Influence of microarc oxidation modes on coatings properties by sodium hexametaphosphate electrolyte
Oleg V. Bashkov, Lyu Lan,2, Xiao Fukun, Zhao Zidong, Tatiana I. Bashkova
(1.Department of Materials Science & Technology of New Materials, Komsomolsk-na-Amure State University, Komsomolsk-on-Amur 681013, Russian Federation; 2.International Cooperation &Exchanges Office, Heilongjiang University of Science &Technology, Harbin 150022, China; 3.School of Mining Engineering,Heilongjiang University of Science &Technology, Harbin 150022, China)
Abstract:The paper aims to study the influence of micro-arc oxidation modes on the properties of the coatings from the plates of aluminum alloy 7075. Employing the dense oxide coatings with low porosity generated by sodium hexametaphosphate electrolyte, the study observes the higher consistence of the linear approximation of the reliability between coating thickness and oxidation time. The results show that along with the increase of oxidation time, the roughness and hardness of the coatings monotonically increases, the pore size increases, and the number of large pores decreases.
Key words:micro-arc oxidation; coatings; aluminum alloy 7075; microhardness; roughness
0 Introduction
Currently, aluminum alloys account for more than 70% of structural materials in the aircraft industry, and are also widely used in other industries that require high specific strength of materials[1-2]. Due to the low hardness of aluminum alloys, their surfaces need to be protected from damage and wear[3- 4]. To protect the surface of aluminum alloys from corrosion and damage, various oxidation methods are widely used, which include chemical oxidation, anodic oxidation, and microarc oxidation (MAO)[5-6]. As a result of oxidation, an oxide layer of a given thickness is obtained on the surface, which is resistant to corrosion and has high strength and hardness, which improves the mechanical properties and ensures a long service life of products or structures.
MAO, like anodic oxidation, is performed in electrolyte solutions under the influence of electric current. A distinctive feature of MAO is that, unlike anodizing, MAO is performed by applying short high voltage pulses from 100 to 500 V. Weak solutions of salts and alkalis are usually used as electrolytes in MAO. Oxide coatings formed in the MAO process are porous and rough, which is associated with the mechanism of action of spark discharges as a result of electrical breakdown of the barrier and oxide layers. The larger the oxide layer is formed on the surface of the alloy, the greater the pulse voltage is required to carry out electrical breakdown of the formed oxide layer. As a result of MAO, an oxide layer with a thickness of up to 100 μm or more can be formed. A thick oxide layer, as a rule, has large pores and deep channels that provide access to the electrolyte to the substrate and are a consequence of local spark breakdowns. During an electrical breakdown, the protective film of the resulting oxide melts, high-temperature oxidation of new adjacent areas of the substrate metal occurs, and the oxide hardens, accompanied by an increase in the thickness of the oxide layer.
The purpose of the work is to study the influence of oxidation time using MAO on the structure and properties of oxide coatings formed on the aluminum alloy 7075 in an electrolyte based on sodium hexametaphosphate with the addition of metasilicate and sodium hydroxide[7].
1 Materials and research methods
To form oxide coatings from sheets of aluminum alloy 7075, samples measuring 30 mm×20 mm were made. The samples were pre-polished to ensure a high quality surface with a roughness of 0.02 μm.
Microarc oxidation was performed on a laboratory digitally controlled MAO setup. The installation software controls thyristors, which provide a given current or electrical voltage pulses[8]. The equipment allows you to generate pulses with a frequency of 300 Hz with variable duty cycle and amplitude, operating in constant voltage or constant current mode. The program controls thyristors that are connected to a three-phase electrical network through a transformer. Therefore, the control of a full-wave rectifier of a three-phase network always provides a frequency of 300 Hz. Current or voltage control is carried out by adjusting the duration of the pulses that control the thyristors.
The coatings were formed at a constant current density of 4.7 A/dm2for various times from 30 to 120 minutes at intervals of 30 minutes. Oxidation was carried out in an aqueous solution of an electrolyte based on sodium hexametaphosphate with the addition of silicate and sodium hydroxide. During the MAO process, a constant electrolyte temperature was maintained at no higher than 30 °C.
The structure and thickness of the coatings were studied using a Hitachi S3400N electron microscope (Japan) in backscattered electrons at a magnification of 1 000 times. The hardness of the coatings was measured using a microhardness tester HMV-2 (Japan), roughness was measured along three lines using a portable meter TR-200 (China).
2 Results and discussions
During the MAO process, the aluminum alloy went through all stages of sparking and the formation of microarc discharges. The sparking process on the surface of the sample is clearly visible in Fig. 1.

Fig. 1 MAO process

Fig. 2 Structure of coating formed on alloy 7075 after oxidation
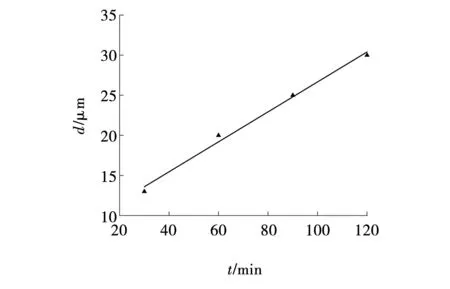
Fig. 3 Dependence of thickness of oxide coating on oxidation time
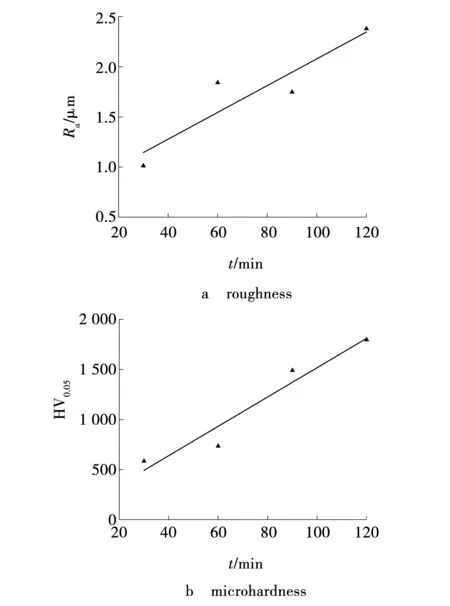
Fig. 4 Influence of MAO time on coating parameters
At the beginning of MAO (15 min), smaller sparks are observed (Fig. 1a). After a significant oxidation time (90 min), the sparks change their color and become rarer and larger (Fig. 1b). This is due to the fact that electrical breakdown of a thicker and denser oxide film requires a higher pulse voltage and higher pulse energy. After spark discharges occurring in the channels and cracks of the already formed coating, high-temperature oxidation of the new substrate metal occurs, the melt splashes out and solidifies on the surface of the already formed oxide. If the channel is clogged and the formed surface layer near this channel does not have cracks and pores, then electrical breakdown occurs in other places where penetration of the electrolyte becomes possible, the thickness of the oxide layer is smaller, or there are cracks and pores.
Electron microscopy of transverse sections showed that the coatings[9-10]have internal pores and channels, which were formed in the process of micro arc and arc discharges (Fig.2).
With increasing thickness of the coatings, an increase in pore sizes is observed, and the continuity of the coating also increases. Coatings of greater thickness are less discontinuous. Using photographs of microstructures, the thickness of the coatings was measured in several areas. The diagram in Fig.3 shows the dependence of the thickness of oxide coatings on the oxidation time obtained for four samples. A high reliability of the linear approximation of the dependence of coating thickness on oxidation time is observed, reaching 0.99. This is a positive factor that allows us to predict the achievement of a given coating thickness under certain oxidation conditions. Previously, an increasing nonlinear relationship between the thickness, roughness of the oxide coating and oxidation time was noted, which is associated with the hereditary roughness of the original samples[11].
The surface roughness of the resulting coatings was measured in three sections of each sample (Fig. 4a). It was found that the roughnessRaincreases monotonically with time[12]. This is due to the fact that, at a constant current density, high energy is required to ensure electrical breakdown, which leads to the plasma process of the ceramic melt splashing out and solidifying on the surface of the already formed oxide. The unevenness of the surface influenced the fact that the roughness values at oxidation times of 60 and 90 minutes are almost the same.
It is necessary to note a significant increase in the hardness of the coatings with increasing oxidation time (Fig. 4b). When the oxidation time changed from 30 to 120 minutes, the microhardness of the coatings increased 3 times. The increase in the hardness of coatings is associated with the mechanism of electrical breakdown, in which in the channels formed during the primary breakdown, with increasing thickness of the coatings, the thickness of the film and the energy of the microarc discharge increase. This leads to an increase in the pressure in the channel and the temperature of the resulting melt, which ensures an increase in the hardness of the oxide coating in its upper layers.
3 Conclusions
During the study, it was established that during the miraculous arc oxidation of aluminum alloy 7075 in an electrolyte based on sodium hexametaphosphate with the addition of metasilicate and sodium hydroxide, a high degree of reliability of the linear approximation ofR2=0.99 is observed for the dependence of the thickness of the oxide coating on time. As the oxidation time increases from 30 to 120 minutes, the thickness of the oxide coatings increases from 13 to 30 μm.
The unevenness of the surface roughness and porosity of the coatings shows a lower reliability value of the approximation of the increasing dependence on time, equal toR2=0.84. The roughness in the studied time range increased and varied from 1.01 to 2.38 μm.
A significant increase in the hardness of the oxide coating from 585 to 1 800 was noted with an increase in the thickness of the coatings from 13 to 30 μm. A significant increase in hardness is associated with an increasing energy of electrical breakdown of an oxide coating of greater thickness and, as a consequence, an increase in temperature and pressure at the site of spark discharges.
Acknowledgments
The work was carried out with the assistance of the Chinese-Russian joint laboratory for monitoring damage to materials and rocks (agreement on the creation of the laboratory between KnAGU (Russia) and PRC (China), dated October 20, 2023), with the support of a grant from the President of the Russian Federation for state support of leading scientific schools of the Russian Federation (project NSh-452.2022 .4).

