Pickering Emulsion Templated Proteinaceous Microsphere with Bio-Stimuli Responsiveness
Weijie Jiang ,Hang Jiang ,*,Wei Liu ,Xin Guan ,Yunxing Li ,*,Cheng Yang ,To Ngai
1 The Key Laboratory of Synthetic and Biological Colloids,Ministry of Education,School of Chemical and Material Engineering,Jiangnan University,Wuxi 214122,Jiangsu Province,China.
2 Department of Chemistry,The Chinese University of Hong Kong,Shatin,N.T.,Hong Kong SAR,China.
Abstract: Bio-stimuli-responsive microspheres,which can encapsulate and release actives in response to physiological triggers,have attracted increasing attention in pharmaceutical,cosmetic,food biotechnology,and agricultural industries.However,most microspheres are based on synthetic polymers and suffer from a lack of biocompatibility due to the residues of harsh organic solvents or crosslinkers used in the synthesis process.Herein,we develop a simple and sustainable method for the construction of proteinaceous microspheres templated from Pickering double emulsions.Specifically,silica nanoparticles with a diameter of 100 nm were synthesized by Stöber method and modified by reacting with dichlorodimethylsilane.The Pickering emulsions are stabilized by hydrophobic silica nanoparticles,while zein protein is dissolved in the middle phase.Subsequent ethanol removal from the emulsion template precipitated the protein skeleton.First,we stained the aqueous ethanol phase with rhodamine B and the oil phase with pyrene to demonstrate the formation of double emulsions by confocal laser scanning microscopy (CLSM).The morphology of microspheres and silica nanoparticles was characterized by scanning electron microscopy (SEM).The obtained microspheres showed high sphericity and uniformity.In addition to acting as particulate stabilizers,the silica nanoparticles could improve the mechanical strength and monodispersity of microspheres.Herein,fluorescein isothiocyanate (FITC)-labeled dextran was chosen as the model active for encapsulation into microspheres.The CLSM images showed that it was uniformly dispersed in the microspheres and had no effect on the structure of the microspheres.Next,we investigated the pH tolerance of the microspheres.Through optical microscope,it was noted that the structure was intact under pH 3–11,and thus,it has a high resistance.Finally,we investigated the bio-stimuli-responsive behavior of microspheres.Zein is rich in sulfur-containing amino acids,which can form intra- and inter-molecular disulfide bonds.Because disulfide bonds can be reduced by glutathione (GSH) and the protein itself has enzymatic hydrolysis characteristics,the proteinaceous microspheres can be triggered release in response to GSH and protease.The release profiles of FITC-dextran from microspheres at different concentrations of GSH and protease were evaluated by fluorescence spectrophotometer.The decomposition behavior of microspheres under certain concentrations of GSH and protease was further verified by CLSM and SEM.To conclude,excellent stability and tunability of emulsion templates render the resulting proteinaceous microspheres with adjustable structures.Meanwhile,the proteinaceous microspheres have high encapsulation efficiency of model actives and have shown excellent bio-stimuli-responsiveness to protease and glutathione.
Key Words: Bio-stimuli-responsive; Proteinaceous microsphere; Pickering emulsion; Protease; Glutathione
1 Introduction
The use of stimulus-responsive materials in the preparations of microspheres has raised considerable attention and research interests in drug delivery,diagnostics,tissue engineering,biomedical devices,etc.1–5.Traditional drug delivery systems have certain drawbacks,such as lack of site-specificity,the necessity for large dosages,and off-target drug accumulation,all of which can have adverse effects on the metabolic systems6.Thus,understanding the biological milieu is critical for developing smart materials that respond selectively to physiological stimuli (pH,enzyme concentrations,redox species,glucose levels) at the organ level,under pathological circumstances,and in diverse intracellular sub-compartments7–11.
Many attempts have been undertaken to explore bioresponsive microspheres,the bulk of which have used synthetic polymer.However,harmful organic solvents or crosslinking agents are always introduced in the synthesis process,raising concerns about their biocompatibility,potential toxicity,and carcinogenicity,which could severely restrict their biomedical uses12,13.Therefore,the ultimate goal from the material standpoint is to build materials that are biocompatible and sensitive to specific biological stimuli (e.g.,biological signals,pathological abnormalities),so that can endow the resultant microspheres with the bio-trigger release7,14,15.Alternatively,the usage of natural polymer has expanded in recent years as a result of the advocacy of green chemistry and technology16,17.Natural polymer is biogenic,and the biologic features,such as cell recognition and interactions,enzymatic degradability,extracellular matrix resemblance,and chemical flexibility,make them ideal materials for microsphere composition18–20.
Plant-based proteins have recently gained increasing attention as prospective biomaterials for tissue engineering due to their biocompatibility,biodegradability,and extraordinary interfacial properties21.Zein is a plant-based biopolymer with excellent biocompatibility and biodegradability,as well as facile film formation and high environmental resistance (light,heat,oxygen)22,23.In addition to the enzymatic hydrolysis properties of the protein itself,zein protein molecules can be linked by strong disulfide bonds and hydrophobic connections during film formation24,25.In the presence of reducing agents,disulfides can be transformed into thiols,and one typical reducing agent is glutathione (GSH).GSH can be utilized as endogenous signals to build drug transporters as studies have revealed that cancer cells have more of it than normal tissues26–29.Similarly,the disorder of enzymes may be linked to a number of disease states,such as increased concentrations and activity of certain enzymes in tumors and inflammatory regions compared to normal tissues,making it a potential target for medicine30,31.Hence,zein is thought to be a potential candidate material for bio-responsive delivery systems since it can respond to endogenous stimuli(protease and redox) simultaneously.However,the present methods for fabricating zein-based microspheres (phase separation,spray drying) have problems with easy aggregation and uncontrollable structure32,33.We recently proposed a facile and green strategy for the fabrication of porous zein microspheres,and the zein microspheres were straightforwardly derived from oil-in-(ethanol/water)-in-oil double emulsion templates stabilized by lecithin34.Although the emulsion templates directly influenced the size,structure,and morphology of the resultant microspheres,the final microsphere formation was significantly affected by the emulsion stability during the ethanol removal process.It was found that conventional surfactants stabilized emulsions should be treated immediately after preparation,otherwise,demulsification severely hampered the microsphere formation.
Herein,we present a simple,and environmental-friendly method for fabricating zein-based proteinaceous microspheres using a Pickering double emulsion approach modified from our earlier work35,36.Hydrophobic silica nanoparticles are used as particulate stabilizers in this work to stabilize aqueous ethanol droplets containing zein protein.After evaporating the ethanol,zein precipitates and proteinaceous microspheres are thus formedinsitu.Furthermore,the proteinaceous microspheres can respond to endogenous stimuli,such as protease and redox,making them a potential target drug carrier in tumors and inflammatory areas.
2 Experimental and computational section
2.1 Materials
Zein (grade Z3625),fluorescein isothiocyanate (FITC),and fluorescein isothiocyanate-labeled dextran (FITC-Dextran,MW70 kDa) were purchased from Sigma-Aldrich (USA).Tetraethyl orthosilicate (TEOS,> 98%),dichlorodimethylsilane (> 99%),ethanol (> 99.7%),ammonia solution (25%–28%),n-hexane(≥ 97%),and toluene (≥ 99.5%) were obtained from Sinopharm Chemical Reagent Co.,Ltd.(China).Rhodamine B (≥ 95%),protease (> 3000 U∙mg-1),and glutathione (GSH,99%) were acquired from Macklin Biochemical Co.,Ltd.(China).Caprylic/capric triglycerides (GTCC) was provided by Guangzhou Chou Qin Biotechnology Co.,Ltd.(China).Deionized water was used for all experiments.
2.2 Preparation of monodisperse silica nanoparticles
Monodisperse silica nanoparticles with a diameter of approximately 100 nm were prepared according to a modified Stöber method37.Firstly,ethanol (90 mL),DI water (5.4 mL),and ammonia solution (2.8 mL) were added to a three-neck round flask.Then,the dispersion of TEOS and ethanol(TEOS/ethanol = 6.6 mL/60 mL) was slowly added within 3 h.The mixture was stirred at room temperature for 12 h.Finally,the as-prepared silica nanoparticles were purified by centrifugation (12000 r∙min-1,10 min),washing 3–4 times with ethanol and deionized water.
2.3 Hydrophobic modification of silica nanoparticles
Hydrophobic modification of the silica nanoparticles was obtained by the addition of dichlorodimethylsilane38.Typically,the synthetic silica nanoparticles (0.5 g) were dispersed in toluene (60 mL) under ultrasonic,followed by adding dichlorodimethylsilane (0.5 mL).The mixture was stirred overnight at room temperature.The modified silica nanoparticles were washed with toluene and n-hexane,separated by centrifugation (12000 r∙min-1,10 min),and dried at 50 °C under vacuum.
2.4 Fabrication of proteinaceous microspheres
Firstly,zein powder was dissolved in ethanol/water mixture(70% (volume fraction)) to prepare a zein solution (20%,w/v).The hydrophobic silica nanoparticles (0.2 g) were dispersed in GTCC (20 mL) as oil phase.The emulsion was obtained by adding zein solution to the oil phase by homogenization under 17000 r∙min-1for 2 min and then was rapidly transferred to a flask.The ethanol was removed by rotary evaporation,during which zein microspheres gradually precipitated.Finally,the microspheres were washed with n-hexane to remove interior GTCC,then dried in vacuum at 50 °C.
2.5 Encapsulation of FITC-dextran in proteinaceous microspheres
According to the above-described procedures,a certain amount of FITC-dextran was added into the zein stock solution with ultrasonic.Afterward,homogeneous emulsification and rotary evaporation were performed.Microspheres loaded with FITC-dextran were collected after washing and freeze-drying.
2.6 GSH-responsive release of FITC-dextran
The release of FITC-dextran from the proteinaceous microspheres was induced by redox-mediated disassembly.Microspheres loaded with FITC-dextran (20 mg) were mixed with a buffered GSH solution (20 mL) at GSH concentrations of 0,5,or 10 mmol∙L-1respectively.Aliquots (1 mL) of the dispersion were collected at different time intervals,and the concentration of released FITC-dextran was determined from the changes in fluorescence intensity measured by a fluorescence spectrometer.
2.7 Protease-responsive release of FITC-dextran
The release of FITC-dextran from the proteinaceous microspheres was induced by protease-mediated disassembly.Microspheres loaded with FITC-dextran (20 mg) were mixed with a buffered protease solution (20 mL) at enzyme concentrations of 0,1000,or 2000 U∙mL-1.Aliquots (1 mL) of the dispersion were collected at different time intervals,and the concentration of released FITC-dextran was determined from the changes in fluorescence intensity measured by a fluorescence spectrometer.
2.8 Characterization
The morphologies of silica nanoparticles and proteinaceous microspheres were characterized by scanning electron microscopy (Hitachi,S-4800,Japan).The droplets and the release behavior of microspheres were observed by optical microscopy (Keyence,VHX-1000C,Japan).The wettability of hydrophobic silica nanoparticles and microspheres was measured by a contact angle analyzer (Dataphysics,OCA15EC,Germany).Fluorescent images were obtained using a confocal laser scanning fluorescence microscopy (Lecia,TCS SP8,Germany),the excited wavelength for pyrene,FITC,and rhodamine B is 405,488,and 530 nm,respectively.A fluorescence spectrometer (Varian; CARY Eclipse; America)was used to measure fluorescence intensity.The size analysis of silica nanoparticles and microspheres was calculated by Image J software.
3 Results and discussion
3.1 Preparation and characterization of the proteinaceous microspheres
The fabrication process of the proteinaceous microspheres is divided into three phases (Scheme 1).First,an oil-in-(ethanol/water)-in-oil Pickering double emulsion was prepared and used as a template by shearing a mixture of oil and an aqueous ethanol solution of zein and active payloads,with hydrophobic silica nanoparticles serving as the particulate stabilizers.Although zein alone can stabilize the oil-in-(ethanol/water)-in-oil double emulsion (Fig.S1a (Supporting Information)),the obtained emulsion droplets tend to coalesce and occur phase separation after one hour (Fig.S2).To improve the stability of the double emulsion stabilized by zein,hydrophobic silica nanoparticles are utilized as an additional stabilizer in the preparation of Pickering emulsions39–41.Hydrophobic silica nanoparticles can effectively stabilize w/o emulsions,as illustrated in Fig.S1b.As a result,as expected,the hydrophobic silica nanoparticles and zein formed a double emulsion and the emulsion droplets maintained exceptional stability (Fig.S1c).To visualize the microstructure of the generated double emulsions,we stained the oil phase with pyrene and the aqueous ethanol phase with rhodamine B,respectively.The CLSM images revealed that a number of small single droplets (blue) were included in each larger ethanol-water droplet (red) dispersed in the continuous oil phase (blue),confirming the formation of oil-in-(ethanol/water)-in-oil double emulsions (Fig.1a–f).Following that,zein molecules precipitated to form the skeleton structure as the ethanol/water phase composition changed.Simultaneously,the silica nanoparticles adhered to the outmost layer (Scheme 1b).After removing the ethanol and oil,the proteinaceous microspheres were successfully obtained.
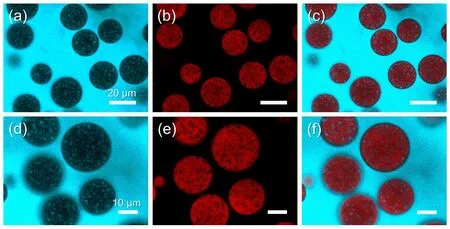
Fig.1 CLSM images of the Pickering emulsions template:blue channel (a,d); red channel (b,e); and overlay of both channels (c,f).The oil phase was stained by pyrene (blue),and the aqueous phase was stained by rhodamine B (red).
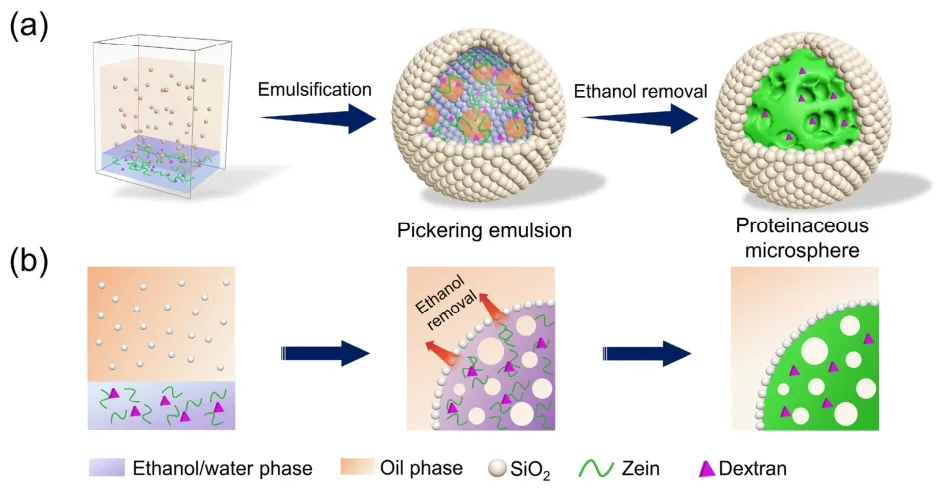
Scheme 1 (a) Schematic illustration of the fabrication process of the proteinaceous microspheres through a Pickering multiple emulsion strategy.(b) Formation of the protein skeleton from silica-stabilized Pickering droplet.
Monodisperse silica nanoparticles with a diameter of ~100 nm(Fig.S3a) were synthesized,and hydrophobically modified by reacting with dichlorodimethylsilane (Fig.2a).In Fig.2b,scanning electron microscopy (SEM) images showed the successful preparation of highly spherical microspheres without collapse even under ultrasound,suggesting their superior robustness.The average size of the as-prepared proteinaceous microspheres was around 8 μm,as determined by a particle sizing instrument (Fig.S3b).The magnified SEM image displayed that the microsphere was surrounded by closelypacked silica nanoparticles (Fig.2c).As revealed by contact angle measurements (Fig.2d–f),the hydrophobic silica nanoparticles (143°) imparted a hydrophobic surface to the proteinaceous microspheres (138°) compared to natural zein protein (76°).Meanwhile,it was found that the resultant proteinaceous microspheres had a similar size to the template droplets,suggesting the great stability of the Pickering double emulsions templates,attributed to the interfacial assembly of silica nanoparticles.
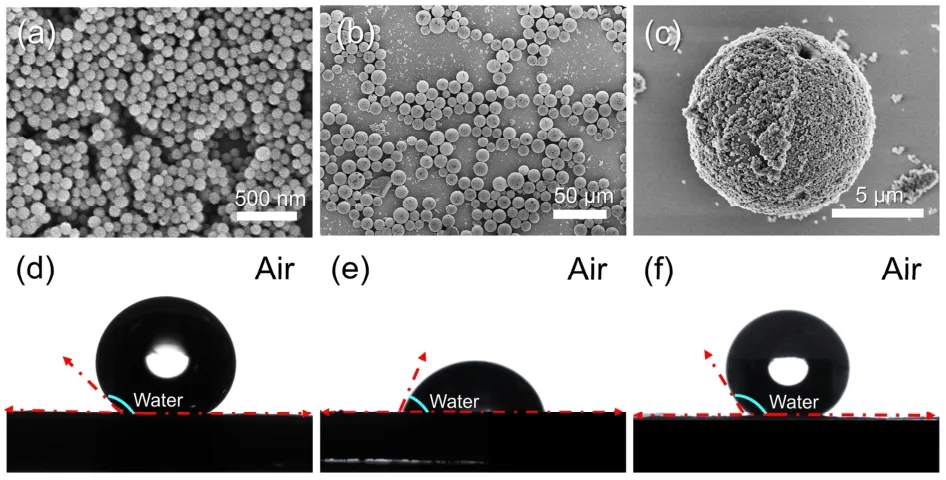
Fig.2 (a) SEM image of hydrophobic silica nanoparticles.(b,c) SEM images of proteinaceous microspheres.Air-water contact angles of hydrophobic silica nanoparticles (d),zein powder (e),and proteinaceous microspheres (f).
3.2 Encapsulation and protection of actives in the proteinaceous microspheres
FITC-labeled dextran (MW= 70 kDa),a hydrophilic fluorescent model active,was chosen for encapsulation into the proteinaceous microspheres.As confirmed by CLSM observation,the FITC-dextran appearing as the green color was thoroughly distributed in the proteinaceous microspheres following precipitation (Fig.3a–d).Furthermore,the dye molecules encapsulated inside the microspheres have almost no influence on the structure of resulting microspheres.

Fig.3 (a–d) CLSM images of the proteinaceous microspheres loaded with FITC-dextran at different magnifications.
Interestingly,zein-based materials were particularly pHresistant,so such proteinaceous microspheres could be expected to tolerate weak acid environment in the body and prevent premature release of the drug at a given period.To investigate the pH tolerance of the microspheres,the pH of the dispersion was varied from 3 to 12.Fig.4a shows the results of a quantitative release assay of FITC-dextran from the proteinaceous microspheres at different pH values.Under pH =12,the FITC-dextran was rapidly released: the cumulative release of FITC-dextran reached 75% within 10 min.Fig.4b further illustrated the fast decomposition process of the proteinaceous microspheres.However,no obvious cargo release was observed in the pH ranging from 3 to 11,as displayed in Fig.4a.
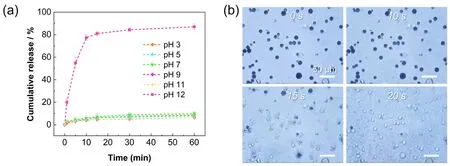
Fig.4 (a) Release curves of FITC-dextran from the proteinaceous microspheres at different pH values.(b) NaOH-triggered disintegration of the proteinaceous microspheres along with time.
3.3 Endogenous release of actives from the proteinaceous microspheres
Although the physicochemical properties of zein offer the proteinaceous microspheres with high stability against pH change (pH 3–11),the trigger release of payloads becomes a difficulty.Conventional procedures42,43,such as grinding and ultrasonication can certainly destroy the proteinaceous microspheres and induce the release of encapsulated actives,however,specific equipment or means are necessary,and they are not suitable for bio-based applications.Improving communication with diseased cells or tissues,especially in response to physiological abnormalities,and achieving the bioresponsive release of actives is a key scientific topic in the field of microsphere preparations.Generally,physiological disorders such as inflammation and tumors often cause abnormalities in specific physiological situations44.In this work,we found that the zein-based proteinaceous microspheres are very sensitive to several endogenous stimuli,such as protease and glutathione(GSH),owing to the nature of the protein skeleton.Hence,we anticipate the payloads in the proteinaceous microspheres will be effectively protected under normal circumstances,but can be triggered release in response to bio-stimuli,such as enzyme and GSH,as depicted in Scheme 2.
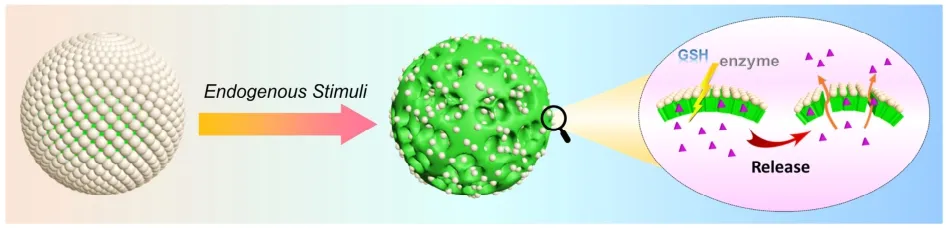
Scheme 2 Endogenous stimuli triggered the release of FITC-dextran from the proteinaceous microspheres.
Zein is rich in sulfur-containing amino acids,and the protein molecules are connected by strong disulfide bond and hydrophobic interaction,which is the basis for the easy formation of a zein film or particle.Therefore,through reductive cleavage of disulfide bridges by additional reducing agents,this can be exploited to disassemble protein microcompartments.The redox-responsive biomimetic carriers for drug delivery and controlled release are widely developed,effectively utilizing the high level of GSH to achieve rapid release within tumor cells45.The release of FITC-dextran was accompanied by the gradual disintegration of the protein skeleton,associated with reductive cleavage.Fluorescence spectroscopy was used to assess the release patterns under different GSH concentrations; as expected,higher GSH concentrations resulted in more effective decomposition of the proteinaceous microspheres and correspondingly faster release rates of FITC-dextran (Fig.5a).When we exposed the microspheres to an aqueous solution of GSH,the proteinaceous microspheres collapsed,causing deformation as well as some structural defects (Fig.5b).Meanwhile,CLSM revealed the release of encapsulated FITCdextran within 8 h as the proteinaceous microspheres were disintegrated (Fig.5c).

Fig.5 (a) Release curves of FITC-dextran from the proteinaceous microspheres under different GSH concentrations.(b) Time series of SEM images showing the dissolution of the proteinaceous microspheres at 10 mmol∙L-1 GSH.(c) Time series of CLSM and optical microscope images showing the release of FITC-dextran from the proteinaceous microspheres at 10 mmol∙L-1 GSH.
Furthermore,we prepared an aqueous dispersion of proteinaceous microspheres containing FITC-labeled dextran and mixed them with protease solutions ranging from 0 to 2000 U∙mL-1.The release profiles were measured at different protease concentrations by fluorescence spectroscopy; as expected,higher concentrations of protease led to more efficient degradation of the protein skeleton and correspondingly faster rates of FITC-dextran release (Fig.6a).SEM images revealed that in the presence of 2000 U∙mL-1protease,the proteinaceous microspheres were gradually broken within 8 h,showing that enzyme-mediated hydrolysis of zein was effective (Fig.6b).As a result,the release of FITC-dextran during approximately 8 h was observed by CLSM as the proteinaceous microspheres were disassembled (Fig.6c).
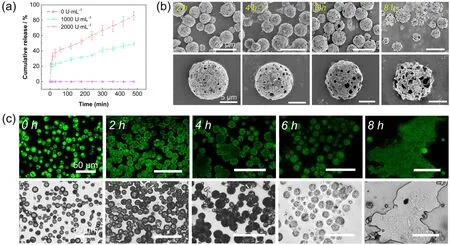
Fig.6 (a) Release curves of FITC-dextran from the proteinaceous microspheres at different concentrations of protease.(b) Time series of SEM images showing the dissolution of the proteinaceous microspheres at 2000 U∙mL-1 protease.(c) Time series of CLSM and optical microscope images showing the release of FITC-dextran from the proteinaceous microspheres at 2000 U∙mL-1 protease.
4 Conclusions
In summary,we have proposed a green and viable strategy to fabricate proteinaceous microspheres with bio-stimuli responsivenessviaa Pickering emulsion template.The method is based on the template of double emulsions with zein solution as the middle phase,followed by ethanol evaporation to precipitate zein in the form of microspheres.This method possesses the advantages of simplicity,sustainability,and controllability.High percentage of sulfur-containing amino acids in zein can form strong intramolecular and intermolecular disulfide bonds to build a protein network.Because the disulfide bonds contained in zein can be broken by reducing agents and the protein itself has enzymatic hydrolysis property,the asprepared proteinaceous microspheres exhibited excellent biostimuli-response (GSH and protease).Given the feasible preparation,extraordinary biocompatibility,and bioresponsiveness of our proteinaceous microspheres,it will find great potential in biomedicine,cosmetics,and food biotechnology.In particular,it is useful for tumor site targeting and tailored drug release.
Supporting Information: available free of chargeviathe internet at http://www.whxb.pku.edu.cn.
- 物理化学学报的其它文章
- 用于高灵敏快速核酸检测的荧光碳点
- Performance Improvement and Antibacterial Mechanism of BiOI/ZnO Nanocomposites as Antibacterial Agent under Visible Light
- 2D/3D S-Scheme Heterojunction Interface of CeO2-Cu2O Promotes Ordered Charge Transfer for Efficient Photocatalytic Hydrogen Evolution
- Construction of a Highly Active Rh/CeO2-ZrO2-Al2O3 Catalyst Based on Rh Micro-Chemical State Regulation and Its Three-Way Catalytic Activity
- Electronic Modulation of Ni-Mo-O Porous Nanorods by Co Doping for Selective Oxidation of 5-Hydroxymethylfurfural Coupled with Hydrogen Evolution
- RuP Nanoparticles Anchored on N-doped Graphene Aerogels for Hydrazine Oxidation-Boosted Hydrogen Production