35]. The value of ΔHwas negative, demonstrating that the binding process was exothermic, and this deduction was in accordance with that of the negative correlation between temperatures andKavalues[36].The negative ΔHand ΔSindicated that hydrogen bonds and van der Waals interactions were the dominate driving forces for the combination process[37].
3.5 CD spectra analysis
CD spectra was analyzed to acquire the content of secondary structures ofα-glucosidase. Fig. 4 shows that two negative bands at around 210 and 220 nm were observed inα-glucosidase system, which was weakened in the presence of PCA. Further analysis found thatα-helix content reduced from 17.75% to 16.17% while the proportions ofβ-sheet,β-turn and random coil increased in the presence of PCA (Table 2). This phenomenon was similar to that of sinensetin,indicating that PCA aroused the rearrangement of secondary structure ofα-glucosidase[38]. Considering that secondary structures are mainly maintained by hydrogen bonds, we inferred that PCA-induced change of secondary structures might be due to the establishment of hydrogen bonds withα-glucosidase.

Table 2 Effect of PCA on the secondary structure of α-glucosidase.
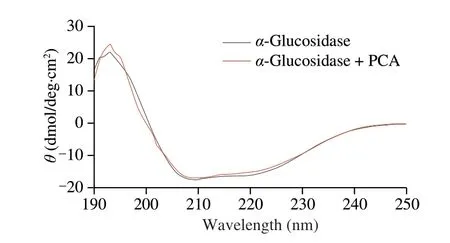
Fig. 4 CD spectra of α-glucosidase in the presence and absence of PCA. The concentrations of α-glucosidase and PCA were 0.27 and 0.06 g/L, respectively.
3.6 Molecular docking analysis
Molecular docking is a powerful tool for visually displaying the binding information of protein and ligands. Fig. 5A showed the predicted binding postures of PCA andα-glucosidase. Obviously,PCA docked into the active cavity ofα-glucosidase, supporting the results obtained from the inhibitory kinetic analysis that PCA was a competitive-dominated mixed-type inhibitor. Besides, PCA was enclosed by amino acid residues Phe 159, Phe 178, Arg 213, Val 216,Glu 277, Phe 303, Arg 315, Tyr 316, Asn 350, Asp 352, Gln 353, Glu 411 and Arg 442 (Fig. 5B). PCA established one hydrogen bond with residues Arg 315 and Asp 352, respectively. Considering that Arg 315 and Asp 352 are catalytic amino acid residues ofα-glucosidase, the interactions between PCA and these residues might directly influence the catalytic activity. Other four hydrogen bonds were also observed by residues Glu 277, Gln 353 and Arg 442 with PCA. Moreover, van der Waals interactions were noticed between PCA andα-glucosidase via residues Phe 159, Phe 178, Arg 213, Val 216, Phe 303, Tyr 316,Asn 350 and Glu 411. In addition, the benzene ring of PCA built Pi-anion interaction with residue Asp 352. These interactions were favorable to the binding stability between PCA andα-glucosidase.These results indicated that hydrogen bonds and van der Waals forces were crucial to the combination betweenα-glucosidase and PCA, which was consistent with the conclusion from the thermodynamic analysis.

Fig. 5 Binding characteristics of PCA with α-glucosidase. (A) The optimal binding posture of PCA in α-glucosidase and the hydrophobic pocket of α-glucosidase. (B) 2D schematic diagram of the interactions between PCA and α-glucosidase.
3.7 MD simulation
MD simulation was utilized to monitor the process of PCA-induced changes in the conformation and stability ofα-glucosidase.Root mean square deviation (RMSD) analysis ofα-glucosidase backbone provided the information on the trajectory stability of freeα-glucosidase andα-glucosidase-PCA complex. Fig. 6A showed that freeα-glucosidase took approximately 7 ns for equilibrium with the RMSD value being around 0.12 nm. In terms of the complex system, the RMSD values oscillated around the average value at the first 12 ns, then there was a large elevation of RMSD values (from 0.10 to 0.21 nm) observed from 12 to 20.1 ns, followed by a new steady status at around 24.4 ns. The sharp rise of RMSD values reflected the binding process of PCA withα-glucosidase, in whichα-glucosidase rearranged with PCA to form a stable complex[38].

Fig. 6 MD simulation of PCA and α-glucosidase. The RMSD (A), RMSF (B)and SASA (C) plots of α-glucosidase-PCA complex and free α-glucosidase.
The root mean square fluctuation (RMSF) value had a positive correlation with the flexibility ofα-glucosidase residues[39]. Fig. 6B showed that the RMSF profile of the complex was similar to that of freeα-glucosidase. However, higher RMSF values in the regions of residues 135−185, 220−255, 280−360 and 400−450 were observed in the complex system, meaning that PCA binding increased the flexibility of above residues. Notably, the interacted residues predicted in the docking analysis were located in above regions, which further verified the sites where PCA andα-glucosidase interacted. The increase of RMSF values might be due to the structural changes of residues at above regions resulting from the van der Waals interactions and the establishment of hydrogen bonds with PCA[40].
Solvent accessible surface area (SASA) represents the area of protein in contact with solvent molecules. As presented in Fig. 6C,the value of SASA reached a steady state at around 12 ns, followed by a fluctuation around the average value of 240 nm2. Notably, the SASA values of freeα-glucosidase and the complex were similar,suggesting that PCA did not cause a significant change in the surface hydrophobicity ofα-glucosidase[41].
3.8 Postprandial hypoglycemic activity of PCA
OSTT was conducted to study the effect of PCA on postprandial blood glucose in STZ-induced diabetic mice. Results showed that after sucrose loading, blood glucose levels of mice in all groups increased and peaked at 30 min (Fig. 7A). Within 15 and 30 min, mice treated with 1 g/kg BW of PCA exhibited a significant lower blood glucose level than those in the control group. After 30 min, blood glucose levels started to decrease. PCA treated group still had a lower blood glucose level compared with the control. As shown in Fig. 7B,1 g/kg BW of PCA could decrease the AUC of blood glucose. Since that sucrose requiresα-glucosidase to be hydrolyze into glucose and fructose,blood glucose levels after sucrose loading would indirectly reflect the activity ofα-glucosidase. Results indicated that PCA might suppress postprandial hyperglycemia via inhibitingα-glucosidase activity[42].
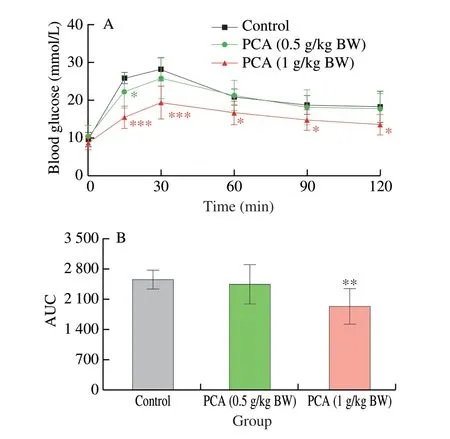
Fig. 7 (A) Blood glucose concentrations in STZ-induced diabetic mice after administration of 4 g/kg BW sucrose along with 0.25% sodium carboxymethyl cellulose (vehicle), 0.5 or 1 g/kg BW PCA, and (B) the AUC of blood glucose.*P < 0.05, **P < 0.01, ***P < 0.001 vs. control group.
4. Conclusion
In summary, this study was the first time to probe the inhibitory mechanism of PCA onα-glucosidase.In vitro, PCA inhibitedα-glucosidase activity in a concentration-dependent pattern(IC50= 0.37 mg/mL) and it was a reversible mix-typeα-glucosidase inhibitor. The fluorescence quenching experiment demonstrated that PCA spontaneously combined withα-glucosidase to form anα-glucosidase-PCA complex, which aroused the conformational arrangement ofα-glucosidase, leading to the decline in fluorescence intensity. Further studies found that there was one site inα-glucosidase for PCA binding and the binding behavior was mainly driven by van der Waals interactions and hydrogen bonds. Molecular simulation analysis found that PCA docked into the active center ofα-glucosidase,which hindered substrates from entering catalytic sites, resulting in decreasedα-glucosidase activity. Besides, PCA established interactions with amino acid residues situated in active sites, which might partly explain the inhibitory effect of PCA onα-glucosidase.The results of MD simulation illustrated that the binding of PCA increased the flexibility of interacted amino acid residues without affecting the hydrophobicity ofα-glucosidase.In vivo, PCA(1 g/kg BW) significantly reduced PBG levels with a decreased AUC of blood glucose in STZ-induced diabetic mice. It was concluded that PCA was an effectiveα-glucosidase inhibitor, which might be used as a hypoglycemic supplement in functional foods.
Conflict of interest
Zhenyu Chen is an editorial board member forFood Science and Human Wellnessand was not involved in the editorial review or the decision to publish this article. The authors declare there is no conflict of interest.
Acknowledgement
This work was supported by the General Research Fund of Hong Kong (14105820).
- 食品科学与人类健康(英文)的其它文章
- Betalains protect various body organs through antioxidant and anti-inf lammatory pathways
- Effects of Maillard reaction and its product AGEs on aging and age-related diseases
- Characterization of physicochemical and immunogenic properties of allergenic proteins altered by food processing: a review
- Polyphenol components in black chokeberry (Aronia melanocarpa)as clinically proven diseases control factors—an overview
- Food-derived protein hydrolysates and peptides: anxiolytic and antidepressant activities, characteristics, and mechanisms
- Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango

