Rebuilding insight into the pathophysiology of Alzheimer’s disease through new blood-brain barrier models
Kinya Matsuo, Hideaki Nshihara
Abstract The blood-brain barrier is a unique function of the microvasculature in the brain parenchyma that maintains homeostasis in the central nervous system.Blood-brain barrier breakdown is a common pathology in various neurological diseases, such as Alzheimer’s disease, stroke, multiple sclerosis,and Parkinson’s disease.Traditionally, it has been considered a consequence of neuroinflammation or neurodegeneration, but recent advanced imaging techniques and detailed studies in animal models show that blood-brain barrier breakdown occurs early in the disease process and may precede neuronal loss.Thus, the blood-brain barrier is attractive as a potential therapeutic target for neurological diseases that lack effective therapeutics.To elucidate the molecular mechanism underlying blood-brain barrier breakdown and translate them into therapeutic strategies for neurological diseases, there is a growing demand for experimental models of human origin that allow for functional assessments.Recently, several human induced pluripotent stem cell-derived bloodbrain barrier models have been established and various in vitro blood-brain barrier models using microdevices have been proposed.Especially in the Alzheimer’s disease field, the human evidence for blood-brain barrier dysfunction has been demonstrated and human induced pluripotent stem cell-derived blood-brain barrier models have suggested the putative molecular mechanisms of pathological blood-brain barrier.In this review, we summarize recent evidence of blood-brain barrier dysfunction in Alzheimer’s disease from pathological analyses, imaging studies, animal models, and stem cell sources.Additionally, we discuss the potential future directions for blood-brain barrier research.
Key Words: Alzheimer’s disease; blood-brain barrier; human induced pluripotent stem cells
Physiology of the Blood-Brain Barrier and its Role in Neurodegenerative Diseases
The brain parenchyma is surrounded by a hard skull, and access to it by external interference in the absence of trauma or other events is limited.The main routes of approach to the central nervous system (CNS) from the outside world or peripheral tissues are transvascular, translymphatic, or transneural.The presence of a lymphatic system in the CNS is controversial and out of the scope of the present review (Proulx, 2021).In these three routes, the vasculature of the CNS is the main interface connecting the isolated CNS with the peripheral milieu.However, the brain microvasculature is not simply a “pipe” in the brain and has unique characteristics.The bloodbrain barrier (BBB) is the specific function of the microvasculature in the brain parenchyma and contributes to maintaining CNS homeostasis.The BBB is composed mainly of brain microvascular endothelial cells (BMECs),pericytes sandwiched between the basement membrane of the BMECs and the basement membrane of the parenchyma and end-feet of astrocytes(Figure 1).These cells are major players in the neurovascular unit (NVU),and system responsible for coordinating the peripheral environment and the CNS (Castro Dias et al., 2019).The choroid plexus also has a barrier function known as the blood-cerebrospinal fluid barrier, in which epithelial cells instead of endothelial cells form the barrier function (Castro Dias et al., 2019).The blood-cerebrospinal fluid barrier is another important transvascular route to the brain, but the present review will focus primarily on the BBB.Within the components of the NVU, BMECs play an essential role in providing BBB functions.BMECs establish continuous tight junctions, exhibiting remarkably diminished pinocytotic activity relative to the microvascular endothelial cells found in peripheral organs.These complex tight junctions inhibit the paracellular diffusion of water-soluble molecules into the CNS.BMECs also express specific transporters and efflux pumps on their surface, which take up essential nutrients from peripheral blood or export harmful molecules from the CNS.Furthermore, the restricted expression levels of endothelial adhesion molecules on the surface of BMECs strictly control immune cell trafficking into the CNS (Marchettiand Engelhardt, 2020).Under pathological conditions, the level of expression of endothelial adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), is upregulated compared with that under physiological conditions and is crucial for immune cell infiltration into the CNS (Marchettiand Engelhardt, 2020).
In dementia, regardless of the underlying disease etiology, dysfunction of the BBB is observed as a common phenomenon, especially in the late stage(Skillback et al., 2017).Recent advances in imaging have suggested that BBB abnormalities are observed from the early stage of the disease (Yuan et al.,2023); however, its detailed pathophysiology remains unclear.Elucidation of the molecular mechanisms underlying disease-specific or disease-common BBB dysfunction could lead to the development of novel therapeutic strategies directly targeting the BBB.This could be particularly important for neurological disorders where effective treatments have been lacking thus far.To this end, investigation of the details of the mechanisms of BBB dysfunction in each disease is warranted.
In this review, we focus on Alzheimer’s disease (AD), the most prevalent cause of dementia and known to be associated with BBB dysfunction.First,we will summarize the evidence of BBB changes in AD from humans, mainly from autopsy samples and imaging or biomarker studies.We then describe the molecular mechanisms of BBB dysfunction that have been elucidated by studies using animal models.Although studies in mice have elucidated many detailed molecular mechanisms, species differences cannot be ignored when considering therapeutic applications in humans.Moreover, due to the complexity of BBB functions maintained by various cell types, an experimental model is required in that it can be developed step-by-step from simple studies on a cell-by-cell basis to more complex systems.Therefore, human induced pluripotent stem cell (hiPSC)-derived BBB models have emerged as new tools in BBB research to address these challenges.Each experimental model has advantages and disadvantages, summarized in Table 1, and researchers must choose appropriate models or methods according to the study.We also discuss the current status and future directions associated with iPSC-derived BBB models.
Search Strategy
We have searched all publications from the PubMed database using the keywords such as Alzheimer’s disease, blood-brain barrier, and human induced pluripotent stem cells on 1stSeptember, 2023.All years were chosen in the search.
Evidence for Blood-Brain Barrier Alterations in Alzheimer’s Disease in Humans
AD is the most prevalent cause of dementia in older adults.Amyloid plaques,consisting of extracellular deposits of polymerized amyloid-β (Aβ) and neurofibrillary tangles, formed by abnormal accumulation of phosphorylated tau in neurons, are pathological hallmarks of the disease (Hyman et al.,2012).From a pathophysiological perspective, there are three major putative alterations of BBB function related to disease, that is, (1) increased paracellular transfer due to disruption of tight junctions causes entry of blood molecules, (2) accumulation of abnormal proteins due to decreased or increased transporter function, and (3) increased infiltration or interaction of peripheral immune cells, resulting in neuroinflammation.First, we review whether the findings regarding BBB alterations are actually observed in the brains of AD patients (Figure 1).Pathological changes in the BBB have been confirmed in numerous human autopsy cases.Plasma proteins such as fibrin,immunoglobulin G (IgG), and prothrombin, which are not present in the CNS under physiological conditions, were found to leak around blood vessels in the brains of AD patients (Hultman et al., 2013; Halliday et al., 2016;McAleese et al., 2019; Kirabali et al., 2020).Perivascular fibrin correlates with interruptions of tight junction proteins, such as ZO-1 (Fiala et al., 2002).An ultrastructural study showed a substantial presence of pinocytotic vesicles and cytoplasmic inclusions in the endothelial cells in the hippocampus of AD patients (Baloyannis and Baloyannis, 2012).An ELISA using a lysate of multiple brain lesions found decreased cortical tight junctional molecules such as claudin-5 and occludin in AD patients compared with normal aging individuals, and the expression levels were negatively correlated with tau protein accumulation (Yamazaki et al., 2019; Liu et al., 2020).Immunostaining highlighted the relationship between vascular amyloid depositions with a disrupted tight junction (Carrano et al., 2012), suggesting a possible relevance of BBB dysfunction to the process of plaque accumulation.
The specific transporters in the endothelium are involved in Aβ transportation.P-glycoprotein (P-gp) and lipoprotein receptor-related protein 1 (LRP-1) are expressed on the luminal (blood-facing) side and abluminal(parenchyma-facing) side of endothelial cells, respectively, and are related to Aβ efflux (Zhang et al., 2022).By contrast, advanced glycosylation endproducts (RAGE) are expressed at the luminal side of brain endothelial cells and mediate transcytosis of plasma-derived Aβ.In AD, the alteration of specific transporters has been observed.Several immunostaining studies performed on autopsied brain samples from AD patients have shown decreased expression of P-gp and LRP-1 but increased expression of RAGE in microvessels (Deane et al., 2004; Vogelgesang et al., 2004; Jeynes and Provias, 2008; Chiu et al., 2015; Halliday et al., 2016; Bourassa et al., 2019).However, a proteomics study using isolated BMECs from the hippocampus and parietal lobe did not observe any significant difference in the protein levels of P-gp and LRP-1 between AD patients and control individuals (Storelli et al., 2021).The potential discrepancy between these findings might be a difference in vascular zonation.Isolated BMECs in the latter study analyzed all types of endothelial cells in the brain parenchyma; however, recent single-cell RNA sequencing studies have shown that transcriptomes differ significantly depending on vascular zonation (Garcia et al., 2022).The expression of specific transporters in AD patients may be altered in a selective population of vascular endothelial cells.
Neuroinflammation also plays a pivotal role in disease progression in AD.In AD, neuroinflammation is often discussed, mainly in the context of brain resident microglial abnormalities (Webers et al., 2020), but infiltrating peripheral immune cells into the CNS also induces neuroinflammation.The BBB is the gatekeeper of peripheral immune cells and actively contributes to immune cell trafficking into the CNS in other neurological diseases(Marchettiand Engelhardt, 2020).Additionally, in AD, there is some evidence in humans of an altered BBB that contributes to immune cell trafficking into the CNS as described following.Isolated microvessels from AD patients highly expressed proinflammatory cytokines, indicating that BMECs contribute to neuroinflammation in AD (Grammas and Ovase, 2001).Furthermore, an increased number of macrophages, neutrophils, natural killer cells, T cells,and B cells infiltrate the vessel wall or perivascular space in brain areas such as the hippocampus and frontal cortex that are typically affected in AD (Fiala et al., 2002; Hultman et al., 2013; Gate et al., 2020; Liu et al., 2023).
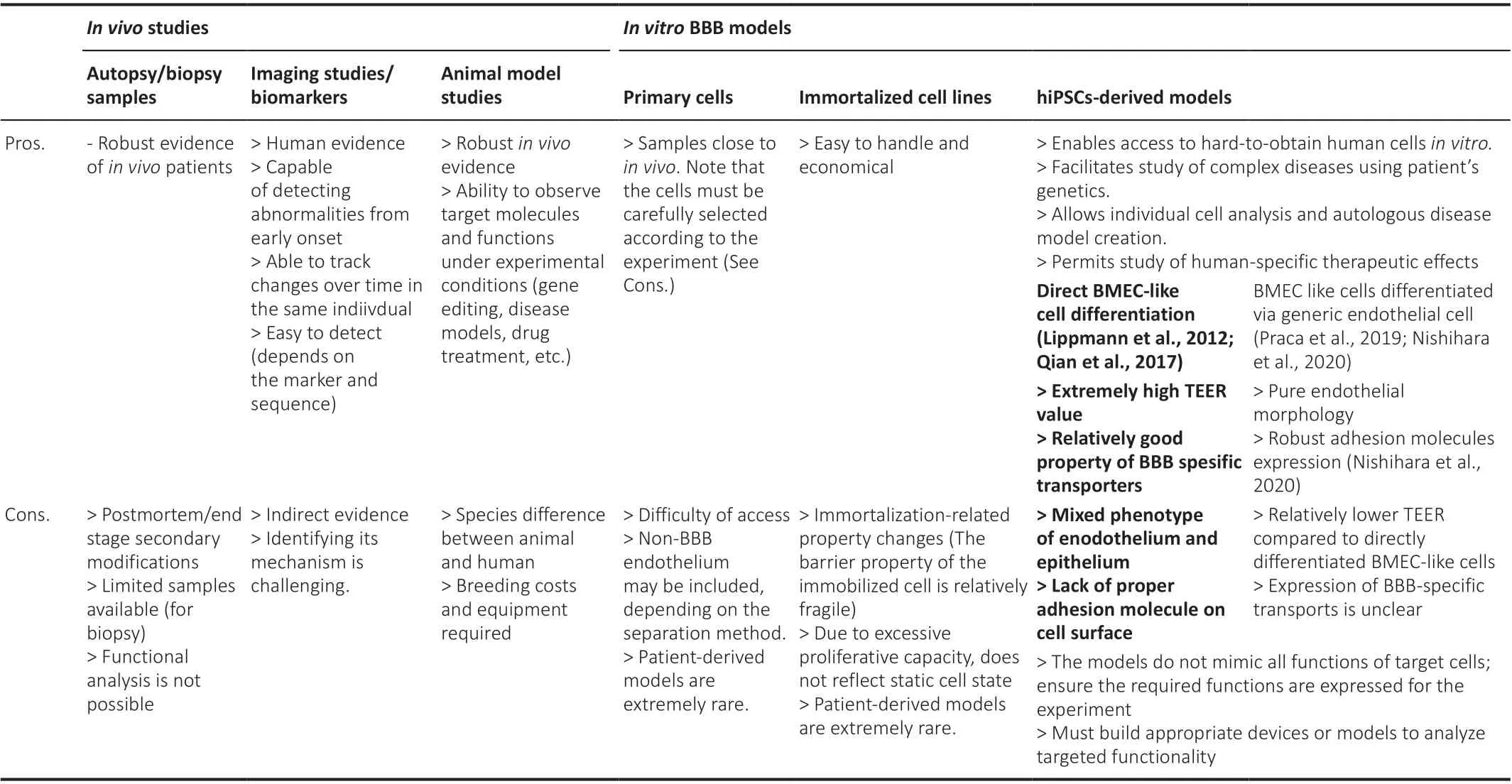
Table 1 |Advantages and disadvantages of different experimental models or studies for blood-brain barrier

Figure 1 | Human evidence for blood-brain barrier alterations in Alzheimer’s disease.Under physiological conditions (right), endothelial cells are interconnected by tight junction proteins with minimal vesicle presence and express suppressed adhesion molecules such as ICAM-1 and VCAM-1 on their surface.Pericytes are embedded in the vascular basement membrane.The postcapillary venule has a perivascular space between the vascular and parenchymal basement membranes.The amyloid peptides are cleared by P-gp and LRP-1 towards the vascular side, while RAGE transports amyloid from the blood to the abluminal (parenchymal) side.Under pathological conditions in Alzheimer’s disease (left), degraded tight junction proteins lead to diffusion of plasma solute molecules.Upregulated adhesion molecules such as ICAM-1 and VCAM-1 may lead to an influx of immune cells into the central nervous system.Although soluble ICAM-1 and VCAM-1 are upregulated in the serum of Alzheimer’s disease patients, it is not clear whether their origin is BMECs or other cells.Expression of P-gp and LRP-1 is decreased, and the protein level of RAGE is upregulated in patients (whether these proteins are polarized to a particular side of BMECs is unclear).Endothelial cells and pericytes are vacuolated and pericytes detached from the vasculature.Immune cells are observed in the perivascular space.The end feet of astrocytes are swollen where the expression of AQP4 is decreased.Created with BioRender.com.AQP4: Aquaporin 4; BM:basement membrane; BMECs: brain microvascular endothelial cells; ICAM-1: intercellular adhesion molecule-1; LRP-1: low density lipoprotein receptor-related protein 1; P-gp:P-glycoprotein; TJ: tight junctions; VCAM-1: vascular cell adhesion molecule-1.
Other cellular components of the BBB are also altered in AD.Pericyte coverage prominently decreases with the progression to the Braak stage of AD and is inversely proportional to plasma protein leakage (Sengillo et al., 2013;Halliday et al., 2016; Kirabali et al., 2020).An ultrastructural study showed an increased number of pinocytotic vesicles and abnormal mitochondria in pericytes (Baloyannis and Baloyannis, 2012).Astrocytes are also structurally and functionally related to AD, and reactive astrocytes, which highly express glial fibrillary acidic protein, are increased in the AD brain (Serrano-Pozo et al., 2013; Gomez-Arboledas et al., 2018; Kirabali et al., 2020).The endfeet of perivascular astrocytes swell (Higuchi et al., 1987); however, the coverage of small capillaries by astrocytes remains intact (Kirabali et al., 2020).Aquaporin 4 is a water channel highly expressed in the end-feet of astrocytes, and its perivascular staining was decreased in small vessels in the frontal cortex of AD patients (Zeppenfeld et al., 2017), suggesting that perivascular astrocytes are also altered even when they are attached.
Most evidence for BBB dysfunction in humans is derived from histological studies obtained from end-stage AD patients.Because the functions of the BBB are maintained by various types of cells in the CNS, the loss and dysfunction of each cell due to neurodegeneration consequently leads to secondary BBB breakdown, and the autopsy sample cannot exclude secondary postmortem effects.In addition, pathological aggregation such as Aβ and tau protein themselves also evokes vascular injury and inflammation (Wang et al., 2021).Therefore, it is difficult to determine whether BBB dysfunction is a causative factor in the onset and progression of the disease or merely a consequence of neurodegeneration using histology.The advanced imaging technology and biomarkers described in the next section may provide a clue.
Evidence for Blood-Brain Barrier Dysfunction in Early-Stage Alzheimer’s Disease: Does Blood-Brain Barrier Dysfunction Proceed with Neurodegeneration?
Biomarkers in cerebrospinal fluid (CSF) have been used to detect BBB dysfunction in living individuals.The Qalb index, which is the ratio of CSF to blood albumin and is an indicator of how much serum Alb is leaking into the CNS, has been a conventional biomarker of BBB leakage (Skillback et al., 2017).AD patients had increased Qalb (Skillback et al., 2017), which correlates with the progression of cognitive impairment (Bowman et al.,2007).A systematic review showed that Qalb levels are not only correlated with aging and diagnosis of AD but are also significantly affected by vascular dementia (Farrall and Wardlaw, 2009).Platelet-derived growth factor beta(PDGFRβ) is a major marker of pericytes, and the soluble form of PDGFRβ in CSF is known to correlate with pericyte damage (Montagne et al., 2015).The elevation of soluble PDGFRβ in the CSF seems to be more sensitive from a very early stage of AD (Miners et al., 2019; Montagne et al., 2020).Although there are some other possible biomarkers to detect BBB breakdown (Kapural et al., 2002; Marchi et al., 2003; Gubern-Merida et al., 2022; Table 2), these biomarkers are indirect evidence of BBB damage and could be upregulated by other conditions.Reliable blood markers are required in the clinical setting that are easier to obtain and less expensive than traditional gadoliniumenhanced magnetic resonance imaging (MRI).

Table 2 | Possible biomarkers of damaged blood-brain barrier
The advanced imaging techniques have allowed us to detect BBB dysfunction at an early stage of the disease, i.e., in patients with clinically mild cognitive impairment (MCI) or before the detective accumulation of pathological proteins.First, dynamic contrast-enhanced MRI using a 3T scanner and a 1 kDa gadolinium-based contrast agent has shown that MCI patients without evidence of hippocampal atrophy have increased BBB permeability in the hippocampus (Montagne et al., 2015).BBB permeability in the hippocampus and parahippocampal gyrus of MCI patients was elevated when a patient had no evidence of increased CSF Aβ and phosphorylated tau levels, suggesting that BBB leakage was likely to be directly related to cognitive function and precedes pathological protein accumulation (Nation et al., 2019).Water permeability in the brain can be estimated by a new MRI technique, in which arterial blood in the cervical spine region is labeled by spin echo, and extravasated water in the brain is estimated by subtracting labeled blood volume in the superior sagittal sinus from total labeled arterial blood (Lin et al., 2021).Water permeability in the brain of MCI patients was elevated even when the Qalb index was not yet elevated, suggesting that BBB dysfunction occurs earlier than previously thought.Although whether Qalb is elevated in the MCI phase remains controversial (Montagne et al., 2015; Lin et al.,2021), the elevation may be due to insufficient sensitivity of Qalb, and advanced imaging techniques may resolve this inconsistency.Additionally,positron emission tomography studies using specific tracers are also useful for detecting the BBB function of each transporter in living patients.A significantly decreased activity of P-gp in the cortices and hippocampus of mild to moderate AD patients was detected (Deo et al., 2014).Thus, BBB dysfunction may not be merely a secondary injury at the end stage but may begin upstream in AD pathophysiology.Moreover, a comprehensive single RNA sequence analysis of the AD brain revealed that 30 of the top 45 genes reported to be associated with AD risk in genome-wide association studies are expressed in brain endothelial cells (Yang et al., 2022), suggesting that endothelial alteration is critical.
While advanced imaging techniques can reveal BBB dysfunction, their use to explore the underlying mechanism remains difficult.In other words, they can estimate BBB function to some extent, e.g., quantitative measurements of permeability and specific uptake of substrates.However, they are not yet sufficient to investigate detailed molecular mechanisms, such as whether increased permeability is due to permeation of paracellular or transcellular pathways or whether decreased transporter function is due to decreased protein expression or altered localization.Therefore, animal models are widely used to study the pathophysiology of BBB dysfunction in AD.
Blood-Brain Barrier Breakdown in Alzheimer’s Disease Model Mice and Underlying Molecular Mechanisms
In this section, we will summarize the studies in vitroandin vivo, mainly using rodent models, which have contributed greatly to elucidating the detailed mechanisms of BBB dysfunction in AD pathophysiology.BBB abnormalities in AD are described along with each of the three major BBB functions.
Increased paracellular transfer due to disruption of tight junctions causes entry of blood molecules
Apolipoprotein E (APOE), a protein involved in the metabolism and transport of lipids such as cholesterol, is secreted mainly by astrocytes in the CNS.APOEhas genetic polymorphisms ε2, ε3, and ε4.APOE3is the most common allele, andAPOE4is known as the most prominent risk factor for AD, whileAPOE2is known to act suppressively against disease onset (Wisniewski and Drummond, 2020).APOE has been studied extensively to understand the pathogenesis of AD, and this section focuses on the effect of APOE on BBB function, especially barrier tightness.Interestingly, in addition to AD,APOE4is also a common risk factor for many diseases associated with BBB dysfunction, including cerebral amyloid angiopathy, Lewy body dementia,multiple sclerosis, vascular dementia, ischemic stroke, and traumatic brain injury, however, in age-related macular degeneration in which excessive angiogenesis causes pathological neovascularization, theAPOE4allele works protectively (Safieh et al., 2019).Thus, APOE might play a critical role in various diseases by suppressing essential angiogenesis or inhibiting the physiological functions of the microvasculature.HumanAPOE4knock-in mice provide evidence of BBB breakdown before neurodegeneration, as described following.BBB breakdown detected by decreased tight junction protein and leakage of 40 kDa dextran and IgG was already seen in 2-week-oldAPOE4mice (Bell et al., 2012), when neuronal density, expression levels of preor postsynaptic proteins, and cortical neural activity were still normal.The same investigators analyzed the transcriptomes, phosphorylation sites, and proteomes of cells composing the NVU from young and middle-aged humanAPOE3orAPOE4knock-in mice (Barisano et al., 2022).Endothelial cells derived from 2–3-month-oldAPOE4mice, in which BBB breakdown already appears, expressed upregulated transcriptomes related to junctions and transporters, which is likely to be a compensatory cell response to stabilize the dysfunctional BBB.However, at 7 months,APOE4mice showed decreased or abnormally phosphorylated proteins related to cell junctions and clathrinmediated transport controlling endocytosis of the transporter at the cell membrane.Subsequently, at 9–12 months, the compensatory transcriptome expression seen in the young mice disappeared and was replaced by increased expression of the transcriptome, which promotes intracellular trafficking and enzymatic BBB disruption.InAPOE4-mice, the disappearing compensatory transcriptome expression over time was also seen in pericytes,astrocytes, and microglia.Such pathway analysis is useful for elucidating the mechanism of BBB dysfunction.Immunohistochemical studies of autopsied human brains revealed that the presence of the ε4 allele is associated with increased fibrin or Aβ deposition in the vessel wall and plasma protein leakage in the brain parenchyma, decreased pericyte coverage (Hultman et al., 2013; Halliday et al., 2016; Liu et al., 2020).Contrast-enhanced MRI detects higher vascular permeability in the hippocampus ofAPOE4carriers than in noncarriers, even when they are not suffering from dementia(Montagne et al., 2020; Moon et al., 2021).How does APOE contribute to BBB dysfunction? Mice selectively transfected with humanAPOE2,APOE3, orAPOE4in astrocytes with an APOE-null background showed leaky BBB only in APOE4-mice (Bell et al., 2012).Interestingly, the APOE-null mice also showed a leaky barrier detected by vital multiphoton microscopy via a cranial window above the parietal lobe.This finding suggests that APOE2 and APOE3, but not APOE4, secreted from astrocytes maintain BBB function.Secreted APOE binds to LRP-1 and inhibits the activation of proinflammatory cytokines such as cyclophilin A (CypA), promoting the production of matrix metalloproteinase-9,which disrupts basement membranes and reduces tight junction proteins.The ability to inhibit CypA is lower in APOE4 than in other isoforms—despite the higher affinity of APOE4 for LRP-1 (Cooper et al., 2021)—suggesting that this pathway is responsible for BBB dysfunction inAPOE4carriers.Although LRP-1 is highly expressed in pericytes and astrocytes in the NVU, the CypA pathway via LRP-1 is also inhibited in endothelial cells (Nikolakopoulou et al.,2021).The expression levels of CypA and matrix metalloproteinase-9 were much higher in the CSF and endothelial cells and pericytes of AD patients carryingAPOE4than it was in healthy individuals and AD patients withAPOE3(Halliday et al., 2016; Montagne et al., 2020).Interestingly, the CypA pathway inAPOE4carriers is activated even when the individuals with AD are cognitively normal (Montagne et al., 2020), indicating that the cascade of BBB breakdown has started in the prodromal stage of AD.APOE is also present in the peripheral blood, and the next question is whether peripheral APOE also affects the BBB.In postoperative liver transplant patients, APOE alleles in blood and cerebrospinal fluid showed that more than 90% of recipient blood APOE was replaced by donor alleles, while the cerebrospinal fluid APOE remained the recipient allele (Linton et al., 1991).Furthermore, transgenic mice in which humanAPOE3orAPOE4was specifically expressed in the liver on anAPOEknockout background showed that human APOE is present only in the periphery (Liu et al., 2022).These data suggest that BBB separates APOE in the CNS and peripheral blood.Expression of human APOE4 in the liver reduced endothelial tight junctions, thinned and fragmented the basement membrane, caused serum protein leakage, and impaired cognitive function(Liu et al., 2022).The investigators suggested that these phenomena are due to changes in APOE isoform-dependent humoral factors in the periphery that affect the BBB rather than APOE itself.Peripheral human APOE3 expression improved cognitive function and reduced amyloid deposition (Liu et al.,2022).Furthermore, serum from young mice expressing peripheral human APOE3 alleviated the failing BBB function in aged mice (Liu et al., 2022), but it is not clear whether peripheral APOE3 expression has the same function in maintaining the BBB as human APOE3 expressed in the CNS (Bell et al., 2012).APOE is expected to be further investigated to help elucidate how the BBB fails in neurodegenerative diseases.
Accumulation of abnormal proteins due to decreased or increased transporter function
Both P-gp and LRP1 expressed on the surface of endothelial cells play major roles in the clearance of Aβ from the interstitial fluid of the brain (Storck et al., 2016, 2018a; Chai et al., 2020).What molecular pathways are involved in the excretion of Aβ by these transporters? When Aβ binds to LRP1 and alters its intracellular domain in mouse and human BMEC lines, phosphatidylinositol binding clathrin assembly protein attaches to the complex and induces clathrin-mediated endocytosis, which further directs the complex into the endosome (Zhao et al., 2015).Inhibition of both LRP-1 and P-gp has no synergistic effect compared with their inhibition, indicating that these molecules act through the same pathway for Aβ excretion (Storck et al.,2018b).It is noteworthy that mutation of phosphatidylinositol binding clathrin assembly protein has been reported as a genetic risk for AD (Bellenguez et al.,2022), and this pathway seems to play an essential role in Aβ transportation.RAGE is known to accumulate Aβ by acting as an influx pump but also through many pathological pathways in AD, such as neuroinflammation and oxidative stress (Juranek et al., 2015).Other specific receptors and transporters of Aβ,such as ATP-binding cassette (ABC)C1, ABCG2, and ABCG4, have also been implicated in Aβ efflux and may be therapeutic targets (Xiong et al., 2009;Krohn et al., 2011; Dodacki et al., 2017).
Increased immune cell infiltration results in neuroinflammation
Neuroinflammation is widely thought to be a secondary event in AD pathophysiology; however, there is accumulating evidence that it plays a strong aggravating role.The plasma proteins that leak across the BBB, such as fibrinogen, strongly activate microglia, resulting in neurodegeneration,as demonstrated in a mouse study using microglia-specific deletion of the fibrinogen-binding motif gene (Mendiola et al., 2023).In addition, because the BBB controls not only the permeation of soluble molecules but also the infiltration of inflammatory cells, the recruitment of peripheral immune cells by the pathological BBB can also exacerbate neuroinflammation.Indeed,immune cells accumulate in the brains of a mouse model of AD (Browne et al., 2013; Wang et al., 2015; Zenaro et al., 2015) as well as AD patients.The Aβ peptide induces lymphocyte function-associated antigen 1 to a highaffinity state and results in increased neutrophil trafficking into the brain parenchyma, and deleting or blocking lymphocyte function-associated antigen 1 reduces neutrophil infiltration and the accumulation of Aβ and phosphorylated tau protein, thereby restoring cognitive status (Zenaro et al.,2015).To study the effect of peripheral immunity on AD, Aβ-specific CD4+T cells were generated and injected into the periphery of amyloid precursor protein (APP)/PS1 mice (Browne et al., 2013).The injected Th1-cells but not Th2- or Th17- cells infiltrated the CNS and activated microglia, Aβ deposition,and abnormal cognitive behavior.By contrast, increased Th17-cells in the peripheral blood in AD patients have been reported (Oberstein et al., 2018),and an adaptive transfer study showed that Th17 cells contribute to disease progression in the same APP/PS1 mice.This discrepancy might arise from differences in the T cell culture environment or markers used to identify cell populations.These findings indicate that peripheral immune cells contribute to neuroinflammation.Moreover, sera from aged mice activated microglia and impaired neural progenitor cell activity in young mice, and deletion of the brain endothelial and epithelial-specific gene for VCAM-1 in young mice reduced the neuroinflammatory effect of sera from aged mice (Yousef et al.,2019).Whether the neuroinflammation is induced by infiltrating immune cells via activated endothelial cells or whether activated endothelial cells themselves activate microglia remains unclear; either way, the findings suggest that BMEC activation induces neuroinflammation.
Limitations of Animal Models and Advantages of a Human Induced Pluripotent Stem Cells-Derived Model In Vitro
Except for some types of nonhuman primates, at least rodents do not develop AD pathology, so heterologous gene transfer is necessary to reproduce ADlike pathology in rodents (Neff, 2019).Most rodent models of AD involve transfected human genes derived from autosomal dominant familial AD,such as APP and presenilin 1/2 (PSEN1/2).However, the familial form of AD is only a part of the AD population, and the majority of sporadic AD develops through a complex mix of multiple genetic and environmental factors.Moreover, although transgenic mice recapitulate some of the specific aspects of AD pathogenesis, they cannot yet fully reproduce human AD pathology,and it is important to understand the characteristics and limitations of each model and to use it accordingly (de Sousa et al., 2023).Moreover, it should be noted that APOE construction and function differ between mice and humans,so humanAPOEis required for transfection (Balu et al., 2019).From a BBB perspective, mouse and human BMECs also differ in specific transporters and endothelial adhesion molecules (Lecuyer et al., 2017; Song et al.,2020).Therefore, models of human originin vitro, in which function can be analyzed, are needed for further study of AD.Advanced stem cell technology has recently allowed us to induce various types of cells from hiPSCs derived from the somatic cells of patients or healthy individuals.There are several major advantages of hiPSC-derived models compared with animal models and primary or immortalized cell lines.(1) Human cells that would otherwise be difficult to obtain from healthy individuals or patients can be producedin vitro.For example, brain tissue is difficult to obtain from patients who do not require biopsy or treatment for diagnosis or treatment.(2) Using cells with all of a patient’s genetic information makes it possible to study the characteristics of diseases, such as AD, in which multiple genetic factors are probably involved(Bellenguez et al., 2022).Conversely, combining gene editing techniques allows the effects of a target gene to be compared in an isogenic background.(3) Through the differentiation of each component cell with the patient’s genetic information, disease gene effects on each cell type can be analyzed individually.Additionally, it is possible to study the interactions between cell types in the disease by creating an autologous disease model that combines multiple cell types from patient-derived hiPSCs or by incorporating patientderived cells one at a time into models derived from healthy individuals.(4)Patient-derived cells can be used to study the effects of therapeutic agents that differ between species or in diseases unique to humans.
Regarding each cell type comprising the BBB, several iPSC-derived pericytes(Stebbins et al., 2019; Jeske et al., 2020) and astrocytes (Perriot et al., 2018;Leventoux et al., 2020) have been established, and in particular, several models for BMECs, the main actor with barrier properties, have been created(Lippmann et al., 2012; Hollmann et al., 2017; Qian et al., 2017; Praca et al., 2019; Nishihara et al., 2020; Lu et al., 2021).Of course, like other methods, the hiPSC-derived model of the BBB is not ideal and currently has some limitations that should be noted.iPSC-derived cells mimic only a subset of target cells and do not currently reproduce all of their functions.It is important to confirm in advance whether the model expresses the functions required for the intended experiment, such as (1) tightness of the paracellular diffusion barrier, (2) low nonspecific transcellular transport or expression of specific transporters and receptors, and (3) expression of adhesion molecules that control immune cell trafficking.Studies using stem cell technology in the field of AD are also accumulating.BMEC-like cells derived from hiPSCs of a familial AD patient with aPSEN1mutation showed a decreased transendothelial electrical resistance, which reflects the ability to inhibit ion diffusion, and showed some transcriptomic distinctions compared to isogenic hiPSC-derived BMEC-like cells (Oikari et al., 2020).These studies indicate that AD patients’ derived BMEC-like cells themselves have a leaky barrier in this genetic background; however, the detailed mechanism of this phenotype remains elusive.Other investigators have also analyzed BBB function in BMEC-like cells derived from hiPSCs withPSENmutations (Kattet al., 2019; Raut et al., 2021).They found hiPSC-derived BMEC-like cells showed lower transendothelial electrical resistance, higher small tracer permeability,and lower transporters and efflux properties, but the studies only compared single, nonisogenic clones, making it difficult to determine whether this phenotype is due to the mutation itself.Interestingly, a recent study using iPSC-derived BBB organoids from patients with AD revealed not only the abovementioned three aspects of barrier functions but also the impact of the BBB on Aβ accumulation (Lin et al., 2018).Using the CRISPR/Cas9 system, the investigators generated hiPSCs carryingAPOE3orAPOE4from an isogenic hiPSC line and differentiated them into BMEC-like cells, pericytes,and astrocytes.Then, these three components of the BBB were cultured in hydrogel together and spontaneously assembled to form a vascularlike network within the gel.APOE4-BBB, when cultured with conditioned medium from neurons derived from theAPPduplication hiPSC line, which produces high levels of Aβ, had increased immunoreactivity for Aβ compared withAPOE3-BBB, suggesting that APOE4 expression may promote Aβ accumulation in this system.To determine which cells were responsible for Aβ accumulation, the investigators converted each component of theAPOE3-BBB model toAPOE4-carrying cells one by one.Ultimately, they found thatAPOE4carriers had upregulated APOE expression in pericytes through alteration of a transcription factor, which induced Aβ accumulation.Such organoid models of the BBB are attractive for studying cell-cell interactions.However, the biggest challenge is to evaluate the function of the BBB as a window connecting the peripheral milieu and the CNS.Evidence that the peripheral milieu impacts AD pathology has accumulated as follows.Sera derived from elderly mice impaired synaptic plasticity, proliferation of neural stem cells, and cognitive functions in young mice (Yousef et al., 2019).Additionally, in humans, the plasma levels of systemic inflammatory markers are negatively correlated with the volume of the brain and cognitive function (Walker et al., 2017), and plasma exchange significantly reduced cognitive progression in patients with moderate AD (Boada et al., 2020).Peripheral immunity is correlated with inflammation in the CNS, and more BBB models are needed to study such a BBB-mediated link between the periphery and the CNS.
Future Directions for Models of the Blood-Brain Barrier, Including Human Induced Pluripotent Stem Cell-Derived Models in Vitro
Traditionally, many studies have used 2D models of the BBBin vitrousing Transwell filters, in which BMECs are plated on the apical side of the filters to form a monolayer, and other cell types such as astrocytes and pericytes can be plated in the lower chamber for coculture (Wu et al., 2021).This type of 2D model is very simple and directly analyzes barrier properties and the impact of the peripheral milieu on the CNS, but it has some limitations that cannot be ignored.First, there is no influence of blood flow, which affects endothelial cell characteristics and immune cell-BBB interactions.To overcome this limitation and observe cell-cell interactions under the physiological flow conditions, several flow models have been established (Coisne et al., 2013;Reinitz et al., 2015), but culturing multiple cell types in these models is difficult.Recent BBB modeling with organ-on-tip uses 3D designs that mimic vascular structures, generating a flow that represents blood flow and applies shear stress to the endothelial layer (Wu et al., 2021).The model consists of two distinct cell culture compartments; the first is an open conduit where endothelial cells are seeded to form a monolayer, while the second is isolated by a porous membrane, and pericytes and astrocytes are seeded.The second limitation of 2D models of the BBBin vitro, which cannot be overcome by the organ-on-tip model, lies in that the model does not mimic the direct cell-cell adhesion that occursin vivo.Recently, a model has been developed to reproduce blood flow under cell-cell adhesion, where cells are mixed with fibrinogen and thrombin and seeded to form a self-organizing vascular structure (Campisi et al., 2018).The big difference from previous organoid models is that the vascular endothelium forms the vascular structure and can actually allow blood flow into the vessel, and the permeability of the tracer can be measured.Challenges remain, such as reproducibility of the vessel diameters and cell assembly, the extracellular matrix being different from thatin vivo, and control of fluid velocity.Moreover, neurons are not yet cocultured in such a BBB model.In the future, cocultured neurons in the abluminal side of such a model would be helpful to study whether the peripheral milieu truly affects neurodegeneration across the BBB.A third limitation, which is present not only in the 2D models but also in all BBB models, is with respect to the hiPSC-derived BMEC-like cells themselves.Well-established methods exist to generate hiPSC-derived BMEC-like cells that exhibit strong diffusion barrier properties and express BBB-specific transporters and efflux pumps,facilitating the study of molecular transport mechanisms and drug delivery in the brain, however, the previously identified cells have mixed phenotypes of endothelium and epithelium and lack a comprehensive ensemble of endothelial adhesion molecules required for immune cell interactions with the BBB (Lippmann et al., 2020; Nishihara et al., 2020).A new approach to differentiation using endothelial precursors to generate BMEC-like cells with high endothelial properties, good barrier function, and expression of robust endothelial cell surface adhesion molecules is necessary for immune cell adhesion (Nishihara et al., 2020, 2021; Matsuo et al., 2023).Such a model would be particularly useful for studying the interactions between immune cells and the BBB endothelium.This model has been shown to phenocopy both the disrupted barrier and accelerated immune cell infiltration into the BBB in multiple sclerosis that occursin vivoand to be useful to confirm the efficacy of therapeutic interventions (Nishihara et al., 2022).Once again,it is important to emphasize that there is still no perfect BBB model, and researchers must be familiar with the strengths and limitations of each model and adapt it to their disease or functional studies (Table 1).The further development and combination of modelsin vitroand methods to differentiate hiPSCs will open new avenues for research and treatment of neurodegenerative diseases.
Conclusions
As more studies accumulate, treating BBB dysfunction is becoming a reality in murine models of various diseases.In experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis, BBB sealing by ectopic expression of a tight junction molecule improved clinical scores in the chronic phase (Pfeiffer et al., 2011).Administration of specific surrogates activating Wnt signaling, which is essential for the barrier genesis of BMECs,restored BBB leakage in a mouse model of ischemic stroke (Ding et al., 2023).Inhibiting immune cell trafficking into the CNS via very late antigen-4 and VCAM-1 interaction with BMECs reduces infarct volume and postischemic neuroinflammation (Liesz et al., 2011).BBB dysfunction is a pathological signature not only in AD but also common to many neurological diseases and appears to be upstream in their pathophysiology.BBB therapy promises to shed light on neurodegenerative diseases for which there have been no effective treatments.
Author contributions:Data collection, manuscript writing, preparing tables and figure: KM; manuscript conception and design, revise manuscript: HN;approval of the final version of the manuscript: KM and HN.
Conflicts of interest:The authors declare no conflicts of interest.
Data availability statement:Not applicable.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- NADPH oxidase 4 (NOX4) as a biomarker and therapeutic target in neurodegenerative diseases
- Circadian rhythm disruption and retinal dysfunction:a bidirectional link in Alzheimer’s disease?
- Interplay between the glymphatic system and neurotoxic proteins in Parkinson’s disease and related disorders: current knowledge and future directions
- Roles of neuronal lysosomes in the etiology of Parkinson’s disease
- Therapeutic advances in neural regeneration for Huntington’s disease
- The advantages of multi-level omics research on stem cell-based therapies for ischemic stroke

