Metformin alleviates spinal cord injury by inhibiting nerve cell ferroptosis through upregulation of heme oxygenase-1 expression
Zhihua Wang, Wu Zhou, Zhixiong Zhang, Lulu Zhang, Meihua Li,*
Abstract
Key Words: acyl-CoA synthetase long-chain family member 4; ferroptosis; glutathione peroxidase 4; heme oxygenase-1; inflammation; iron; lipid peroxidation;metformin; neuroprotection; spinal cord injury
Introduction
Studies have shown that patients with traumatic spinal cord injury (TSCI) often die from acute respiratory failure or a combination of other severe injuries(Bach, 2012; Xie et al., 2022; Yu et al., 2022).In addition, TSCI survivors often exhibit varying degrees of concomitant motor dysfunction.Despite surviving the acute phase of injury, many patients with tetraplegia or paraplegia continue to face various complications, including pulmonary infections,spasticity, autonomic dysreflexia, hypertension, venous thromboembolism,and pain (Adegeest et al., 2022).These secondary health conditions are common long-term complications of TSCI that negatively affect quality of life.Studies have shown that various forms of cell death play a role in histopathologic deterioration after TSCI (Shi et al., 2021).Ferroptosis is a novel type of non-necrotic regulatory cell death regulated by various molecular mechanisms (Stockwell et al., 2017) that aggravates the deteriorating microenvironment following spinal cord injury (SCI; Li et al., 2022).Both invasive and non-invasive interventions can effectively promote functional recovery in experimental animal models of TSCI by inhibiting ferroptosis (Ge et al., 2021, 2022; Ying et al., 2023).
Several recent studies have reported that the ferroptosis-related gene (FRG)heme oxygenase-1 (HMOX1) is significantly upregulated after SCI (Kang et al., 2022; Dong et al., 2023; Li et al., 2023).HMOX1, an inducible enzyme,is known to have anti-inflammatory, antioxidant, and neuroprotective properties.The prevailing factors contributing to ferroptosis induction and progression include marked down-regulation of glutathione peroxidase 4(GPX4) and upregulation of acyl-coenzyme A synthetase long-chain family member 4 (ACSL4) (Wei et al., 2020; Liu et al., 2022a).In contrast, the role or overall effect of HMOX1 in regulating ferroptosis is controversial.Some studies have suggested that HMOX1 promotes ferroptosis, as it can cause iron accumulation by catalyzing heme degradation to iron, carbon monoxide,and biliverdin (Fang et al., 2019; Fernández-Mendívil et al., 2021; Zeng et al., 2021).In contrast, other studies have shown that HMOX1 inhibits ferroptosis, thereby preventing disease development and progression, by alleviating oxidative stress injury and inflammation (Ma et al., 2020; Cai et al., 2022; Meng et al., 2022; Ryter, 2022).In addition, many reports have suggested that HMOX1 upregulation has beneficial effects on the injured spinal cord (Yamauchi et al., 2004; Vargas et al., 2005; Kanno et al., 2009;Lin et al., 2017, 2021; Wang et al., 2017).However, a recent study suggested that HMOX1 upregulation after SCI aggravates oxidative stress by increasing ferrous iron release, which leads to the accumulation of reactive oxygen species (ROS) (Dong et al., 2023).Nevertheless, other reports suggest that HMOX1 upregulation in the injured spinal cord inhibits ferroptosis (Zhou et al., 2020; Ge et al., 2021).Thus, animal experiments based on bioinformatics analysis results are needed to determine the effect of HMOX1 on ferroptosis after SCI.
Previous studies have shown that metformin (Met), a widely used hypoglycemic drug and classic adenosine 5′-monophosphate-activated protein kinase activator, has anti-ferroptotic effects (Lee et al., 2020; Ma et al.,2021; Yan et al., 2022; Zhao et al., 2023).In addition, Met has been shown to have a neuroprotective effect in many central nervous system diseases (Leech et al., 2019; Dziedzic et al., 2020; Cao et al., 2022; Du et al., 2022; Ning et al.,2022).A recent meta-analysis concluded that treatment with Met results in significant locomotor function recovery in rats with SCI (Chen et al., 2021a).We previously reported that Met promotes functional recovery after SCI by inhibiting ferroptosis (Wang et al., 2022b).Other groups have shown that Met effectively treats cardiovascular and digestive diseases by promoting HMOX1 upregulation (Sauvé et al., 2010; Wu et al., 2018; Yan et al., 2019; Patel et al.,2021).In this study, we explored the correlation between Met administration and HMOX1 expression after SCI and the mechanism by which Met modifies the injury microenvironment by regulating HMOX1 expression.In addition, we investigated the anti-ferroptotic effect of HMOX1 after SCI and the effect of HMOX1 knockdown on Met-mediated inhibition of ferroptosis.
Methods
Data acquisition and gene expression analysis
All SCI data were obtained from the GEO database (https://www.ncbi.nlm.nih.gov/gds/).Ferroptosis markers and regulators were downloaded from the FerrDb V2 database (http://www.zhounan.org/ferrdb/current/; Zhou et al., 2023).Additional Table 1 shows detailed information on all datasets.In brief, the degree of normalization among samples was detected by box plot analysis.Differences in clusters between the sample groups were analyzed by principal component analysis.Analysis of differential expression between the two groups was performed using the “limma” package in R software (https://www.r-project.org/; version 4.0.1, R Foundation, Auckland, New Zealand).The results were visualized by volcano plot with |logFC| > 1 and adjustedP< 0.05.
Enrichment analysis and protein-protein interaction network construction
Using the Gene Ontology database (http://geneontology.org/) (Thomas et al.,2022) and the Kyoto Encyclopedia of Genes and Genomes database (https://www.genome.jp/kegg/) (Kanehisa and Goto, 2000), enrichment analysis was performed for 17 ferroptosis-related differentially expressed genes (DEGs) to investigate their role in SCI.The Gene Ontology annotation analysis included biological process, molecular function, and cellular component.Functional and pathway enrichment analysis was performed using the clusterProfiler package in R software.To detect protein-protein interactions, network analysis was performed using the online STRING database (https://cn.stringdb.org/) with the default settings (Szklarczyk et al., 2019).
Animals
All animal care and experimental protocols were carried out in accordance with the Animal Management guidelines of the Ministry of Health of the People’s Republic of China.This study was approved by the Medical Ethics Committee of the Animal Experimental Center of Nanchang University on March 10, 2021.Because men predominate patients with TSCI (Wang et al.,2013, 2022a), only male rats were used in this study.In total, 220 specific pathogen-free male Sprague-Dawley rats (weight 220–250 g, age 8 weeks)were purchased from the Experimental Animal Center of Jiangxi University of Chinese Medicine (Nanchang, Jiangxi, China; license No.SCXK (Gan) 2018-0003).The rats were randomly grouped and housed in standardized cages (five rats per cage) for at least 7 days before surgery, in a controlled environment at 22–24°C, 50% relative humidity, and a 12/12-hour light/dark cycle.
SCI model and Met intervention
As shown in Figure 1, this study involved three animal experiments (Parts 1–3).In Part 1, to identify ferroptotic characteristics at different time points after SCI, the rats were randomly assigned to sham and SCI groups.Three rats were included in each group for transmission electron microscopy(TEM), five rats were included in each group for western blotting, and six rats were included in each group for Prussian blue staining, Fluoro-Jade B(FJB), and immunohistochemistry.Anesthesia was induced by inhalation of 5% isoflurane (RWD, Shenzhen, China) and maintained with 1.5% isoflurane.The sham group underwent laminectomy at the T9 level without injury to the spinal cord, whereas the SCI group underwent laminectomy followed by compression injury at the T9 level delivered by application of an aneurysm clip (No.FT220T; Am Aesculap-Platz, Tuttlingen, Germany) for 10 seconds.Subsequently, the spinal cord was removed at a predetermined time point.In Part 2, rats were randomly assigned to sham, SCI, and Met groups to characterize the anti-ferroptotic effect of Met after SCI.The sham group underwent laminectomy only; the SCI group underwent laminectomy and compression SCI followed by intraperitoneal injection of about 1 mL of normal saline 30 minutes after injury and then once a day until the rats were sacrificed (up to 14 days after injury); and the Met group underwent laminectomy, compression SCI, and intraperitoneal injection of 200 mg/kg Met (Selleck, Houston, TX, USA) 30 minutes after SCI and then once a day until the rats were sacrificed (up to 14 days after SCI).Six rats were included in each group for immunofluorescence staining and PCR detection, and five rats were included in each group for western blotting.In Part 3, rats were randomly assigned to normal control (NC) and knockdown (KD) groups to determine the effect of target gene knockdown on ferroptosis and the anti-ferroptotic capacity of Met.Animals in the sham, SCI, and Met subgroups were treated as described above.Three rats were included in each subgroup for spinal cord sectioning, and five rats were included in each subgroup for western blotting, immunofluorescence staining, and FJB.After each surgical procedure, the skin and muscles were carefully stitched together.As a precaution against infection, penicillin (Dingjian, Sichuan, China) was injected subcutaneously for 3 days at a dosage of 2.5 mg/kg daily.The bladder was evacuated manually three times a day until autonomous urinary function was regained, which generally took 10 to 14 days.Thein vivoexperimental process is shown in Figure 1.

Figure 1 |Experimental flowchart for the in vivo experiments.4HNE: 4-Hydroxynonenal; ACSL4: acyl-coenzyme A synthetase long-chain family member 4; Arg1: arginase 1; GPX4: glutathione peroxidase 4; HMOX1: heme oxygenase-1; IF:immunofluorescence; IHC: immunohistochemistry; Iba1: ionized calcium binding adaptor molecule 1; IL-1β: interleukin 1β; IL-6: interleukin 6; iNOS: inducible nitric oxide synthase;LV: lentivirus; PCR: polymerase phain peaction; SCI: spinal cord injury; TEM: transmission electron microscopy; WB: western blotting.
Cell culture
The PC-12 cell line was purchased from Otwo Biotechnology Co., Ltd.(Shenzhen, Guangdong, China, Cat# HTX1783, RRID: CVCL_0481).This is a rat-derived cell line that was used as alternative to neurons for thein vitroexperiments.Briefly, cells were cultured in high-glucose Dulbecco’s modified Eagle’s medium (Gibco, Beijing, China) supplemented with fetal bovine serum(Cat# 10100147C, Gibco, Grand Island, NY, USA), 100 U/mL penicillin G, and 0.1 mg/mL streptomycin in a 5% CO2and 95% air incubator at 37°C.
Construction of stable cell line and in vivo transfection
Lentivirus (LVs) containingHmox1-shRNA (NM_012580) were constructed and synthesized by Genechem Co., Ltd.(Shanghai, China).The LV sequences and titer information are shown in Additional Table 2.In addition, an LV-GFPHmox1-shRNA green fluorescent protein (GFP)-tagged LV was constructed to evaluate transfection efficiency.In brief, stable HMOX1 knockdown cell line were generated by transfecting cells with one of the two LVs.HMOX1 expression at the mRNA and protein level in the resulting two stable cell lines was evaluated to determine transfection effectiveness.To generate anin vivomodel of HMOX1 knockdown, rats were randomly assigned to the NC and KD groups and injected with one of the two LVs, using the same injection site,technique, and dosage.In addition, all procedures were performed by the same investigator.Each rat was subjected to full anesthesia with isoflurane,after which the T9 segment was fully exposed and 10 µL of LV containing the target gene (shRNA) was injected into the spinal cord using a micro-syringe(outer diameter/inner diameter of the needle: 0.16/0.06 mm, 207434; RWD,Shenzhen, China).No hind limb motor function loss was observed when the rats had recovered from the anesthesia.Fourteen days after injection with the LVs, the rats were euthanized by inhaling excessive isoflurane, and the range of LV infection in tissue slices was assessed by evaluating green fluorescence.Knockdown was validated at the protein level.
Frozen section preparation
Anesthesia was induced by inhalation of 5% isoflurane and maintained with 1.5% isoflurane; then, the right atrial appendage was excised, and the rats were perfused with 250–300 mL of 4°C normal saline.Once the effluent liquid ran clear, the whole spinal cord was harvested and dried with gauze.The spinal cord tissue was then snap-frozen in liquid nitrogen for 15 seconds,embedded in optimal cutting temperature compound, cut into large pieces,then sliced into 8- to 10-µm-thick sections.
Prussian blue staining
Iron deposition was detected by Prussian blue staining (Perls’ staining) as described previously, with minor modifications (Li et al., 2017; Xie et al.,2019).For spinal cord tissues, the region of interest was cut into 3-µmthick coronal sections.Then, the sections were placed into xylene, absolute ethanol, and 75% alcohol, followed by washing with tap water and rinsing three times with distilled water.Next, the sections were placed in a 1:1 mixture of 2% potassium ferrohydride and 2% hydrochloric acid and then washed twice with distilled water.Next, the sections were stained with diaminobenzidine (Servicebio, Wuhan, China) for 5–10 minutes.The staining solution was removed, and the stained sections were rinsed once with 0.01 M phosphate-buffered saline, followed by three washes with distilled water.Next, the degree of color development was visualized under an optical microscope (Axio Lab.A1 + ERC5S, ZEISS, Jena, Germany).Sections were counterstained with hematoxylin (Servicebio) for 1 minute, followed by washing with tap water.The sections were differentiated with a hydrochloric acid aqueous solution followed by washing with tap water.Next, the sections were immersed in an aqueous ammonia solution and washed with tap water.Then, they were soaked in absolute ethanol and sealed with neutral balsam.The sections were viewed and photographed under a microscope (Axio Lab.A1 + ERC5S).
FJB staining
For FJB staining, spinal cord tissue sections were incubated in two changes of xylene, dehydrated in two changes of pure ethanol, and dehydrated by passaging through 85% and 75% ethanol.After washing in distilled water,the sections were air-dried.Freshly prepared 50% glacial acetic acid was used as the solvent for the 1:400 FJB working solution (Servicebio).The sections were incubated with dilute FJB solution at 4°C overnight in a humid box.After washing three times with distilled water on a shaker, the sections were incubated with 4′,6-diamidino-2-phenylindole solution (Servicebio)at about 25°C and stored in the dark.They were then rinsed with distilled water, dried with a hair dryer, cleared with xylene, and mounted with resin mounting medium.Representative images were captured using an inverted fluorescence microscope (Axio Observer + Axiocam 208, ZEISS).
Western blotting
Western blotting was performed to detect levels of 4-hydroxynonenal (4HNE),GPX4, ACSL4, and HMOX1 in spinal cord tissues and HMOX1 in PC12 cells.The primary antibodies used were rabbit polyclonal antibody against 4HNE(1:1000, Abcam, Cambridge, UK, Cat# ab46545, RRID: AB_722490), rabbit monoclonal antibody against ACSL4 (1:10,000, Abcam, Cat# ab155282, RRID:AB_2714020), rabbit monoclonal antibody against GPX4 (1:10,000, Abcam,Cat# ab125066, RRID: AB_10973901), rabbit monoclonal antibody against HMOX1 (1:2000, Abcam, Cat# ab189491, RRID: AB_1267209), and rabbit polyclonal antibody against β-actin (1:10,000, Affinity, Cincinnati, OH, USA,Cat# AF7018, RRID: AB_2839420).The membranes were incubated with the primary antibodies overnight (12 hours) at 4°C.Goat anti-rabbit IgG(H+L)(1:2000, Immunoway, Plano, TX, USA, Cat# RS0002, RRID: AB_2938759) was used as a secondary antibody for 1-hour incubation at about 25°C.Then, the proteins were subjected to SDS-PAGE then transferred to a nitrocellulose filter membrane at 200 mA constant current for 120–150 minutes in an ice bath.An enhanced chemiluminescence system (ChemiDoc XRS+System; Bio-Rad, Hercules, CA, USA) was used to visualize the target protein bands.The relative protein levels were normalized to optical density of β-actin and were quantified using ImageJ (v2021.8.0, National Institutes of Health, Bethesda,MD, USA; Schneider et al., 2012).
TEM
For TEM, spinal cord tissues were rapidly fixed in phosphate-buffered glutaraldehyde (2.5%), followed by osmium tetroxide (1%).Then, they were dehydrated in graded ethanol solutions (50–70–80–90–95–100–100%) and acetone (100%) before embedding in epoxy resin.After resin penetration and embedding, the samples were incubated at 65°C for more than 48 hours in an oven to polymerize.The resin blocks were cut into 60- to 80-nm-thick slices using an ultramicrotome (Leica UC7; Leica, Wetzlar, Germany).Finally, the mitochondrial ultrastructure was examined, and images were captured, by TEM (HT7700; Hitachi, Tokyo, Japan).
Immunohistochemistry
For immunohistochemistry, spinal cord slices mounted on slides were immersed in sodium citrate antigen retrieval solution (pH 6.0), deparaffinized,rehydrated, and maintained at a sub-boiling temperature.Then, they were immersed in 3% H2O2and incubated at room temperature in the dark to block the activity of endogenous peroxidase.The slides were then incubated with primary rabbit monoclonal antibody against HMOX1 (1:20,000, Abcam, Cat#ab189491, RRID: AB_1267209) at 4°C overnight in a humid box.Next, the slides were incubated at about 25°C for 50 minutes with a secondary antibody(1:500, goat anti-rabbit IgG(H+L), RS0002, Immunoway, RRID: AB_2938759)labeled with horseradish peroxidase.After developing with diaminobenzidine and counterstaining the nucleus with hematoxylin staining solution, the sections were embedded in resin mounting medium.As described previously(Li et al., 2020), HMOX1 expression in the spinal cord was quantified by two pathologists blinded to the group assignments.The immunostaining intensity was scored as negative = 0, weak = 1, moderate = 2, or strong = 3.The percentage of positively stained cells was scored as follows: < 25% = 1, 25–50% = 2, 50–75% = 3, and > 75% = 4.The overall score was then calculated by multiplying the intensity score by the proportion score (score = intensity ×percentage of positively stained cells).Images were captured using an optical microscope (Axio Lab.A1 + ERC5S).
Quantitative polymerase chain reaction
For quantitative polymerase chain reaction (PCR), the expression levels of FRGs including glutathione peroxidase 4 (Gpx4), acyl-coenzyme A synthetase long-chain family member 4 (Acsl4), and heme oxygenase-1 (Hmox1) were analyzed in injured rats and sham controls 3 days after SCI.Briefly, total RNA was isolated from spinal cord samples using Trizol reagent (R401-01; Vazyme,Nanjing, China).The extracted RNA was quantified using a NanoDrop One(Thermo Fisher Scientific, MA, USA).cDNA was synthesized using a Hifair 1st Strand cDNA Synthesis SuperMix Kit (11141ES60; Yeasen, Shanghai, China).mRNA expression was detected with CFX96 Touch (Bio-Rad) using Hieff qPCR SYBR Green Master Mix (11201ES08; Yeasen).Gene expression was normalized to dehydrogenase (GAPDH).The specific primers are listed in
Additional Table 3.
Immunofluorescence staining
For immunofluorescence staining, spinal cord sections were deparaffinized by sequential incubation in xylene, anhydrous ethanol, 85% ethanol, and 75%ethanol.After washing in distilled water, they were microwaved in citric acid antigen retrieval buffer (pH 6.0) for antigen retrieval.Next, the sections were placed in hydrogen peroxide and bovine serum albumin, and then the primary antibody was added, and the sections were incubated at 4°C overnight in a humid box.The primary antibodies used for immunofluorescence were rabbit monoclonal antibody against GPX4 (1:500, Abcam, Cat# ab125066,RRID: AB_10973901), rabbit monoclonal antibody against ACSL4 (1:500,Abcam, Cat# ab155282, RRID: AB_2714020), rabbit polyclonal antibody against interleukin-1β (1:200, Affinity, Cat# AF5103, RRID: AB_2837589),rabbit polyclonal antibody against interleukin-6 (1:200, Affinity, Cat# DF6087,RRID: AB_2838055), rabbit polyclonal antibody against neuronal nuclei(1:2000, Servicebio, Cat# GB11138, RRID: AB_2868432), rabbit monoclonal antibody against ionized calcium binding adaptor molecule 1 (Iba1; 1:3000,Abcam, Cat# ab178846, RRID: AB_263685), rabbit monoclonal antibody against inducible nitric oxide synthase (iNOS, 1:300, Abcam, Cat# ab178945,RRID: AB_2861417), and rabbit polyclonal antibody against arginase 1(Arg1, 1:300, GeneTex, San-Antonio, TX, USA, Cat# GTX109242, RRID:AB_2036264).The secondary antibodies used for immunofluorescence were Cy3-conjugated goat anti-rabbit IgG (H+L) (1:300, Servicebio, Cat# GB21303,RRID: AB_2861435) and Alexa Fluor 488-conjugated goat anti-rabbit IgG(H+L) (1:400, Servicebio, Cat# GB25303, RRID: AB_2910224).Next, the cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole, followed by autofluorescence quenching and mounting with anti-fluorescence quenching mounting tablets.The fresh tissues were embedded and in optimal cutting temperature compound (G6059-110ML; Servicebio, Wuhan, China) and sliced into 8- to 10-µM-thick sections once the compound turned white and hard.Then, a coverslip was placed on top of the tissue slice and sealed.Representative images were captured using the fluorescence microscope (Axio Observer + Axiocam 208).During image analysis, three people who were unaware of the grouping counted protein-positive neurons or microglia and averaged the results.The main inclusion criterion is that the cells have distinct red (neurons or microglia), green (GPX4, ACSL4, IL-1β, or IL-6), and blue (DAPI)fluorescence at the same time.
Statistical analysis
No sample size calculation was performed to determine the small sample size for this study; however, our sample sizes are similar to those used in previous studies (Chen et al., 2022; Li et al., 2023).The evaluator was blinded to the group assignments, and no rats were excluded from the analyses.All statistical analyses were performed using SPSS software (version 25.0, IBM Corp.,Armonk, NY, USA), R software, and GraphPad Prism (version 8.02, Graphpad,San Diego, CA, USA, http://www.graphpad.com).All data are expressed as the mean ± standard error of the mean (SEM).Data distribution was assessed using the Shapiro-Wilk normality test.Differences between the two groups were analyzed using the independent samplesttest.Differences between multiple groups were analyzed using one-way or two-way analysis of variance with Bonferroni or Tukey’spost hoctest.P< 0.05 was considered statistically significant.
Results
Bioinformatics analysis reveals significant Hmox1 upregulation in rats after SCI
To identify differentially expressed FRGs in the injured spinal cord microenvironment in rats at different time points after SCI, two eligible datasets (GSE45006 and GSE2599) were downloaded from the GEO database,and differential expression analyses were performed.Preliminary analysis of the GSE45006 dataset identified 808 DEGs in the SCI group at five different time points (1, 3, 7, 14, and 56 days post-injury (dpi)) (Figure 2A).Further analysis identified 17 differentially expressed FRGs, includingHmox1,Cybb,Atf3,Capg,Lrrfip1,Gls2,Tlr4,Plin2,Gch1,Cd44,Ano6,Sat1,Ripk1,Chmp6,Tgfbr1,Gria3, andStat3(Figure 2B).The GSE2599 dataset only contained gene expression data from 35 days after SCI.Only seven differentially expressed FRGs (Hmox1,Cp,Tgfbr1,Srebf1,Gch1,Ifng, andGot1) were identified (Figure 2C).Comparison of these two datasets reveled three shared DEGs (Hmox1,Tgfbr1, andGch1) (Figure 2D).The differential expression of these genes was analyzed using volcano plots (Figure 2E–J).Hmox1was upregulated at each time point assessed (1, 3, 7, 14, 35, and 56 dpi).Analysis of three other datasets (GSE92657, GSE42828, and GSE93561) showed thatHmox1is also upregulated in mice after SCI (Additional Figure 1).
Bioinformatics analysis reveals dynamic changes in the expression of inflammatory factors and FRGs after SCI
Further analyses focused on the GSE45006 dataset.Gene Ontology and Kyoto Encyclopedia of Genes and Genomes enrichment analyses were performed to determine the function of the 17 DEGs.The results confirmed an association between ferroptosis and SCI (Figure 3A and B).Next, the DEGs were uploaded to the STRING database to construct a protein-protein interaction network using the default settings (Figure 3C), which showed that HMOX1 may play a critical role in cellular ferroptosis after SCI.Several classical indicators of inflammation were selected and analyzed to investigate dynamic changes in the expression level of genes associated with the inflammatory response and FRGs after SCI.The results showed that, after normalization to the sham group, interleukin 1β (Il-1β) and interleukin 6 (Il-6) expression levels increased in the SCI group on day 1, peaked on day 3, and returned to baseline levels on day 7 (Figure 3D and E).Interleukin 10 (Il-10) expression decreased continuously for 7 days and fluctuated around baseline levels for 49 days,remaining at roughly normal levels throughout the follow-up period (Figure 3F).Although the fold change (FC) value was not significant, expression ofGpx4, a key protein involved in ferroptosis, continued to decrease after SCI(Figure 3G).Slc7a11expression fluctuated around baseline, with an overall increasing trend, whereasSlc3a2remained upregulated (Figure 3H and I).Acsl4increased on day 1, peaked on day 3, and returned to baseline on day 14 (Figure 3J).Lpcat3was upregulated at several time points, although this change was not significant on day 3 (Figure 3K).Hmox1was upregulated throughout the follow-up period (Figure 3L).

Figure 2 | Hmox1 is significantly upregulated in a rat model of SCI.(A) Common DEGs at five time points in the GSE45006 dataset.(B) Ferroptosis-related DEGs (from the FerrDb V2 database (http://www.zhounan.org/ferrdb/current/)) in the GSE45006 dataset.(C) Ferroptosis-related DEGs in the GSE2599 dataset.(D) Common ferroptosis-related DEGs in the GSE45006 and GSE2599 datasets.(E–I) Visualization of DEGs at different time points in the GSE45006 dataset, including three common ferroptosis-related DEGs.(J) Visualization of DEGs in the GSE2599 dataset, containing three common ferroptosis-related DEGs.|logFC| > 1, adjusted P value < 0.05.DEGs:Differentially expressed genes; dpi: day(s) post injury; FC: fold change; Gch1: GTP cyclohydrolase 1 gene; Hmox1: heme oxygenase-1; SCI: spinal cord injury; Tgfbr1:transforming growth factor beta receptor 1.
SCI-induced lipid peroxidation and iron deposition aggravate ferroptosis
The schematic image shown in Figure 4A depicts the morphological changes observed in the spinal cord on days 1 and 7 after SCI.The representative images were selected from the approximate area of the anterior horn of the spinal cord within the region of interest (Figure 4B).We detected iron deposition starting on day 1 after SCI, and iron deposition levels were significantly elevated a week later (Figure 4C and D).Unlike iron deposition,which appeared later, neuron degeneration was readily apparent within the first 3 days after SCI and did not increase significantly thereafter (Figure 4E and F).Western blotting showed that 4HNE and ACSL4 were upregulated in the first 3 days and returned to near-normal levels on day 7 (Figure 4G and H).GPX4 expression decreased slowly over the follow-up period, while HMOX1 remained upregulated (Figure 4I and J).During the initial 3-day period, we did not observe any mitochondria with typical characteristics associated with ferroptotic cell death; instead, we identified damaged mitochondria showing reduced or absent cristae (Figure 4K).Owing to the inflammatory response, lipid peroxidation and neuronal cell death were more pronounced in the first 3 days after SCI than at later time points, suggesting that this is the appropriate time frame for assessing the role of ferroptosis in SCI.
Met attenuates SCI-induced ferroptosis in vivo
Based on the results of bioinformatics and western blotting analyses, we assessed HMOX1 expression in the gray matter and white matter of the spinal cord at different time points after SCI.The immunohistochemistry results showed that HMOX1 was uniformly and consistently upregulated in the SCI group compared with the sham group (Figure 5A–D).Studies have suggested that GPX4 downregulation and ACSL4 upregulation induce and promote the progression of ferroptosis (Chen et al., 2021b; Liu et al., 2022a).Here, we found that SCI induced a significant decrease inGpx4expression on day 3,rather than on day 1, after SCI.In addition, Gpx4 expression was significantly upregulated within the first 3 days after Met treatment (Figure 5E).ACSL4 and HMOX1 were upregulated after SCI, while Met treatment downregulated ACSL4 and upregulated HMOX1 (Figure 5F–I).Met treatment also downregulated the trauma-induced 4HNE expression, further demonstrating that Met effectively regulates lipid peroxidation levels after SCI (Figure 5J).To determine the extent of ferroptotic neuronal cell death at the tissue level,we performed double immunofluorescence labeling of GPX4, ACSL4, IL-1β,and IL-6 with neuronal marker and found that, compared with the SCI group,neurons from rats in the Met treatment group exhibited upregulated GPX4 expression and downregulated ACSL4, IL-1β, and IL-6 expression (Figure 6A–H).This indicated that Met treatment reduced both ferroptosis and inflammation in neurons after SCI.In conclusion, these findings show that Met inhibit ferroptosis at the tissue and neuronal levels.
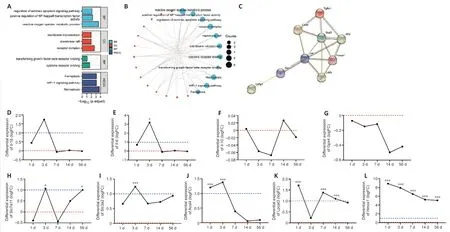
Figure 3 | Dynamic changes in the expression of inflammatory cytokine genes and ferroptosis-related genes (FRGs) after SCI.(A, B) Functional annotation and pathway enrichment analysis of 17 ferroptosis-related DEGs from the GSE45006 dataset.Red represents molecules, and blue represents GO and KEGG items.(C) Protein-protein interaction network of 17 ferroptosis-related DEGs.(D–L) Dynamic changes in nine FRGs of interest after SCI.Red line: baseline value, indicating that the expression level of this gene at this time point was 1 time that of the sham group (20).Blue line: |logFC| = 1, indicating that the expression level of this gene at this time point was 2 time that of the sham group (21).Adjusted *P < 0.05, adjusted ***P < 0.001 (independent samples t test).Acsl4: Acyl-coenzyme A synthetase long-chain family member 4; Atf3:activating transcription factor 3; BP: biological brocess; CC: cell component; CD44: cluster of differentiation 44; Cybb: cytochrome b-245 beta chain; DEGs: differentially expressed genes; FRG: ferroptosis-related gene; Gpx4: glutathione peroxidase 4; HIF-1: hypoxia inducible factor-1; Hmox1: heme oxygenase-1; Il-10: interleukin 10; Il-1β: interleukin 1β; Il-6: interleukin 6; KEGG: Kyoto Encyclopedia of Genes and Genomes; Lpcat3: lysophosphatidylcholine acyltransferase 3; Lrrfip1: lrr binding flii interacting protein 1; MF: molecular function; Ripk1: receptor-interacting protein kinase 1; SCI: spinal cord injury; Slc3a2: solute carrier family 3 member 2; Slc7a11: solute carrier family 7 member 11; Stat3: signal transducer and activator of transcription 3; Tgfbr1: transforming growth factor beta receptor 1; Tlr4: Toll-like receptor 4.
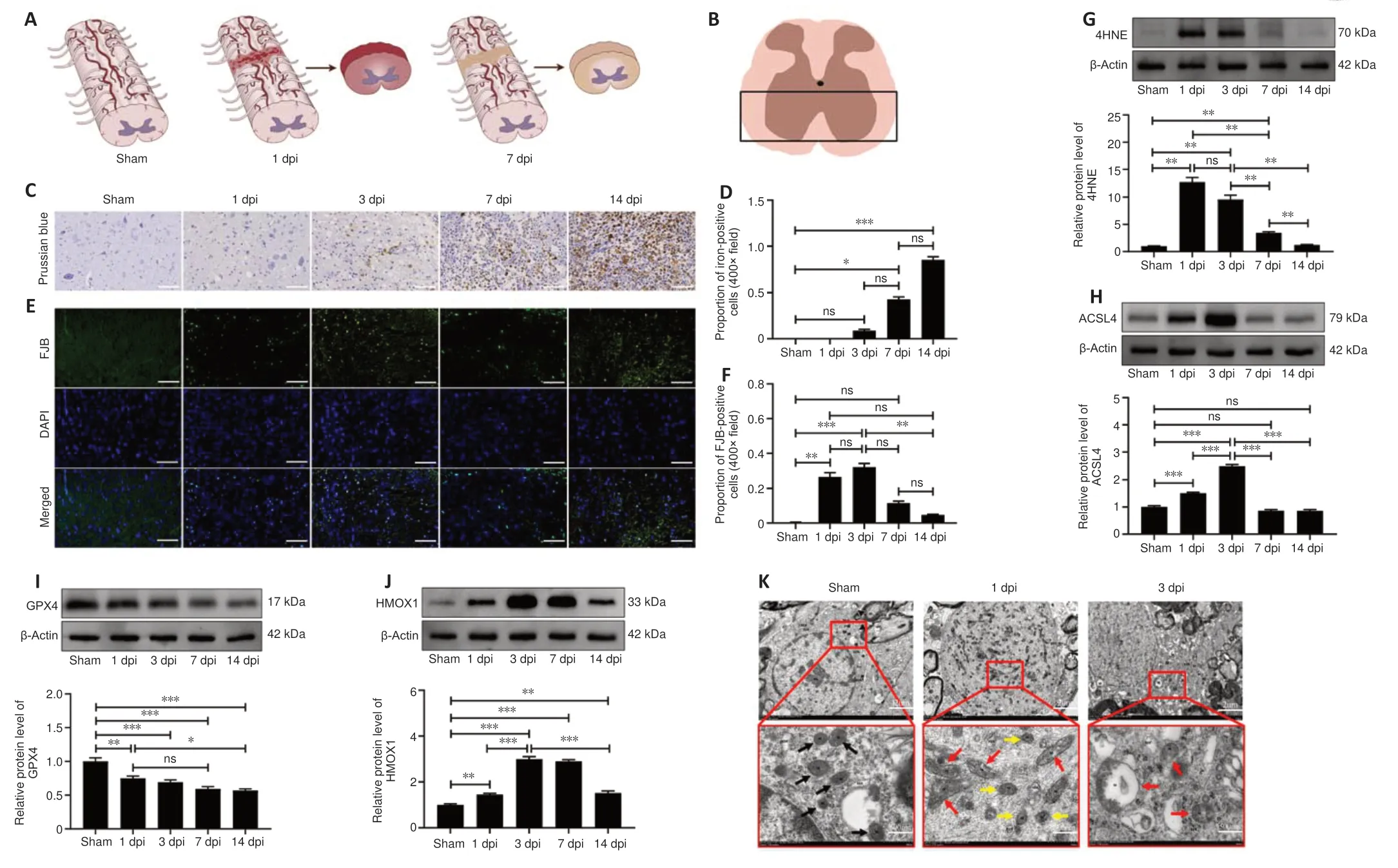
Figure 4 |SCI-induced lipid peroxidation and iron deposition aggravate ferroptosis.(A) Schematic diagrams of the sham, 1 dpi, and 7 dpi groups and the main region of interest (ROI).(B) Representative area of interest in the ROI.(C, D) Representative images of Prussian blue staining and quantification of iron-positive cells.Iron deposition was not detected until 3 days after SCI and was significantly elevated on days 7 and 14 compared with the sham group (n = 6).Scale bars: 50 µm.(E, F) Representative images of Fluoro-Jade B (FJB) staining and quantification of FJB-positive neurons.The number of degenerating neurons was significantly increased on day 3, but not on days 7 and 14, after SCI compared with the sham group (n = 6).Scale bars: 50 µm.(G–J) Relative protein expression levels of 4HNE, ACSL4, GPX4, and HMOX1, normalized to the sham group (n = 5).(K) Mitochondrial ultrastructure was examined by transmission electron microscopy (n = 3).Scale bars: 2µm (upper panel), 500 nm (lower panel).Black arrows: normal mitochondria, red arrows: damaged mitochondria, yellow arrows: smaller mitochondria (that did not exhibit shrunken morphology).The data are expressed as the mean ± SEM.*P < 0.05, **P < 0.01, ***P < 0.001 (one-way analysis of variance with Bonferroni post hoc test).4HNE: 4-Hydroxynonenal;ACSL4: acyl-coenzyme A synthetase long-chain family member 4; dpi: day(s) post injury; GPX4: glutathione peroxidase 4; HMOX1: heme oxygenase-1; hpi: hours post-injury; ns: not significant; SCI: spinal cord injury.
HMOX1 knockdown partially reverses the neuroprotective effects of Met after SCI
To minimize the use of laboratory animals, we first used the PC-12 cell line to evaluate the effectiveness of the LV vector.HMOX1 expression was reduced in all transfected PC-12 cells compared with NC cells, indicating the effectiveness of the knockdown construct (Additional Figure 2A–C).LV-Hmox1-RNAi(c) was selected for use in the animal experiments owing to its superior knockdown effect.On day 14 after LV injection, rat spinal cord tissues were harvested and prepared for western blotting.The western blot results demonstrated the effectiveness of LV-mediated knockdown in downregulating HMOX1 expressionin vivo(Additional Figure 2D–F).To assess the role of HMOX1 in ferroptosis and the anti-ferroptotic effects of Met, rats were injected with LV 14 days prior to compression injury, and spinal cord tissues were collected on day 3 after SCI.HMOX1 expression was then evaluated in all six subgroups:the NC group (sham, SCI, and Met subgroups) and the KD group (sham, SCI,and Met subgroups).In the NC group, HMOX1 expression was significantly upregulated after SCI, and it was further increased after Met treatment.LVmediated knockdown of HMOX1 expression was stable and long-lasting.Neither SCI nor Met treatment was effective in reversing this effect (Figure 7A).In both the NC and KD groups, 4HNE was upregulated after SCI, but it was downregulated after Met treatment.In the SCI subgroups (SCI subgroup of the KD groupvs.SCI subgroup of the NC group), 4HNE expression was not significantly increased after HMOX1 knockdown.In the Met subgroups (Met subgroup of the KD groupvs.Met subgroup of the NC group), however, 4HNE expression was significantly upregulated after HMOX1 knockdown (Figure 7B).Similarly, compared with the sham group, ACSL4 levels were upregulated after SCI in both the NC and the KD group.However, Met treatment effectively reduced ACSL4 expression levels in the NC group compared with the KD group.In the SCI and Met subgroups, ACSL4 expression increased significantlyafter HMOX1 knockdown (Figure 7C).Finally, compared with sham group, we found that GPX4 levels were significantly downregulated after SCI in both the NC and KD groups.However, Met treatment effectively increased GPX4 expression levels in the NC group compared with the KD group.In addition, in both the SCI and Met subgroups, GPX4 expression was significantly downregulated after HMOX1 knockdown (Figure 7D).These results show that HMOX1 has at least some anti-ferroptotic effect after SCI, and that the anti-ferroptotic effect of Met depends partly on HMOX1 upregulation.
After TSCI, activated M1 microglia release nitric oxide (NO), causing oxidative stress and a neurotoxic cascade in neurons.It is thought that Arg1 blocks uncoupling of nitric oxide synthase (NOS) in M2 microglia (Ma et al., 2017).HMOX1 expression in macrophages is induced by stress and promotes their differentiation into an M2 phenotype, with associated anti-inflammatory activities (Campbell et al., 2021).In both the NC and KD groups,the proportion of Iba1+/iNOS+microglia/macrophages was significantly upregulated after SCI, and significantly downregulated after Met therapy.In the SCI and Met subgroups (SCI subgroup of the KD groupvs.SCI subgroup of the NC group, Met subgroup of the KD groupvs.Met subgroup of the NC group), the proportion of Iba1+/iNOS+microglia/macrophages was significantly upregulated after HMOX1 knockdown.This indicates that HMOX1 upregulation and Met treatment prevented overactivation of M1-type microglia in SCI rats (Figure 8A and B).Similarly, in both the NC and KD groups, the ratio of Iba1+/Arg1+microglia/macrophages was significantly increased after SCI, and Met treatment further increased this ratio.However,in the SCI and Met subgroups, the ratio of Iba1+/Arg1+microglia/macrophages was significantly decreased after HMOX1 knockdown.This indicates that HMOX1 upregulation and Met treatment promoted activation of M2-type microglia cells in SCI rats (Figure 8C and D).These findings suggest that both HMOX1 upregulation and Met treatment after SCI can promote the polarization of M1 microglia/macrophages towards an M2 phenotype, and that the effects of Met may not be dependent on HMOX1 upregulation.Finally, we examined the effect of HMOX1 knockdown on neuronal damage.As depicted in Figure 9, the proportion of FJB-positive neurons increased significantly following SCI in the NC and KD groups.In the NC group, Met treatment noticeably reduced neuronal damage, but this effect was not pronounced in the KD group.In the SCI and Met subgroup, HMOX1 knockdown led to a considerable increase in neuronal damage.These findings indicate that HMOX1 upregulation following SCI helps attenuate nerve damage, and that the effectiveness of Met treatment partly relies on HMOX1 upregulation.

Figure 5 |Met treatment increases HMOX1 expression after SCI.(A, B) Representative images of immunohistochemistry (IHC) staining and quantification of HMOX1 expression in the gray matter.HMOX1 expression was significantly upregulated in the first 7 days after injury in the SCI group compared with the sham group (n = 6).Scale bars: 50 µm.(C, D) Representative images of IHC staining and quantification of HMOX1 expression in the white matter.HMOX1 expression was significantly upregulated in the first 14 days after injury in the SCI group compared with the sham group.HMOX1 expression was highest on day 3 after SCI compared with the Sham group (n = 6).Red arrows: positively stained cells, black arrows: non-stained cells.Scale bars: 50 µm.(E–G) Relative mRNA levels of Gpx4, Acsl4, and Hmox1 were determined by PCR (n = 6).(H–J) Relative protein expression levels of ACSL4, HMOX1, and 4HNE in the indicated groups (n = 5).Expression levels shown in E–G were normalized to those in the sham group.The data are expressed as the mean ± SEM.*P < 0.05, **P < 0.01, ***P < 0.001 (one-way analysis of variance with Bonferroni post hoc test (B, D) or two-way analysis of variance with Tukey’s post hoc test (E–J)).4HNE: 4-Hydroxynonenal; ACSL4: acyl-coenzyme A synthetase long-chain family member 4; dpi: day(s) post injury; GPX4: glutathione peroxidase 4; HMOX1: heme oxygenase-1; Met: metformin; ns: not significant; SCI: spinal cord injury.
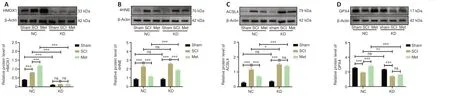
Figure 7 |HMOX1 knockdown partially reverses the anti-ferroptotic effects of Met after SCI.(A–D) Representative western blot showing HMOX1, 4HNE, ACSL4, and GPX4 expression levels in the indicated groups at 3 dpi (n = 5).Expression levels were normalized to the β-actin expression level in the same lane.The data are expressed as the mean ± standard error of the mean.*P < 0.05, **P < 0.01, ***P < 0.001 (two-way analysis of variance with Tukey’s post hoc test).4HNE: 4-Hydroxynonenal; ACSL4: acyl-coenzyme A synthetase long-chain family member 4; dpi: day(s) post injury; GPX4: glutathione peroxidase 4; HMOX1: heme oxygenase-1; KD: knockdown; Met: metformin; NC: normal control; ns: not significant; SCI: spinal cord injury.

Figure 8 |Met promotes the polarization of M1-type microglia/macrophages towards an M2 phenotype in an HMOX1-independent manner.(A, B) Representative IF staining images and the proportion of iNOS+-stained microglia/macrophages in the indicated groups at 3 dpi.In both the NC and KD groups, the ratio of M1-type to M2-type microglia in the SCI group was increased compared with that in the sham group, and this effect was dramatically reversed by Met treatment.After HMOX1 knockdown, the ratio in the SCI and Met subgroups of the KD group was higher than that in the NC group (n = 5).Scale bars: 50 µm.(C, D) Representative IF staining images and the proportion of Arg1+-stained microglia/macrophages in the indicated groups at 3 dpi.In both the NC and KD groups, the ratio of M2-type to M1-type microglia in the SCI group was increased compared with that in the sham group, and this ratio was further increased by Met treatment.After HMOX1 knockdown, the ratio in the SCI and Met subgroups of the KD group was lower than that in the NC group (n = 5).Red indicates Iba1 (stained with Cy3), and green indicates iNOS or Arg1 (stained with Alexa Fluor 488).Scale bars: 50 µm.*P <0.05, **P < 0.01, ***P < 0.001 (two-way analysis of variance with Tukey’s post hoc test).Arg1: Arginase 1; dpi: day(s) post injury; IF: immunofluorescence; iNOS: inducible nitric oxide synthase; KD: knockdown; Met: metformin; NC: normal control; ns: not significant; SCI: spinal cord injury.

Figure 9 |Met exerts a neuroprotective effect in an HMOX1-dependent manner.(A, B) Representative FJB staining images and the proportion of FJB+ neurons at 3 dpi.In both the NC and KD groups, the ratio of FJB+neurons to unstained neurons in the SCI group was increased compared with that in the sham group.This effect was dramatically reversed by Met treatment in the NC group but not in the KD group.After HMOX1 knockdown, the ratio in the SCI subgroup and the Met subgroup of the KD group was higher than that in the NC group (n = 5).Scale bars: 50 µm.**P < 0.01,***P < 0.001 (two-way analysis of variance with Tukey’s post hoc test).dpi: Day(s) post injury; FJB: Fluoro-Jade B; KD: knockdown;Met: metformin; NC: normal control; ns: not significant; SCI: spinal cord injury.
Discussion
In our previous study, we demonstrated that Met has anti-ferroptotic effects after SCI (Wang et al., 2022b).This neuroprotective effect was ascribed to the antioxidant mechanism mediated by the nuclear factor erythroid2-related factor 2 (Nrf2) signaling pathway.In further studies, we found that HMOX1, a downstream target gene of the Nrf2 signaling pathway, is stably expressed at high levels in the early and late period of SCI.HMOX1 is generally believed to play a protective role in various diseases (Puentes-Pardo et al., 2020; Ryter,2021, 2022).However, it is considered to be harmful to tissues owing to its promotion of heme degradation, leading to excess iron production (Fang et al., 2019; Zeng et al., 2021).In the present study, we found that it plays anti-ferroptotic roles in TSCI than it does in other diseases.Direct mechanical trauma and subsequent lipid peroxidation, as well as inflammation, are the main drivers of nerve cell death (Hu et al., 2021; Dong and Yong, 2022).Here we found that cell death occurred primarily within 1 week after SCI,as confirmed by FJB staining.Mechanical trauma was also the main cause of erythrocyte rupture and iron deposition, which was significantly elevated 1 week after SCI.Therefore, there does not appear to be a significant relationship between the death of nerve cells in the early stage of injury and iron deposition.However, we cannot completely rule out the possibility that free iron does not contribute to nerve damage.Seven days after SCI, oxidative stress and inflammation had returned to normal levels.GPX4 expression levels also leveled off, and at this stage, high levels of HMOX1 seemed even less likely to contribute to neuronal cell ferroptosis.
Apoptosis has been considered to be the main form of cell death that occurs after SCI.Given that enhanced oxidative stress, lipid peroxide accumulation,and inflammation were detected in the post-injury microenvironment, it is likely that other types of cell death are also involved.The earliest report on a possible association between ferroptosis and TSCI was published in 2019(Zhang et al., 2019), and the results from our current study support this association.However, the 2019 study showed that 4HNE expression remained elevated on the 14thday, and mitochondria with ferroptotic morphology were detected at multiple time points after.In contrast, we only detected high 4HNE expression levels 7 days after SCI, with a peak on day 3, which is basically consistent with the conclusions from several previous studies (Xiong et al., 2007; Carrico et al., 2009; Ge et al., 2021; Liu et al., 2022b).Unlike previous reports, we did not observe any mitochondria with typical ferroptotic features on days 1 and 3 after SCI.This was confirmed by morphological analysis showing no marked iron deposition in tissues within 24 hours, or indeed up to day 3, while significant iron deposition was observed on the 7thday after SCI.This differs from previous studies, which reported that significantly increased iron content can be detected at 1, 4, and 24 hours after SCI, presumably because of massive erythrocyte stasis induced by trauma in the acute stage of SCI.This discrepancy may be attributable to the use of different animal models and detection methods.
Inflammation in the microenvironment is an important factor associated with worse outcomes after SCI.Inflammatory cytokines have been reported to participate in and promote ferroptosis (Bin et al., 2021; Han et al., 2021;Yao et al., 2021).IL-1β can induce the accumulation of ROS and lipid ROS in chondrocytes and significantly alter the expression of ferroptosis-related proteins.Ferrostatin-1, a specific inhibitor of ferroptosis, can reduce IL-1βinduced cytotoxicity and the accumulation of ROS and lipid ROS, as well as modulate the expression of ferroptosis-related proteins and promote activation of the Nrf2 antioxidant system (Yao et al., 2021).Disc degeneration can cause low back pain, often accompanied by severe inflammation.Studies have shown that the inflammatory cytokine IL-6 can aggravate intervertebral disc degeneration by inducing chondrocyte ferroptosis (Bin et al., 2021).In addition, IL-6 has been implicated in the pathogenesis of asthma, owing to its ability to promote reactive oxygen-dependent lipid peroxidation and disrupt iron homeostasis, which promotes ferroptosis in bronchial epithelial cells(Han et al., 2021).In the present study, expression levels of the inflammatory factors IL-1β and IL-6 were significantly increased in the injured spinal cord on the 3rd day after SCI.Met treatment significantly reduced the overall levels of inflammatory factors, alleviated inflammation-induced damage to neurons,and inhibited ferroptosis in the injury microenvironment.
As mentioned earlier, HMOX1 plays dual roles in some diseases (Choi and Kim,2022; Yang et al., 2022).Its overall role in ferroptosis after SCI is currently controversial.In the current study, we explored HMOX1 expression after SCI using different methods.We demonstrated that its expression is significantly increased over a long period of time after SCI.In addition, we found that Met treatment promoted motor function recovery in a rat model of SCI by inhibiting ferroptosis.The anti-ferroptotic effect of Met treatment was mediated by the increase in HMOX1 levels.In further tests, we knocked down HMOX1 expression in the spinal cord of rats.In our study, all rats received 10µL LV by injection into the spinal cord at the T9 level after laminectomy.Once satisfactory outcomes were obtained, we divided the injected rats into two main groups and six subgroups, some of which were subjected to SCI and some of which were also treated with Met.On day 3 after SCI, spinal cordtissues were collected for western blotting and immunofluorescence analysis.It demonstrated that the anti-ferroptotic effect of Met is partly mediated by increased HMOX1 expression by western blotting analysis.It also implies that HMOX1 has a polarized anti-inflammatory effect in the early phase of SCI and promotes the conversion of M1-polarized Iba1+/iNOS+microglia/macrophages to M2-polarized Iba1+/Arg1+microglia/macrophages by immunofluorescence analysis.Moreover, our results suggest that the anti-inflammatory effect of Met was not reversed by HMOX1 knockdown.This demonstrates that,even when HMOX1 is knocked down, Met treatment effectively reduced the proportion of M1-polarized Iba1+/iNOS+microglia/macrophages and increased the proportion of M2-polarized Iba1+/Arg1+microglia/macrophages.Finally,we demonstrated that HMOX1 upregulation following SCI plays a vital role in combating nerve damage, and that the effectiveness of Met treatment is partially dependent on HMOX1 upregulation.
We did not screen for drug targets or for relevant signaling pathways.In addition, we did not perform behavioral experiments or explore the molecular mechanism underlying the effects of HMOX1 knockdownin vitro.Taken together, the findings from our study suggest that Met inhibits ferroptosis after SCI, and that this effect is partly dependent on high levels of HMOX1 expression.Met exerted an anti-inflammatory effect that mitigated further damage after SCI through HMOX1-independent pathways.In addition,high levels of HMOX1 expression played a neuroprotective role after SCI by reducing inflammation, alleviating oxidative stress, and inhibiting ferroptosis.Therefore, HMOX1 is a promising therapeutic target for inhibiting nerve cell ferroptosis after SCI.
Author contributions:Study design and conception, experiment implementation and data collection, analysis and visualization, article writing and revising: ZW.Experiment implementation, data collection and analysis,and article review: WZ and ZZ.Experiment implementation and article review:LZ.Study design and conception and article review: ML.All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare that they have no conflicts of interest.
Data availability statement:All data generated or analyzed during this study are included in this published article and its Additional files.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:George M.Smith, Temple University, USA; Omar Tawakol,Illinois Institute of Technology, USA.
Additional files:
Additional Table 1: The detailed information of all datasets.
Additional Table 2: Sequences and titer information of lentiviruses.
Additional Table 3: Primer sequences used in PCR analysis.
Additional Figure 1: Hmox1 is significantly upregulated in SCI rat models.
Additional Figure 2: Lentivirus-mediated knockdown reduced HMOX1 expression both in vitro and in vivo.
Additional file 1: Open peer review reports 1 and 2.
- 中国神经再生研究(英文版)的其它文章
- NADPH oxidase 4 (NOX4) as a biomarker and therapeutic target in neurodegenerative diseases
- Circadian rhythm disruption and retinal dysfunction:a bidirectional link in Alzheimer’s disease?
- Interplay between the glymphatic system and neurotoxic proteins in Parkinson’s disease and related disorders: current knowledge and future directions
- Roles of neuronal lysosomes in the etiology of Parkinson’s disease
- Therapeutic advances in neural regeneration for Huntington’s disease
- The advantages of multi-level omics research on stem cell-based therapies for ischemic stroke

