大蕉特异性分子靶标的开发及其评价
王芳 崔广娟 吕顺 曾莉莎 陈东仪 黄晓彦 曾国玲 刘文清 何建齐


摘 要:【目的】由于香蕉高度不育和無性繁殖,经过长期的进化,导致许多资源来源不清晰。开发大蕉资源特异性分子靶标,为香蕉资源鉴定和遗传改良提供技术支撑。【方法】利用香蕉线粒体cox2/2-3基因序列,根据大蕉在该序列的特异性位点进行分子靶标设计,采用37份大蕉,以及香牙蕉、粉蕉、贡蕉、尖苞片蕉(Musa acuminata)、长梗蕉(M. balbisiana)、芭蕉(M. basjoo)以及阿宽蕉(M. itinerans)等共计59份其他类型香蕉资源进行鉴定筛选,获得特异性鉴定大蕉的分子靶标DcR/DcF,并进行评价。【结果】通过对共计96份香蕉资源的检测,发现37份大蕉均出现634 bp特异性条带,香牙蕉、粉蕉、贡蕉未出现该条带。在应用该标记对野生蕉检测中发现仅有阿宽蕉出现该特异性条带,长梗蕉、尖叶蕉、芭蕉均未出现该特异条带,同时大蕉和阿宽蕉的杂交后代出现了该特异条带。【结论】成功开发了一个大蕉特异性分子靶标DcR/DcF,可以在栽培蕉中特异性地鉴定大蕉,并具有快速、简便、准确的特点,该技术对香蕉种质资源鉴定、新品种选育等具有重要的应用价值。
关键词:大蕉;分子靶标;特异性鉴定,线粒体基因,评价
中图分类号:S668.1 文献标志码:A 文章编号:1009-9980(2023)12-2661-11
收稿日期:2023-09-05 接受日期:2023-10-23
基金项目:东莞市2021年度省乡村振兴战略专项资金“大专项+任务清单”(20211800400052);广东省级农业科技创新及推广项目-香蕉菠萝产业技术体系创新团队(2023KJ109);广东省基础与应用基础研究基金项目(2022A1515140114)
作者简介:王芳,女,正高级农艺师,硕士,研究方向为香蕉分子生物技术。E-mail:29333689@qq.com。#为共同第一作者。崔广娟,女,硕士,研究方向为分子育种。
*通信作者 Author for correspondence. E-mail:shunlv@qq.com
Development and evaluation of specific molecular target of Dajao
WANG Fang, CUI Guangjuan#, L? Shun*, ZENG Lisha, CHEN Dongyi, HUANG Xiaoyan, ZENG Guoling, LIU Wenqing, HE Jianqi
(Dongguan Agricultural Research Centre, Dongguan 523000, Guandong, China)
Abstract: 【Objective】Banana is an important fruit and food crop in the world, but it is facing the technical bottleneck of resource identification and genetic improvement. Banana plants are asexual and highly sterile. Because of long-term cultivation and exchanging between different regions, the origin of banana varieties is not clear. China has abundant cultivated and wild banana resources. Dajiao (Musa) is one of widely distributed banana resources in China which is different from plantain abroad, and there are many different types of Dajiao in different growing areas. Dajiao has many advantages, such as high yield, cold resistance, and strong disease resistance, so Dajiao is an important genetic resource. The aim of this study was to develop a specific molecular target of Dajiao for the rapid identification and genetic improvement of Chinese banana resources. 【Methods】 The 96 samples of different banana resources used in this experiment included 6 cultivars of Cavendish, 9 cultivars of Pisang Awak, 1 cultivar of Longya banana, 4 cultivars of Pisang Mas, 37 cultivars of Dajiao, 3 wild resources of Musa acuminata, 6 wild resources of Musa balbisiana, 5 wild resources of Musa basjoo, 21 wild resources of Musa itinerans and 3 hybrids, which were collected from different producing areas of China. The genomic DNA from each sample was isolated from fresh young cigar leaves using CTAB method. The concentration and purity of each DNA were checked with BioDrop μLite. First, we selected eight varieties of four groups, including Huanong Zhongba Dajiao, Dongguan Zhongba Dajiao, 8818-1, Beida Aijiao, Zhongfen No. 1, Fenza No. 1, Gongjiao and Gongxuan as representatives, through cloning and sequencing of the mitochondrial gene cox2/2 -3, and aligning the sequences by Mega 5.0. We found the specific base sequence in Dajiao from the results. Then Primer Premier 5.0 was used to design the specific primer, the optimal PCR amplification system and agarose gel electrophoresis detection method were optimized. At last, we obtained the specific detection target through a certain range of screening and expanded range of validation. 【Results】 The concentration of DNA extraction reached 500-1000 ng·μL-1, OD260/OD280 = 1.8-2.0, and the quality was good, which met the requirements of the experiment. The DNA was finally diluted into 50 ng·μL-1 and used for the experiments. PCR amplification of the cox2/2-3 region produced a single fragment of about 750-1200 bp in all the samples, and the gene fragment of Dajiao was longest, about 1200 bp. Through comparing the gene sequence of eight banana resources, we found 9 different insertional mutations (175 bp in total) in Dajiao, located at 229-985 bp of this gene. The abundant variation facilitated the design of specific primers. According to the specificity of cox2/2-3 gene sequence in Dajiao, a pair of primers was designed, the forward primer was DCR: TATTGACCGGTATGTCGGTA, and the rewerse primer was DCF: AGGTATTAATTGGCGGCCTAA. The optimal PCR procedure was: 94 ℃ predenaturation for 3 min, 94 ℃ denaturation for 30 s, 60 ℃ annealing for 30 s, 72 ℃ extension for 1 min, 30 cycles, 72 ℃ extension for 10 min, 94 ℃ denaturation for 30 s, 60 ℃ annealing for 30 s, 72 ℃ extension for 10 min. The optimal PCR system was: 10×PCR reaction buffer 2.5 μL, 2.5 nmol·L-1 dNTPs 2 μL, 10 μmol·L-1 primer 1 μL, 50 ng·μL-1 template DNA 1 μL, 5 U·μL-1 TaqDNA polymerase 0.5 μL, the volume was replenished to 25 μL with sterilized double-distilled water. The optimal detection method was: 1.2% agarose gel, 0.5×TBE electrode buffer, 110 V electrophoresis for 30 min. Through the examination, a 634 bp specific band was found in all 37 banana resources of Dajiao, but not in banana resources of Cavendish, Pisang Awak, Pisang Mas and Longyajiao. The target band was clear, no miscellaneous band and the detection accuracy was 100% in cultivated species. Only M. itinerans showed this specific band in the detection of wild banana using this marker, no specific band was found in M. acuminata, M. balbisiana and M. basjoo. At the same time, the specific band appeared in 3 hybrid progenies, so this fragment would be also suitable for the identification of hybrid progenies from Dajiao × M. itinerans. Banana had a unique inheritance mode of mitochondrial paternal inheritance as reported early, and this specific molecular target was derived from mitochondrial genes. On the whole, the 634 bp special band appeared in 37 cultivars of Dajiao and 22 wild resources of M. itinerans, so there should be a certain relationship between the paternal origin of Dajiao and M. itinerans. 【Conclusion】 Compared with traditional evaluation method using morphological markers, this specific molecular target of Dajiao would be more stable, sensitive and accurate and could be efficiently used in selection of parents and early identification of hybrid offspring in cross breeding , the results of this study about Dajiao and M. itinerans would provide information for studying the origin and evolution of bananas.
Key words: Dajiao; Molecular target; Specificity identification; Mitochondrial gene; Evaluation
香蕉(Musa spp.)是芭蕉科(Musaceae)芭蕉属(Musa L.)植物,不仅是世界上重要的水果之一,更是世界第四大粮食作物,全球4亿人的主食[1-2]。香蕉栽培种主要是由尖苞片蕉Musa acuminata Colla.(记为A基因组)和长梗蕉M. balbisiana Colla.(记为B基因组)这两个原始野蕉种内或种间杂交后代演化发展而来的[3]。香蕉属于无性繁殖,高度不育,经过长期的栽培,再加上不同地域之间的交流,导致了许多来源不明的品种和资源,并且出现了很多同名异种和同种异名的现象[4-6],这种情况给香蕉种质资源的鉴定和遗传育种的研究带来了很多困难。1955年,Simmonds等[7]根据不同香蕉品种的形态,包括叶片、蕉蕾和假茎等相關性状,结合染色体倍性,将栽培蕉分为了AA、BB、AB、AAA、AAB、ABB、AAAA、AAAB、AABB、ABBB。在之后广大学者的研究中,不断地补充和完善该种分类方法。然而,仅通过这些形态性状判定并不精确,并且该方法难以准确反映不同基因型香蕉的基因组来源和组成[8]。之后的研究结果证实了这一点,利用形态特征和分子标记技术手段,发现了香蕉中还具有不同于A和B基因组的其他基因组,如有的香蕉品种带有S(M. Schizocarpa)或者T(M. Textilis)基因组的特征[9-10]。
据报道,我国香蕉的种质资源间的遗传多样性比较丰富,种类繁多[11],栽培蕉主要有香牙蕉(AAA)、粉蕉(ABB)、粉大蕉(ABB)、大蕉(基因型不确定)、龙牙蕉(AAB)、贡蕉(AA)等[12]。而形态分类法在调查研究时工作量大,并且耗费时间久,容易受环境影响,存在一定的主观性,因此需要进一步发展其他更可靠的研究方法。随着科学技术的进步、分子生物学的快速发展,各类分子标记已经被应用于分析香蕉品种(系)的种群鉴定与分类、遗传多样性研究[13-16],利用分子手段研究香蕉的基因组可以更直观地呈现,并且分子生物技术手段的出现加速了植物品种改良进程[17]。笔者研究团队前期收集了大量我国各香蕉产区的大蕉资源,在对香蕉线粒体基因片段cox2/2-3序列的研究中(论文尚未发表),发现大蕉的该序列具有特异性,根据这段序列设计了可以特异性鉴定大蕉的靶标引物,并对其检测效果进行了分析评价,以期为香蕉种质资源的鉴定和新品种的选育提供更加快速精准的技术手段。
1 材料和方法
1.1 试验材料
于2019年3—6月采样于东莞市农业科学研究中心万江基地香蕉资源圃以及广东省农科院果树所国家果树种质广州香蕉荔枝圃。其中杂1、杂2、杂4为大蕉和阿宽蕉类野蕉的杂交后代。
1.2 方法
1.2.1 香蕉基因组的DNA提取 选取香蕉无病虫害的嫩叶,分别进行DNA提取。DNA提取方法采用十六烷基三甲基溴化铵(cetyltrimethylammonium bromide,CTAB)法[18]。
1.2.2 特异性序列测序 目的片段扩增:采用线粒体基因组中细胞色素氧化酶亚基Ⅱ基因中的1个内含子(cox2/2-3),PCR反应体系及电泳检测参考Duminil等[19]的方法。
目的片段测序:cox2/2-3片段经DNA胶回收试剂盒回收纯化后,克隆到pMD18-T载体中,阳性克隆送至Invitrogen公司广州分公司测序。为确保测序结果的准确性,分别用引物对每个扩增片段进行正反链测序,将两条链比对拼接。
1.2.3 特异性靶标引物设计及PCR扩增 采用MEGA 5进行序列比对;引物设计采用Primer premier 5.0,并对PCR退火温度及体系中DNA模板和酶量进行优化;检测采用琼脂糖凝胶电泳。
1.2.4 香蕉资源的鉴定评价 采用1.2.3引物及扩增检测方法,进行96个香蕉资源(表1,表2)的鉴定评价。
2 结果与分析
2.1 香蕉基因组DNA的提取
采用改良CTAB法提取香蕉叶片DNA,用BioDrop μLite(超微量蛋白核酸分析仪)检测其质量浓度和纯度,DNA质量浓度为500~1000 ng·μL-1,OD260/OD280=1.8~2.0,质量较好,符合试验要求;
根据测得的浓度,吸取适量体积的DNA样品,稀释成质量浓度50 ng·μL-1的工作液备用。
2.2 香蕉cox2/2-3基因测序
采用cox2/2-3基因对不同香蕉资源进行分析,不同香蕉资源该片段大小在750~1200 bp;大蕉与其他香蕉资源相比,基因片段最长,约1200 bp,大蕉的序列基本一致,序列为:TATGAGAGCCTTTCA
GCTCGTACTGCTCACACTCCTAGATCTGAACTAAGAGACCTCTGCGACCATAGTTTGAGCTGGGAGTTGCTCCTAGAATCCTTCCAAATGAGCTTGAAAGTCAACGTCAACAACACGAAAAGTACACGTTGGTTGCCTACTAATCAGATAATAGGTGAAATCCCTTCGCCTCTCGGAAGCTTGAAAGGGAGCTGAAAGTTTAGGGTGAGAAAGGTGAGAAAAGAGATTAGCTGGAGGTAGGGCGGGTCCTGAAACTAAGGTGTACATACATCAAAGCAGATTATGTCGGTATCCTTCCAATCCATATTGACCGGTATGTCGGTATCCTTCCAATCCATATTGACCGGGAAGAGTGGGGAGGCTAATGCAGAAGTATCTATGTATTAGAGAGATCCCTTATATTGATGATTGCTGGCTTCCCGGACTTGTCACAGATGGCTAGGAAGAAGAATAGGAGAAGTAGTCTCTGCCGTAGCAGGTCCTTCTCCTGTAGCTAAGACTGCCCTTACTTTGATTATTGTTCGTTCAGTTCACCGCGGCACTAATGAATAAGCTTGAGAATAACTTAGAGTGGCGCCTAACCTTTGAGAGCGTCTCTTGTCTTTGAATTTCAGAAGAAGAGTTGTAGATCTTGGACTGGCCCCCTTCGCATGACCTAGAATGAAAGGTCTGTGCTACTATAAGGCCTCTAAACTCCTTCCTCAGGACACTGTTGCGTTGCCATGGGACGGGGTATCCCCGACTTCTATAGTTCCTTGGTTCGACCTCCTAATGAGAATTGAGGTCCTTGCGCGGGCGTCTCATCCCTAAGACGAGTTTGCCTTTGTTTGTATGGAGTGTCCCGTGGTTACTCTAGTGCCAGCCGCAGAGAGGAATGCCATCAACTAGGGCGCTATTTGCCACTAACCACTCGCTCTTAGGCCGCCAATTAATACCTCCTCCGCGTTTCAAGTTGGTTATCCTAACCATTTCCCCTGCTCTACCGGGAGCCTGGCCCAATATTCGATCTTATATACTGCCTTGCTCCTCGGCTCCCTACTGCTCAAGCGGCTCGCTGTAATAGCTTGCTTATCGGGTGGCTCGCACCCCGACCACGGGTGGTGCGGC
TAAGCCAGAGTGGGCTCAGCTGTCGGCCTATG
TATCCGG。
2.3 特异性靶标引物设计及检测方法
通过对东莞中把大蕉、华农中把大蕉、8818-1、北大矮、中粉1号、粉杂1号、贡蕉、贡选等8个香蕉资源cox2/2-3核苷酸序列的比对,发现在该基因229~985 bp处,大蕉存在9处的插入突变(图1),共计175 bp,可以进行特异性靶标引物设计。根据大蕉cox2/2-3核苷酸序列的特异性,设计了1对引物,上游引物为DcR:TATTGACCGGTATGTCGGTA;下游引物为DcF:AGGTATTAATTGGCGGCCTAA。上游引物位于336~355 bp处;下游引物位于948~969 bp处(见图1黑色方框)。
经过优化获得最优的PCR程序:94 ℃预变性3 min;94 ℃变性30 s,60 ℃退火30 s,72 ℃延伸1 min,循环30次;72 ℃延伸10 min;其最优的PCR体系:10×PCR反应缓冲液2.5 μL、2.5 nmol·L-1 dNTPs 2 μL、10 μmol·L-1引物各1 μL、50 ng·μL-1模板DNA 1 μL、5 U·μL-1 TaqDNA聚合酶[TIANGEN,天根生化科技(北京)有限公司]0.5 μL,灭菌双蒸水补足体积至25 μL;最优检测方法为:1.2%琼脂糖凝胶、电极缓冲液为0.5×TBE、110 V电压电泳30 min。
2.4 特异性靶标的PCR扩增
对46份不同基因型的香蕉资源进行分析,分别选用部分大蕉和香牙蕉、粉蕉、贡蕉等栽培蕉和BB、AAw、basjoo类野蕉类进行对比检测,结果显示只有大蕉在634 bp处出现特异的条带,而其余蕉类均没有条带出现(图2、图3)。
2.5 扩大群体进行特异性靶标引物准确性的检测
对37份大蕉资源以及3个杂交种进行特异性扩增,所有供试大蕉品种均出现634 bp特异性条带(图4、图5)。杂1、杂2、杂4为大蕉和阿宽蕉的杂交后代,这3份资源也出现了634 bp特异性条带(图4),检测准确率100%。
2.6 利用特异性靶标引物对阿宽蕉类野蕉的检测
对从我国各地收集的22份阿宽蕉类野蕉资源进行特异性扩增,所有供试品种均出现634 bp特异性条带(图6)。
3 讨 论
近年来,应用单个或多个分子标记技术来鉴定香蕉种质资源的研究逐渐增多[20-23],但是多数是针对不同香蕉基因组的鉴定,而对某一类香蕉的快速鉴定分子标记研究比较少。本研究利用cox2/2-3基因序列,根据大蕉在序列上的特异性位点进行大蕉特异性分子靶标设计,获得了可以在香蕉栽培种中特异性鉴定大蕉的一对引物,具有快速、简便、准确的特点,该类分子标记对香蕉种质资源鉴定、新品种选育等具有重要的应用价值。
研究表明,根据香蕉的植株形态和经济性状,栽培蕉主要分为香牙蕉、龙牙蕉、粉蕉、大蕉、粉大蕉、和贡蕉等[12]。国内易把我国大蕉和国外煮食蕉中作粮食用的饭蕉(Plantain,AAB)混淆[24-25],我国的大蕉一般鲜食用,不同于国外的饭蕉。受到消费市场的限制,大蕉的种植相对较少,传播范围也较小,对其研究不多,但是我国的大蕉具有抗病、高产、耐贫瘠、抗寒等多个优点[26-29]。大蕉大多为三倍体,具有单性结果和高度不育性。目前研究表明少量三倍体的栽培品种具有较微弱的雌性可育性[30-32],其中大蕉与野生蕉杂交可少量结籽[33]。笔者研究组也开展了相关杂交试验,大蕉和尖苞片蕉、长梗蕉及阿宽蕉等野生蕉杂交均能结籽,结籽率较低。可见我国大蕉是一类具有特殊性及重要研究利用价值的香蕉种质资源。通常,国内外学者普遍认为大蕉是ABB基因型,与粉蕉的基因型一致;但是王正询等[34]通过对广东大蕉的形态、染色体配对及核型分析,认为是BBB型;也有研究人员通过形态学指标观察、测量,按照“Simmonds”标准分类法评分,结合染色体计数,判定三江大蕉基因型为AAB[35];笔者研究组按照“Simmonds”标准分类法对香蕉品种资源表型基因型进行调查和评价,大部分大蕉的得分介于AAB与ABB之间,偏向AAB,利用流式细胞技术对香蕉倍性分析结果显示大蕉DNA相对含量(或倍性值)与其他ABB基因型资源有一定差异,基因组更大一些[36]。笔者在本研究中通过分析发现该来源于线粒体的大蕉分子靶标在栽培种香蕉资源中只有大蕉出现特异性条带,在野生种香蕉资源中仅有阿宽蕉类野蕉出现了该特异性条带,同时以大蕉为母本、阿宽蕉为父本获得的杂交种也出現了该特异性条带,而香蕉具有线粒体父本遗传的独特遗传方式[37],因此推测阿宽蕉类野蕉与大蕉的父本来源存在一定的关系。
4 结 论
栽培蕉遗传背景狭窄,病虫害高发,亟须引入新的遗传基因,杂交育种是根本、有效的途径。目前国内育种者开始尝试香蕉杂交育种,从大量的杂交后代中筛选优良种质,工作量大、周期长,精确高效的早期筛选技术对杂交种的鉴定和筛选十分重要,目前这方面的研究还比较少,笔者在本研究中成功开发了一个大蕉特异性分子靶标(DcR/DcF),可以在栽培蕉中特异性地鉴定大蕉,并具有快速、简便、准确的特点,该特异性分子靶标为具有多种优异性状的大蕉类资源的应用提供了技术支撑,该类技术的开发在香蕉改良工程中具有重要的意义。
参考文献 References:
[1] MAYMON M,SELA N,SHPATZ U,GALPAZ N,FREEMAN S. The origin and current situation of Fusarium oxysporum f. sp. cubense tropical race 4 in Israel and the Middle East[J]. Scientific Reports,2020,10(1):1590.
[2] 黄媛媛,徐小俊. 全球香蕉产业现状与发展趋势[J]. 热带农业工程,2021,45(5):34-38.
HUANG Yuanyuan,XU Xiaojun. Current situation and development trend of banana industry in global[J]. Tropical Agricultural Engineering,2021,45(5):34-38.
[3] SIMMONDS N W. The evolution of the bananas[M]. London:Longmans,1962:1-16.
[4] 牟海飞,林贵美,邹瑜,李小泉,李朝生,韦华芳,吴代东,张进忠,王趣有,潘永杰,陈忠良. 利用 ISSR 分子标记分析香蕉品种的遗传多样性[J]. 西南农业学报,2010,23(4):1206-1210.
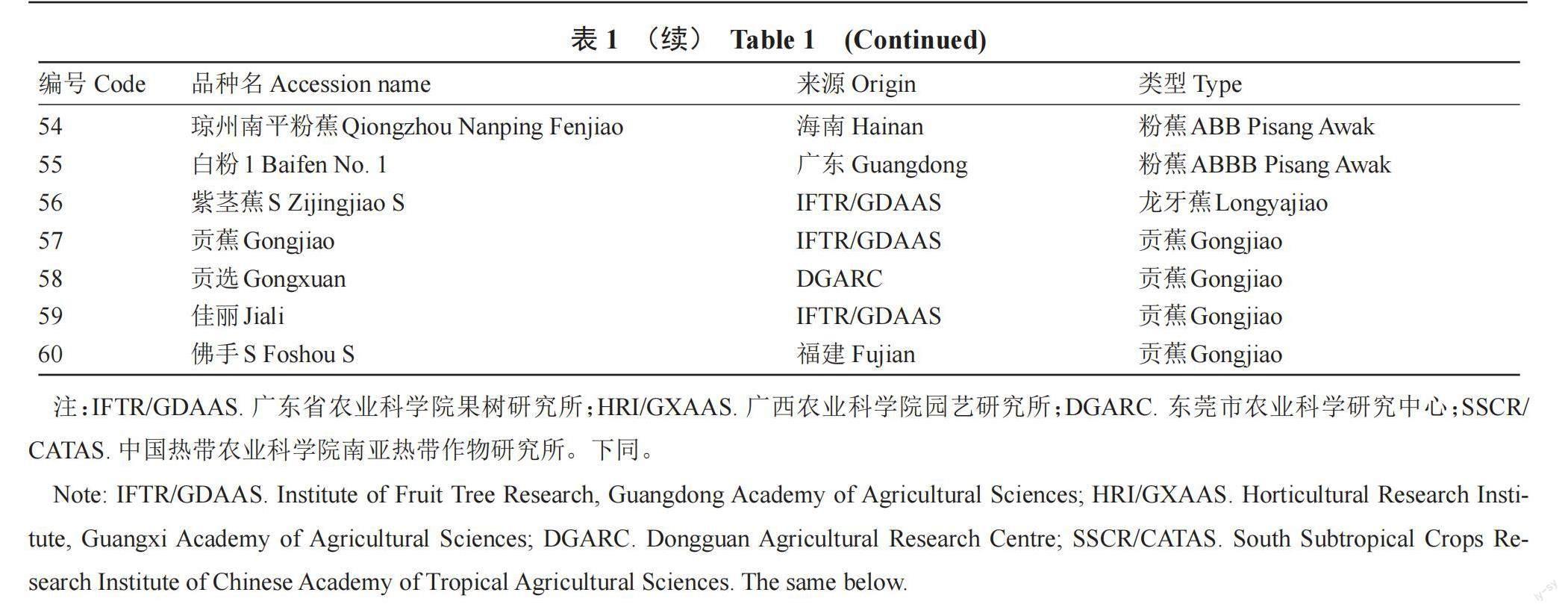
MOU Haifei,LIN Guimei,ZOU Yu,LI Xiaoquan,LI Chaosheng,WEI Huafang,WU Daidong,ZHANG Jinzhong,WANG Quyou,PAN Yongjie,CHEN Zhongliang. Genetic diversity analysis of banana (Musa spp.) based on ISSR molecular marker[J]. Southwest China Journal of Agricultural Sciences,2010,23(4):1206-1210.
[5] 郭计华. 不同基因组类型香蕉抗旱的遗传物质分析[D]. 海口:海南大学,2012.
GUO Jihua. Genetic material analysis of drought resistance different genotypes banana[D]. Haikou:Hainan University,2012.
[6] 姚锦爱,蔡鸿娇,石妞妞,甘林,杨秀娟,陈福如. 香蕉种质资源亲缘关系的ISSR分析[J]. 福建农业学报,2012,27(1):37-42.
Yao Jinai,CAI Hongjiao,SHI Niuniu,GAN Lin,YANG Xiujuan,CHEN Furu. Genetic Relationship among Banana (Musa spp.) germplasms revealed by iSSR analysis[J]. Fujian Journal of Agricultural Sciences,2012,27(1):37-42.
[7] SIMMONDS N W,SHEPHERD K. The taxonomy and origins of the cultivated bananas[J]. Botanical Journal of the Linnean Society,1955,55(359):302-312.
[8] PILLAY M,OGUNDIWIN E,NWAKANMA D C,UDE G,TENKOUANO A. Analysis of genetic diversity and relationships in East African banana germplasm[J]. Theoretical and Applied Genetics,2001,102(6):965-970.
[9] SHEPHERD K,FERREIRA F R. The Papua New Guinea biological foundations banana collection at Laloki,Port Moresby,Papua New Guinea[J]. IBPGR/SEAN Newsletter,1982,8(4):28-34.
[10] DHONT A,PAGET-GOY A,ESCOUTE J,CARREEL F. The interspecific genome structure of cultivated banana,Musa spp. revealed by genomic DNA in situ hybridization[J]. Theoretical and Applied Genetics,2000,100(2):177-183.
[11] 李锡文. 云南芭蕉科植物[J]. 植物分类学报,1978,16(3):54-64.
Li Xiwen. Musaceae from Yunnan[J]. Acta Phytotaxonomica Sinica,1978,16(3):54-64.
[12] 宁淑萍,许林兵,魏平,葛学军. 中国主栽香蕉品种和INIBAP引进品种的SSR分析研究[J]. 热带亚热带植物学报,2007,15(1):16-22.
NING Shuping,XU Linbing,WEI Ping,GE Xuejun. Genetic diversity of Chinese main banana cultivars (Musa spp.) and introduced accessions from INIBAP using simple sequence repeats(SSRs)[J]. Journal of Tropical and Subtropical Botany,2007,15(1):16-22.
[13] 孫嘉曼,尹玲,张进忠,韦绍龙,李朝生,刘金成,卢江. 广西香蕉种质资源的SSR分析[J]. 西南农业学报,2016,29(8):1952-1957.
SUN Jiaman,YIN Ling,ZHANG Jinzhong,WEI Shaolong,LI Chaosheng,LIU Jincheng,LU Jiang. SSR analysis of banana cultivars/accessions in Guangxi[J]. Southwest China Journal of Agricultural Sciences,2016,29(8):1952-1957.
[14] 譚卫萍,张秋明,于晓英,曾继吾,黄秉智,易干军. 香蕉种质遗传多样性与亲缘关系的AFLP分析[J]. 植物遗传资源学报,2010,11(6):715-720.
TAN Weiping,ZHANG Qiuming,YU Xiaoying,ZENG Jiwu,HUANG Bingzhi,YI Ganjun. Studies on the genetic diversity and relationship of banana by AFLP[J]. Journal of Plant Genetic Resources,2010,11(6):715-720.
[15] 蔺维成,郭计华. ISSR技术应用于香蕉种质资源的研究进展[J]. 兴义民族师范学院学报,2021(3):116-119.
LIN Weicheng,GUO Jihua. Research progress of ISSR technology applied to banana germplasm resources[J]. Journal of Xingyi Normal University for Nationalities,2021(3):116-119.
[16] 漆艳香,张欣,彭军,张贺,谢艺贤. 香蕉种质资源的SRAP遗传多样性分析[J]. 分子植物育种,2017,15(10):4220-4227.
QI Yanxiang,ZHANG Xin,PENG Jun,ZHANG He,XIE Yixian. Genetic diversity analysis of banana germplasms based on SRAP molecular markers[J]. Molecular Plant Breeding,2017,15(10):4220-4227.
[17] 李欢,张文洋,田志强,张震,叶文超,周子键,陈甲法,吴建宇. 高通量分子标记检测方法的研究进展[J],玉米科学,2022,30(3):1-9.
LI Huan,ZHANG Wenyang,TIAN Zhiqiang,ZHANG Zhen,YE Wenchao,ZHOU Zijian,CHEN Jiafa,WU Jianyu. Research progress of high-throughput molecular marker detection methods[J]. Journal of Maize Sciences,2022,30(3):1-9.
[18] 李英豪. 福建香蕉种质资源遗传多样性的RAPD分析[D]. 福州:福建农林大学,2007.
LI Yinghao. RAPD analysis of genetic diversity in Fujian banana germplasm resources[D]. Fuzhou:Fujian Agriculture and Forestry University,2007.
[19] DUMINIL J,PEMONGE M H,PETIT R J. A set of 35 consensus primer pairs amplifying genes and introns of plant mitochondrial DNA[J]. Molecular Ecology Notes,2002,2(4):428-430.
[20] 吴斌,陈汉清,王必尊,曾会才,谢艺贤,黄秉智. 19个华蕉类栽培品种的SCAR标记鉴别[J]. 分子植物育种,2015,13(5):1053-1059.
WU Bin,CHEN Hanqing,WANG Bizun,ZENG Huicai,XIE Yixian,HUANG Bingzhi. Identifying 19 cultivars of Musa acuminata subgroup cavendish (AAA) by using SCAR molecular markers[J]. Molecular Plant Breeding,2015,13(5):1053-1059.
[21] 王芳,牛玉清,黄秉智,崔广娟,张珂恒,曾莉莎,刘文清,吕顺,郭权香,何建齐. 香蕉与B基因组相关的SCAR标记研究[J]. 园艺学报,2019,46(3):577-589.
WANG Fang,NIU Yuqing,HUANG Bingzhi,CUI Guangjuan,ZHANG Keheng,ZENG Lisha,LIU Wenqing,L? Shun,GUO Quanxiang,HE Jianqi. Studies on SCAR marker related with B genome in Musa[J]. Acta Horticulturae Sinica,2019,46(3):577-589.
[22] BORBORAH K,SAIKIA D,REHMAN M,ISLAM M A,MAHANTA S,CHUTIA J,BORTHAKUR S K,TANTI B. Comparative analysis of genetic diversity in some non-commercial cultivars of Musa L. from Assam,India,using morphometric and ISSR markers[J]. International Journal of Fruit Science,2020,20(Suppl. 2):1814-1828.
[23] 况梦宇,詹妮,范迎康,易干军,罗充,盛鸥. 香蕉栽培品种基因组类型的分子标记鉴定体系建立[J]. 分子植物育种,2022,20(9):3011-3018.
KUANG Mengyu,ZHAN Ni,FAN Yingkang,YI Ganjun,LUO Chong,SHENG Ou. Establishment of an integrated molecular marker system for identifying genomic composition of banana accessions[J]. Molecular Plant Breeding,2022,20(9):3011-3018.
[24] 许林兵,黄秉智,杨护. 香蕉品种与栽培彩色图说[M]. 北京:中国农业出版社,2008:28-29.
XU Linbing,HUANG Bingzhi,YANG Hu. Color illustration of banana varieties and cultivation [M]. Beijing:China Agriculture Press,2008:28-29.
[25] 陈才林. 乌干达农产品降价[J]. 世界热带农业信息,2002(9):22.
CHEN Cailin. Uganda cuts prices for agricultural products [J]. World Tropical Agriculture Information,2002(9):22.
[26] 黄秉智,许林兵,杨护,唐小浪,魏岳荣,邱继水,李贯球. 香蕉种质资源枯萎病抗性田间评价初报[J]. 广东农业科学,2005,(6) :9-10.
HUANG Bingzhi,XU Linbing,YANG Hu,TANG Xiaolang,WEI Yuerong,QIU Jishui,LI Guanqiu. Prelimiary results of field evaluation of banana germplasm resistant to Fusarium wilt disease[J]. Guangdong Agricultural Science,2005,2(6):9-10.
[27] 刘文清,余铭,李洪波,吕顺,周建坤,向欣叶,刘建平,何建齐. 与东莞大蕉加工适宜性相关的农艺性状研究[J]. 广东农业科学,2012,39(14):29-31.
LIU Wenqing,YU Ming,LI Hongbo,L? Shun,ZHOU Jiankun,XIANG Xinye,LIU Jianping,HE Jianqi. Study of the procession-associated agronomic characteristics of Musa sapientum Linn. (Dongguan)[J]. Guangdong Agricultural Sciences,2012,39(14):29-31.
[28] 刘凯,胡春华,杜发秀,张玉娥,魏岳荣,易干军. 东莞大蕉超表达拟南芥CBF1基因及其抗寒性检测[J]. 中国农业科学,2012,45(8):1653-1660.
LIU Kai,HU Chunhua,DU Faxiu,ZHANG Yue,WEI Yuerong,YI Ganjun. Over-expression of the Arabidopsis CBF1 gene in Dongguandajiao (Musa spp. ABB group) and detection of its cold resistance[J]. Scientia Agricultura Sinica,2012,45(8):1653-1660.
[29] 吳烁凡,何维弟,李春雨,董涛,毕方铖,盛鸥,邓贵明,胡春华,窦同心,高慧君,刘思文,易干军,姚振,杨乔松. 香蕉抗寒分子机制研究进展[J]. 果树学报,2022,39(3):483-494.
WU Shuofan,HE Weidi,LI Chunyu,DONG Tao,BI Fangcheng,SHENG Ou,DENG Guiming,HU Chunhua,DOU Tongxin,GAO Huijun,LIU Siwen,YI Ganjun,YAO Zhen,YANG Qiaosong. Research progress in molecular mechanism of cold resistance in banana[J]. Journal of Fruit Science,2022,39(3):483-494.
[30] RABOIN L M,CARREEL F,NOYER J L,BAURENS F C,HORRY J P,BAKRY F,DU MONTCEL H T,GANRY J,LANAUD C,LAGODA P J L. Diploid ancestors of triploid export banana cultivars:molecular identification of 2n restitution gamete donors and n gamete donors[J]. Molecular Breeding,2005,16(4):333-341.
[31] PILLAY M,TRIPATHI L. Banana breeding[M]. Boston:Blackwell,2007:393-428.
[32] PILLAY M,TENKOUANO A. Banana breeding:Progress and challenges[M]. Boca Raton:CRC Press,2011:21-39.
[33] 李伟明,胡会刚,胡玉林,段雅婕,陈晶晶,谢江辉,王文华. 3个野生近缘种与不同栽培蕉的杂交亲和性[J]. 热带作物学报,2021,42(12):3500-3507.
LI Weiming,HU Huigang,HU Yulin,DUAN Yajie,CHEN Jingjing,XIE Jianghui,WANG Wenhua. Cross-compatibility among three wild banana species and various cultivars[J]. Chinese Journal of Tropical Crops,2021,42(12):3500-3507.
[34] 王正询,骆慧明,吴淑淳,潘坤清. 广东大蕉分类位置的探讨[J]. 热带亚热带植物学报,1995,3(1):49-53.
WANG Zhengxun,LUO Huiming,WU Shuchun,PAN Kunqing. The taxonomic position of Guangdong plantain[J]. Journal of Tropical and Subtropical Botany,1995,3(1):49-53.
[35] 孙长君,程志号,孙佩光,吴琼. ‘三江大蕉植物学性状观察及分类学地位[J]. 中国热带农业,2018(2):35-38.
SUN Changjun,CHENG Zhihao,SUN Peiguang,WU Qiong. Botanical characters and taxonomic status of ‘Sanjiang Dajiao[J]. China Tropical Agriculture,2018(2):35-38.
[36] 吕顺,任毅,王芳,胡桂兵,黃秉智,刘文清,何建齐,刘建平,曾莉莎,周建坤,麦景郁,张珂恒. 利用流式细胞术快速鉴定 169 份香蕉种质资源的染色体倍性[J]. 果树学报,2018,35(6):668-684.
L? Shun,REN Yi,WANG Fang,HU Guibing,HUANG Bingzhi,LIU Wenqing,HE Jianqi,LIU Jianping,ZENG Lisha,ZHOU Jiankun,MAI Jingyu,ZHANG Keheng. Ploidy identification of 169 Musa germplasms by flow cytometry[J]. Journal of Fruit Science,2018,35(6):668-684.
[37] FAUR? S,NOYER J L,CARREEL F,HORRY J P,BAKRY F,LANAUD C. Maternal inheritance of chloroplast genome and paternal inheritance of mitochondrial genome in bananas (Musa acuminata)[J]. Current Genetics,1994,25(3):265-269.

