The advantages of multi-level omics research on stem cell-based therapies for ischemic stroke
Yiqing Wang, Chuheng Chang, Renzhi Wang, Xiaoguang Li, Xinjie Bao
Abstract Stem cell transplantation is a potential therapeutic strategy for ischemic stroke.However, despite many years of preclinical research, the application of stem cells is still limited to the clinical trial stage.Although stem cell therapy can be highly beneficial in promoting functional recovery, the precise mechanisms of action that are responsible for this effect have yet to be fully elucidated.Omics analysis provides us with a new perspective to investigate the physiological mechanisms and multiple functions of stem cells in ischemic stroke.Transcriptomic, proteomic, and metabolomic analyses have become important tools for discovering biomarkers and analyzing molecular changes under pathological conditions.Omics analysis could help us to identify new pathways mediated by stem cells for the treatment of ischemic stroke via stem cell therapy, thereby facilitating the translation of stem cell therapies into clinical use.In this review, we summarize the pathophysiology of ischemic stroke and discuss recent progress in the development of stem cell therapies for the treatment of ischemic stroke by applying multi-level omics.We also discuss changes in RNAs, proteins, and metabolites in the cerebral tissues and body fluids under stroke conditions and following stem cell treatment, and summarize the regulatory factors that play a key role in stem cell therapy.The exploration of stem cell therapy at the molecular level will facilitate the clinical application of stem cells and provide new treatment possibilities for the complete recovery of neurological function in patients with ischemic stroke.
Key Words: ischemic stroke; mesenchymal stem cells; metabolomics; multilevel omics; neural stem/progenitor cells; neuroinflammation; pathophysiology; proteomics; stem cell therapy; transcriptomes
Introduction
Ischemic stroke is usually caused by a thrombus that occludes cerebral blood vessels (Jolugbo and Ariëns, 2021; Morris and Sutherland, 2023) and is the primary type of stroke associated with long-term neurological impairment and a poor prognosis (GBD 2016 Stroke Collaborators, 2019).Treatment options are limited to the intravenous administration of tissue plasminogen activator and mechanical thrombectomy.Nevertheless, narrow therapeutic windows limit the number of patients who can benefit from these procedures (Phipps and Cronin, 2020).No effective clinical treatment is currently available to restore the long-term functional loss caused by ischemic stroke.
Preclinical studies have confirmed that stem cells promote nerve repair and regeneration and can relieve behavioral dysfunction in animal models of stroke (Huang and Zhang, 2019; Hu and Wang, 2023).Although accumulating data confirms the efficacy of cell-based therapy, clinical translation has not yet been achieved (Kawabori et al., 2020).The reasons for this include the complexity of stroke pathogenesis and the unknown mechanistic actions of stem cells following transplantation.Therefore, understanding the mechanism of action of stem cells is essential for their clinical translation.
Over recent years, omics technologies have revolutionized healthcare systems by providing a new platform to investigate the molecular aspects of an individual.Genomes have been applied to disease diagnosis and personalized therapies.In addition, transcriptomes, proteomes, and metabolomes have become important methods to discover biomarkers and analyze molecular variations under pathological conditions (Subramanian et al., 2020).Integrating omics analysis into cell-based therapy has led to the discovery of novel pathways and processes that are mediated by stem cells; these events may also be relevant for patients with ischemic stroke.
This review summarizes recent studies that have applied omics analysis with cell-based therapies for ischemic stroke.We mainly explore how transcriptomics, proteomics, and metabolomics have contributed to our understanding of stroke pathophysiology, monitoring the efficacy of stem cell therapy, and the mechanistic of action of stem cells.Enhancing our understanding of the mechanistic effects exerted by stem cells may promote their clinical translation (Figure 1).
Search Strategy
We conducted an electronic search of the PubMed database in December 2022 to gather relevant literature.Our search strategy involved the following search terms: ischemic stroke, IS, stem cell, cell-based, transcriptome,proteome, metabolome, and bioinformatics.We utilized multiple combinations of these terms to ensure a thorough and comprehensive search.Following retrieval, we screen articles by reading their abstracts and titles.If they were deemed relevant, the full-text papers were assessed.Articles published in English that focused on applying omics analysis to stem cell-based therapy were included, while those that did not include stem cellbased therapy or omics data, as well as non-English articles, were excluded.
The Pathophysiology of Ischemic Stroke
After an occlusion in a cerebral blood vessel, the reduced blood flow causes abrupt oxygen and glucose deprivation in the ischemic area.Cell injuries arise from insufficient blood flow, leading to a cascade of pathophysiological and interconnected changes, including neuroinflammatory responses, the disturbance of metabolites, neuronal apoptosis, endogenous neurogenesis,and angiogenesis.To understand the therapeutic effects of stem cell transplantation, it is imperative to gain a thorough understanding of the pathophysiological cascades that accompany ischemic stroke.
Neuroinflammation
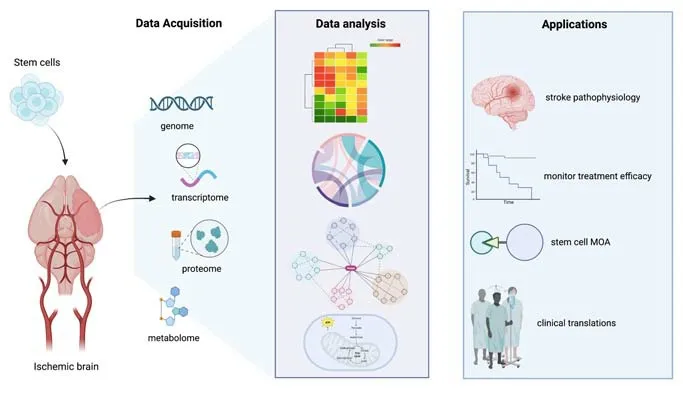
Figure 1 |Schematic representation of integrating omics analysis in the study of stem cell-based therapy for ischemic stroke.Genomic, transcriptomic, proteomic, and metabolomic data can be obtained from animal models with or without stem cell treatment.Data analysis contributes to a clear understanding of stroke pathophysiology, stem cell therapeutic efficacy, and the MOA mediated by stem cells to aid in their clinical translations.Created with BioRender.com.MOA: Mechanism of action.
Injured cells release damage-associated molecular patterns that promote the early onset of the innate immune response by attracting resident microglial cells and astrocytes to the lesion area.Although the traditional view of microglial activation is regarded as deleterious in the brain, emerging evidence has suggested that early microglial recruitment plays a crucial role in clearing cellular debris and promoting subsequent neuronal repair; in contrast, depleting microglial infiltration during the acute phase has been shown to exacerbate brain injury by increasing leukocyte activation and the astrocyte response (Jin et al., 2017; Brennan et al., 2022).Astrocytes account for most glial cells in the brain and are essential in maintaining metabolic homeostasis under physiological conditions (Colombo and Farina, 2016; Hao et al., 2023).Astrocytes aggregate to the peri-infarct area, where a border is formed, known as a glial scar, to seal off additional neuronal death and restrict the size of the infarct area.Within hours of ischemia, the blood-brain barrier becomes compromised, thus leading to an increase in adhesion molecules.This alteration facilitates the infiltration of peripheral leukocytes, specifically neutrophils, monocytes, and adaptive immune cells, into the site of the injury,thereby amplifying the neuroinflammatory pathways (Kuroiwa et al., 1985;Courties et al., 2015).
Disturbance of metabolites and neuronal apoptosis
Cell death in the ischemic core initiates soon after hypoxic onset.The depletion of oxygen and glucose disrupts mitochondria and energy production, thus resulting in ionic concentration disturbances intra- and extra-cellularly (Woodruff et al., 2011).Excessive glutamate release in the synaptic cleft causes delayed excitotoxic injury due to glutamate-mediated calcium influx.This overload leads to multiple neurotoxic effects, including the activation of proteases, lipases, nitric oxide synthase, and endonuclease,ultimately triggering cell apoptosis.Mitochondria are the primary source of reactive oxygen species (ROS) production; this can result in damage to the mitochondrial membrane and the subsequent release of apoptosis-inducing factors and apoptotic protein cytochrome c into the cytosol (Kalogeris et al.,2014; Ding et al., 2023).The release of cytokines and neuronal apoptosis also causes significant changes in metabolites within brain tissues post-stroke.Compared with controls, serum from ischemic stroke patients has been shown to contain higher levels of glutamate, lactate, and pyruvate and lower levels of alanine, citrate, glycine, valine, isoleucine, leucine, serine, phenylalanine,tyrosine, and methionine (Qureshi et al., 2017; Hu et al., 2019).Similar results were obtained in rats subjected to middle cerebral artery occlusion (MCAO),in which the levels of branched chain amino acids (valine, leucine, and isoleucine) were significantly reduced in the plasma and cerebrospinal fluid of model rats (Kimberly et al., 2013).
Endogenous neurogenesis and angiogenesis
Despite the brain having a limited capacity for repair, spontaneous recovery in the central nervous system is known to occur following ischemic stroke.The subventricular zone (SVZ) of the lateral ventricles and the sub-granular zone of the hippocampus contain a group of nascent neurons that are capable of self-renewal throughout life (Feliciano et al., 2015).Under an ischemic or traumatic condition, the proliferation and migration of neuroblasts are significantly induced in SVZ regions in response to the neural injury (Macas et al., 2006).Migrating neuroblasts have been observed in close proximity to the vasculature, thus suggesting that blood vessels provide directional guidance to SVZ-derived neurons (Massouh and Saghatelyan, 2010).In support of these observations, approaches targeting endogenous neurogenesis and angiogenesis have offered potential treatments for stroke recovery (Nih et al., 2018).However, recent studies have found that neurogenesis is mainly restricted to neurogenic niches and that the integration of mature neurons into functional circuits is hindered by the hostile environment and longdistance connections of the adult brain (Dillen et al., 2020; Xie et al., 2020).
Stem Cell-based Therapies
Despite the partial reversibility of brain impairment, achieving complete recovery from ischemic stroke remains a significant clinical challenge due to the complex and interconnected nature of its pathophysiology.Many studies have confirmed that stem cell transplantation improves neurological dysfunction in models of ischemic stroke.The potential mechanisms of stem cell therapy differ according to stem cell lineage but mainly include the direct replacement of damaged neurons and the bystander effect of transplanted cells through paracrine secretion (Eckert et al., 2015; Boese et al., 2018).Recent studies have shown that the paracrine function of stem cells plays the most important role in modulating the microenvironment and ultimately leads to better tissue recovery (Xie et al., 2020; Salikhova et al., 2021; Asgari Taei et al., 2022).Preclinical data have suggested that stem cells could reduce neuroinflammation(Dabrowska et al., 2019), confer neuroprotection by inhibiting apoptosis (Bao et al., 2011; Zhang et al., 2019), restore metabolic equilibrium, and enhance both neurogenesis and angiogenesis (Ryu et al., 2019; Xia et al., 2020).At present,the most commonly used stem cells in clinical research are mesenchymal stem cells (MSCs), neural stem/progenitor cells (NSPCs), embryonic stem cells (ESCs),and induced pluripotent stem cells (iPSCs).
MSCs
Extensive preclinical and clinical experiments have utilized MSCs because of their ease of collection, lack of ethical issues, and potential for multipotent differentiation.Allogenic bone marrow-derived MSCs (BMSCs) are among the most commonly obtained sources; other sources of MSCs include the umbilical cord and fat tissues.For example, transforming growth factor beta secreted from MSCs has been shown to suppress the upregulation of monocyte chemoattractant protein-1 in the ischemic brain, thereby inhibiting the infiltration of peripheral immune cells (Yoo et al., 2013).An increased number of neural progenitor cells in the SVZ and sub-granular zone of the hippocampus have been observed following the transplantation of BMSCs and their enhanced migration towards a lesion area suggests additional roles of MSCs in promoting endogenous neurogenesis (Shiota et al., 2018).Recent studies have also indicated that extracellular vesicles might be the most important mediator of MSCs to exert their protective effects (Zhao et al., 2019; Kim et al., 2020; Tian et al., 2021).Thus, the potential of using MSCderived exosomes as a neuroprotective therapy has attracted much attention.
NSPCs
NSPCs can differentiate into neuroblasts, astrocytes, and oligodendrocytes(Cheng et al., 2015; O’Shea et al., 2022).Such multipotency in generating various types of neurons in the central nervous system renders NSPCs an appropriate choice for cell replacement.In addition, preclinical studies have shown that similar to MSCs, NSPCs can exert neurogenic and immunomodulatory effects (Huang et al., 2014; Tian et al., 2021).The transplantation of NSCs during the acute phase of stroke has been shown to reduce proinflammatory cytokines and destruction of the blood-brain barrier while also inducing angiogenesis and endogenous neurogenesis (Hur et al.,2022).NSPCs are thus suggested to not only replace lost neurons but also facilitate post-stroke repair via multifaceted effects.The majority of the NSPCs that are currently used in preclinical research are obtained directly from the neuroectoderm of the fetal brain.Inevitably, clinical trials using fetal neural stem cells have been plagued by ethical problems.
ESC- and iPSC-derived NSPCs
Isolated from the inner cell mass of the blastocyst, ESCs can differentiate into neuroectodermal cells that give rise to neuroblasts, glioblasts,ependymal cells, and microglia (Thomson et al., 1998).Under the presence of fibroblast growth factor 2 and epidermal growth factor, ESCs have been shown to generate a homogenous culture of NSCs with a symmetrical selfrenewal ability (Conti et al., 2005).Over recent years, iPSCs have gradually become an important source of stem cells for preclinical experiments.iPSC transplantation avoids ethical issues and immune rejection because the cells are generated from autologous somatic cells of the patients themselves.Yuan et al.(2013) successfully obtained mature neurons and astrocytes from iPSCs cultured in serum-free medium with retinoic acid.In another study,Eckert et al.(2015) found that hiPSC-derived NPC transplantation during the acute phase of stroke ameliorated inflammatory damage by reducing proinflammatory cytokines and microglial activation while improving functional recovery.Similarly, iPSC-derived NSPCs transplanted into a rat model of spinal cord injury (SCI) differentiated into mature neurons and astrocytes, thus improving functional recovery (Zheng et al., 2022).
Applying Omics to Stem Cell-based Therapy for Ischemic Stroke
Transcriptomes, or the sequencing of all transcribed RNAs in a given cell or organism, permits the large-scale quantification of gene expression and the identification of novel gene products.Proteomes identify changes in protein profiles and therefore reflect changes downstream of RNA expression.Metabolomics refers to the analysis of a complete set of metabolites of a biological system, including the end products and intermediates in various cellular processes.In recent preclinical studies involving cell-based therapies, comprehensive analyses including transcriptomics, proteomics,and metabolomics have been employed to investigate alterations in gene expression, protein profiles, and metabolic changes of stem cells and in animal models of stroke.Omics analysis offers several advantages in uncovering the mechanisms underlying stem cell-based therapies.Furthermore, omics analysis serves as a complement to traditional experimental methods by offering molecular-level evidence that supports the efficacy of stem cell transplantation.Moreover, omics analysis facilitates the identification of key regulatory factors involved in immunomodulation,neuronal apoptosis, and neuro/angiogenesis.These identified factors hold potential as candidates for optimizing the therapeutic effects of stem cells,thereby addressing the challenges encountered in the clinical application of stem cell therapy (Table 1).
The modulation of neuroinflammation
While the early activation of innate immune responses is necessary for limiting neural apoptosis around the infarct foci, immoderate neuroinflammation during the sub-acute stages after ischemic stroke may interfere with neuronal repair and ultimately leads to diminished functional recovery.Immunomodulation is one of the most important proposed mechanisms underlying cell-based therapy.Applying omics analysis further supports the role of stem cells in modulating neuroinflammation by activating anti-inflammatory pathways and regulating the functions of supporting cells.Hamblin et al.(2022) showed that the transplantation of hNSCs into the aged mouse hippocampus 24 hours after the onset of stroke significantly reduced infarct size and cytokine levels.Ingenuity pathway analysis further indicated that genes involved in neuroinflammation, phagosome formation,and acute phase response pathways were down-regulated, while peroxisome proliferator-activated receptor signaling, liver X receptors/retinoid X receptor activation pathways, and matrix metalloprotease inhibition pathways were significantly activated in NSC treatment groups when compared with MCAO sham controls (Hamblin et al., 2022).Peroxisome proliferator-activated receptors and liver X receptors exert an immune-modulatory effect in the central nervous system by inhibiting the transcription of proinflammatory genes, while promoting an M2-like phenotype in immune cells (Zelcer and Tontonoz, 2006; Cuartero et al., 2013).A similar study found that cell cycle and inflammatory-related pathways were significantly upregulated in the brains of a rat model of MCAO.However, human umbilical mesenchymal stromal cell transplantation two hours after the onset of stroke attenuated such alterations in gene expression (Choi et al., 2016).The effect of stem cells on neuroinflammation is also assessed by measuring protein levels.Proteomic analysis of rat spinal cords subjected to SCI found that a large number of complement proteins, such as complement protein H, C5, and C6, were down-regulated in the SCI rat model injected with exosomes from BMSCs(Zhao et al., 2019).RNA quantification by reverse transcription-quantitative polymerase chain reaction further verified that the mRNA expression levels of the down-regulated complement proteins were significantly reduced in the BMSC group, thus indicating that the factors secreted by BMSCs could attenuate the complement pathway induced in rats with SCI.Collectively,these studies indicated that stem cell transplantation exerted an antiinflammatory effect by modulating multiple cellular pathways associated with the pathological process of stroke.
Gene expression analysis can also be applied to the analysis of transcriptional changes in microglia and astrocytes.Transplanting three-dimensional cultured MSCs intravenously on day 3 after MCAO was shown to reduce the expression of ionized calcium-binding adapter molecule 1, CD5, and proinflammatory cytokines (interleukin 1β and interleukin-6) in the rat brain(Li et al., 2021).Further transcriptomic analysis of microglia isolated from brain tissue identified genes linked to the immune system with significantly altered expression levels.Down-regulated microglial genes in the MSC group were involved in the response to lipopolysaccharide, inflammatory responses, and chemokine-mediated signaling pathways, thus indicating that the transplantation of MSCs reduced proinflammatory responses and enhanced the neuroprotective properties of the host microglia.Hu et al.(2022)discovered that mice injected with extracellular vesicles (EVs) isolated from adipose-derived stem cells showed a higher ratio of M2 phenotype microglia measured by immunohistochemistry staining.To further investigate the relationships between adipose-derived stem cells and microglia, microRNAs from the EVs were sequenced.Ingenuity pathway analysis showed that signal transducer and activator of transcription 1 (STAT1) and phosphatase and tensin homolog (PTEN) were the main downstream targets of the upregulated microRNAs in the microglia, thus suggesting that the EVs from adiposederived stem cells could modulate microglial polarization by suppressing the expression of STAT1 and PTEN (Hu et al., 2022).Anin vitrostudy further identified a regulatory effect of human dental pulp stem cells and human BMSCs on human astrocytes (Song et al., 2017).Gene expression analysis demonstrated that multiple pathways involved in the positive regulation of cell proliferation and cell migration were upregulated in astrocytes cocultured with human dental pulp stem cells or BMSCsin vitro.Specifically,genes related to the mitogen-activated protein kinase signaling pathway and the transforming growth factor beta signaling pathway were significantly induced, thus suggesting the beneficial effect of stem cells in promoting the survival of ischemic astrocytes (Song et al., 2017).Further studies are now needed to address the effect of such increased ischemic astrocyte activities on modulating the processes responsible for global inflammation.The effect of stem cells on regulating peripheral immune cell infiltration has been characterized by analyzing transcriptomic data.Human nonhematopoietic umbilical cord blood stem cells injected intravenously at 48 hours after MCAO were found to exert a beneficial effect by reducing the upregulated expression of genes associated with the blood-brain barrier, ECM remodeling, apoptosis, and cytokine signaling in the ipsilateral hemisphere(Shiao et al., 2019).Immune cell subtype analysis using CIBERSORT further indicated an increase in the expression of genes associated with monocytes,neutrophils, dendritic cells, and T cells in a MCAO group.Nevertheless, the group treated with stem cells isolated from umbilical cord blood showed the reversal of the upregulation of these transcripts and increased expression of genes associated with M2 macrophages, thus indicating the capacity of stem cells from umbilical cord blood to modulate immune cell infiltration after ischemia.Similar results were also observed after intravenously injecting human umbilical mesenchymal stromal cells 24 hours after the induction of MCAO (Oh et al., 2018).Compared with the control group, the numbers of microglia, monocytes, and neutrophils were significantly reduced in the peri-infarct area of the IV-human umbilical mesenchymal stromal cell group.mRNA microarray analysis further revealed inflammation-related genes, such as glycosylation dependent cell adhesion molecule 1 (GLYCAM1), interleukin 1 receptor antagonist (IL1RN), and prostaglandin E synthase (PTGES), were significantly upregulated.Furtherin vitroexperiments showed that the increased expression of interleukin 1 receptor antagonist mainly originated in macrophages, thus confirming the effect of MSCs in upregulating antiinflammatory genes in macrophages (Oh et al., 2018).
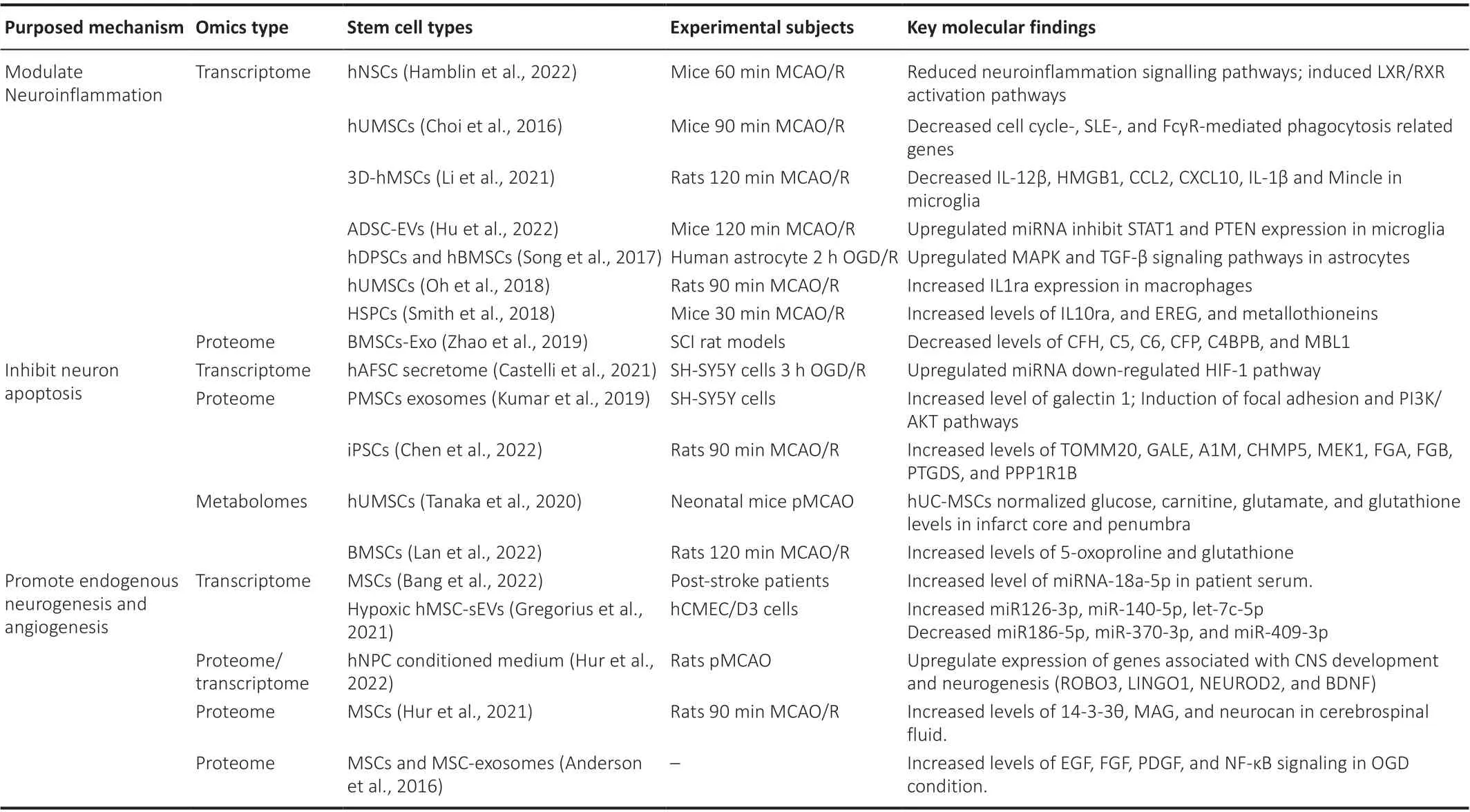
Table 1 |The mechanism of stem cell-based therapies for ischemic stroke elucidated by omics analysis
The inhibition of neuronal apoptosis
Cells in the penumbra region, which are close to the lesion site but not directly deprived of oxygen, experience metabolic disturbance, structural breakdown, and apoptosis due to ATP depletion and the production of ROS(Uzdensky, 2019).However, these injuries are believed to be reversible with appropriate and timely treatment.The intracerebral transplantation of hMSCs during the acute phase of ischemia has been shown to modulate the expression of multiple proteins associated with ATP metabolism, signal transduction, synaptic activity, and ROS metabolism in the rat brain (Mitaki et al., 2020).Metabolomic analysis combined with mass spectrometry imaging of neonatal rats exposed to MCAO revealed substantial alterations in the concentrations of various metabolites.Glucose accumulation was predominantly observed at the core of the infarct, while higher levels of carnitine and Na adducts of glutamate were detected in the penumbra; this was consistent with the excitotoxic effect arising from glutamate release after brain ischemia.However, the administration of MSCs to model mice reversed these metabolic changes, effectively normalizing the excessive levels of these metabolites (Tanaka et al., 2020).Lan et al.(2022) found that the levels of 5-oxoproline and glutathione were significantly increased in the brains of MCAO rats following the administration of BMSCs.Increased levels of glutathione have been observed in stroke patients during the acute phase;this is regarded as an important antioxidant that acts by scavenging free radicals (Ozkul et al., 2007).Therefore, the enhanced levels of glutathione induced by the administration of BMSCs implies the neuroprotective effect of cell-based therapy acts by counteracting oxidative distress.Pathway analysis of upregulated microRNAs (miRNAs) in the MSC secretome has been used to classify multiple cellular pathways involved in cell survival and inflammation,including phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) and hypoxiainducible factor 1 signaling pathways (Castelli et al., 2021).Specifically, the majority of genes involved in the hypoxia-inducible factor 1 pathway were identified as targets of upregulated microRNAs within EVs.This observation suggests a potential global switch-off of the hypoxia-inducible factor 1 signaling pathway, which has been previously associated with the production of ROS (Qin et al., 2022).Co-culturing neuroblast cells with placental MSCs resulted in enhanced cell viability, as determined by cytotoxicity assays, and a reduction in caspase activity.The functional analysis of exosomes from placental MSCs further revealed the enrichment of miRNAs involved in the negative regulation of neuronal apoptosis and axonal guidance (Kumar et al., 2019).Furthermore, network analysis of proteome data demonstrated a strong association of proteins with focal adhesion and PI3K/AKT pathways.
The promotion of endogenous neurogenesis and angiogenesis
Multiple preclinical experiments have consistently demonstrated that stem cell transplantation enhances endogenous neurogenesis in the SVZ and hippocampal regions.The utilization of omics analysis can significantly contribute to uncovering additional mechanisms underlying the neurogenic activities of stem cells.In a clinical trial conducted by Bang et al.(2022),chronic stroke patients who received MSCs intravenously showed an increase in the number of EVs in their blood samples.Importantly, the increased levels of EVs correlated directly with the integrity of the cerebrospinal tract and functional recovery (Bang et al., 2022).Further analysis of these EVs by miRNA sequencing revealed the significant enrichment of microRNAs associated with neurogenesis.Notably, miRNA-18a-5p was identified to target multiple pathways, including neurotrophin signaling and axonal guidance, thus highlighting its potential role in promoting neuronal growth and development.The transcriptome profile of rat brain tissues subjected to MCAO and treated with a medium conditioned with neural progenitor cells revealed the upregulation of genes associated with central nervous system development, synaptic transmission, and neurogenesis, while genes associated with inflammatory responses were down-regulated (Hur et al.,2022).Furthermore, comparative analysis demonstrated that multiple injections of a medium conditioned with neural progenitor cells resulted in the increased expression of numerous markers of neurogenesis just 15 days after treatment, including roundabout guidance receptor 3 (ROBO3), leucine rich repeat and Ig domain containing 1 (LINGO1), neuronal differentiation 2 (NEUROD2), and brain-derived neurotrophic factor (BDNF).This study demonstrated that the administration of multiple injections of a medium conditioned with neural progenitor cells is a feasible approach that can promote neurogenesis after ischemic stroke.
Alterations in protein expression have also been observed in the cerebrospinal fluid after ischemic stroke.In a study by Hur et al.(2021), MCAO rats that received the transplantation of MSCs exhibited significant changes in protein expression.The MSC group showed notable enrichment in neurotrophin and glycosaminoglycan metabolism, as well as nerve growth factor-associated signalling pathways (Hur et al., 2021).Of the proteins associated with these pathways, the levels of 14-3-3θ, myelin-associated glycoprotein, and neurocan core proteins were significantly upregulated.Although these authors proposed the potential role of these proteins as biomarkers for monitoring the neurogenic effect of MSC-based therapy, further clarification is needed.
Multiple studies have demonstrated the notable impact of cell-based therapy for enhancing angiogenesis, highlighting the beneficial role of vascularization in facilitating neurogenesis following ischemic stroke.Proteomic analysis has revealed the significant upregulation of proteins involved in angiogenesisrelated pathways, including epidermal growth factor, fibroblast growth factor,and platelet-derived growth factor, within both MSCs and MSC-derived exosomes under ischemic conditions (Anderson et al., 2016).Network analysis has further revealed that exosomes derived from MSCs exhibit enrichment of networks associated with the nuclear factor kappa B signalling pathway, which is known to play a pivotal role in the regulation of angiogenesis.Another study demonstrated that human cerebral microvascular endothelial cells exposed to EVs isolated from hypoxic MSCs showed alterations in miRNA expression;the upregulated miRNAs were mainly associated with angiogenesis, whereas those with anti-angiogenic effects were down-regulated (Gregorius et al.,2021).
Challenges to the Application of Omics Analysis to Stem Cell-based Therapy
Although omics data have provided many insights into the pathophysiology of stroke and the mechanistic action of stem cells, there is some skepticism when new omics technologies are compared with traditional research methodologies.Karczewski and Snyder (2018) summarized the common barriers confronted by the application of omics studies for clinical research,including analytical problems, data accuracy, interpretation, the selection of relevant tissues, and actionability.Similar challenges are faced by stem cell research.The analysis of molecular samples in omics studies requires high-throughput analytical approaches, thus generating huge amounts of data when compared to the limited sample size available.In addition, cell heterogeneity presents a significant challenge to the accurate interpretation of omics data.Stem cells, being a diverse population, undergo dynamic changes throughout differentiation and development.Consequently, single sequencing analysis can fail to capture the time-dependent molecular events occurring within the brain.Achieving data reproducibility becomes even more difficult in a clinical setting due to variabilities among cohorts,rendering molecular events in one individual incomparable to those in others.Moreover, tissue availability poses a major barrier to the application of omics in clinical trials for stem cell research.Obtaining brain tissue is arduous in a clinical setting and associated with high operational risks.Therefore, it is crucial to identify a more relevant tissue type that reflects changes in the brain and discover stable biomarkers that can be investigated in future clinical research.
Suggestions on Future Directions
Most previous studies only analyzed one type of omics data at a specific time point, thus limiting our comprehensive understanding of stem cell activities after cell transplantation.Recent advancements in omics methodologies offer some potential solutions to overcome these limitations.For instance, singlecell sequencing enables the investigation of cellular heterogeneity among transplanted stem cells.Spatial transcriptomes provide spatially resolved gene expression profiles and protein distributions, thus allowing researchers to monitor the activities of stem cells within complex tissue architectures.Moreover, the application of advanced computational approaches would also improve the integration of different types of data.Multi-omics correlation analysis involves investigating the relationships and associations between different omics layers, thus providing valuable insights into the interplay and regulatory connections within stroke models following stem cell transplantation(Campbell et al., 2022).However, standardized methods to integrate disparate omics data into a collective result are lacking and are further complicated by the dynamic nature of RNAs, proteins, and metabolites.Artificial intelligence and machine learning show great promise in accelerating the process of data standardization and integration (Takahashi et al., 2021).Machine learning can be trained on large datasets to learn the patterns of omics data and identify anomalies.Artificial intelligence can also ensure reproducibility and reduce human error by automating standardized analysis pipelines for processing omics data.Taken together, the new methodologies and computational tools of omics have enormous potential to accelerate discovery in stem cell research.Nevertheless, data quality control and consistency also need to be fully considered to ensure that data is reliable and interpretable.
Conclusion
The pathophysiology of ischemic stroke is complicated and involves the neuroinflammatory process, neuronal apoptosis, and various types of cells that modulate the ischemic microenvironment.In addition to the known aspects of ischemic stroke, omics analysis can assist in the discovery of biomarkers for the onset of stroke and indicators for patient prognosis.Transcriptomes have also been used to determine the heterogeneity of the major cell types involved in the pathogenesis of ischemic stroke, including microglia, astrocytes, and endogenous neural stem cells.The complexity of ischemic stroke requires multiple approaches to restore physiological functions in post-stroke patients.Stem cell-based therapy has shown great promise in promoting stroke recovery by modulating neuroinflammation,mediating neuroprotection, and facilitating angiogenesis and neurogenesis.However, despite years of preclinical research, there are still restrictions that prevent stem cells from becoming a treatment option.Recent omics analysis of stem cell transplantation has augmented our understanding of the effects of stem cells in stroke models.The effects of stem cells on the brain or specific cells have been well demonstrated at transcriptomic, proteomic, and metabolomic levels.Such clarification of stem cell mechanisms is essential for future treatment modifications and improvements in stem cell efficacy.
Author contributions:Initial literature search and original draft preparation:YW; manuscript writing and revision: CC, XL, XB; funding acquisition: RW, XB.All authors approved the final version of this manuscript.
Conflicts of interest:There are no conflicts of interest.
Data availability statement:Not applicable.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- NADPH oxidase 4 (NOX4) as a biomarker and therapeutic target in neurodegenerative diseases
- Circadian rhythm disruption and retinal dysfunction:a bidirectional link in Alzheimer’s disease?
- Interplay between the glymphatic system and neurotoxic proteins in Parkinson’s disease and related disorders: current knowledge and future directions
- Roles of neuronal lysosomes in the etiology of Parkinson’s disease
- Therapeutic advances in neural regeneration for Huntington’s disease
- Emerging role of galectin 3 in neuroinflammation and neurodegeneration

