Paired-related homeobox 1 induces epithelial-mesenchymal transition in oesophageal squamous cancer
Jin-Bao Guo,Ming Du,Bin Wang,Li Zhong,Zhong-Xue Fu,Jin-Lai Wei
Abstract BACKGROUND It is unclear that paired-related homeobox 1 (PRRX1) induces epithelialmesenchymal transition (EMT) in oesophageal cancer and the specific function of PRRX1 in oesophageal cancer metastasis.AIM To assess the significance of PRRX1 expression and investigate the mechanism of EMT in oesophageal cancer metastasis.METHODS Detect the expression of PRRX1 by immunohistochemistry in oesophageal tumour tissues and adjacent normal oesophageal tissues;the PRRX1 short hairpin RNA (shRNA) or blank vector lentiviral gene delivery system was transfected into cells;cell proliferation assay,soft agar colony formation assays,cell invasion and migration assays and animal studies were used to observe cells biological characteristics In vitro and in vivo;XAV939 and LiCl were used to alter the activity of Wnt/β-catenin pathway.Immunofluorescence staining and western blot analysis were used to detect protein expression of EMT markers and Wnt/β-catenin pathway.RESULTS PRRX1 is expressed at high levels in oesophageal cancer specimens and is closely related to tumour metastasis in patients with oesophageal cancer.Regulation of PRRX1 expression might exert obvious effects on cell proliferation,especially the migration and invasion of oesophageal cancer cells.Moreover,silencing PRRX1 expression using a shRNA produced the opposite effects.In addition,when PRRX1 was overexpressed,inhibition of the Wnt/β-catenin pathway with XAV939 negated the effect of PRRX1 on EMT,whereas when PRRX1 was downregulated,activation of the Wnt/β-catenin pathway with LiCl impaired the effect on EMT.CONCLUSION PRRX1 is upregulated in oesophageal cancer is closely correlated with cancer metastasis.Additionally,PRRX1 induces EMT in oesophageal cancer metastasis through activation of Wnt/β-catenin signalling.
Key Words: Paired-related homeobox 1;Oesophageal squamous cancer;Epithelial-mesenchymal transition;Cancer metastasis
INTRODUCTION
Esophageal cancer is one of the most common cancers with high morbidity and mortality.Esophageal cancer includes squamous-cell carcinoma and adenocarcinoma.At present,the treatment strategies of esophageal carcinoma mainly include surgery,chemotherapy,radiotherapy and molecular targeted therapy.However,the prognosis remains poor and overall Five-year survival rate is very low,with metastasis and recurrence being the main causes[1].Metastasis is a sign of the progression of malignant tumours and remains the greatest challenge for cancer treatment[2].Epithelial-mesenchymal transition (EMT) is a set of complex and variable transitional states between the epithelial and mesenchymal phenotypes,which acts a key role in carcinoma metastasis,and increasing reports show that metastasis is caused by EMT in preinvasive stage of most primary cancer[3].According to reports,the ability of cancer cells to invade and metastasize can be obtained by transforming into the mesenchymal phenotype.EMT is a type of transdifferentiation characterized by decreased expression of epithelial markers such as E-cadherin and increased expression of mesenchymal markers such as N-cadherin and vimentin[4].The invasive ability of cancer cells is obtained by transforming into a mesenchymal phenotype,accompanied by changes of EMT markers (N-cadherin,E-cadherin and vimentin) were observed[4].EMT is caused by the changes of genetic,epigenetic changes and the tumour microenvironment.WIST1,SNAI1,ZEB1 and ZEB2 are EMT inducers involved in EMT in most of cancer[5-7].It is reported that the EMT is a key step in inducing the metastasis of highly aggressive cancer cells,and its molecular mechanism must be extensively investigated.
Recently,paired-related homeobox 1 (PRRX1),which is revealed as a EMT inducer,could induce EMT in some cancers.Surprisingly,the function of PRRX1 is markedly different in these cancer types,and high expression of PRRX1 may predict less metastasis in breast cancer and a better prognosis in breast cancer[8].At the same time,we observed the opposite relationship in gastric cancer[9],which was also found in pancreatic cancer and colorectal cancer[10,11].Researchers have not determined whether PRRX1 induces EMT in oesophageal cancer and the specific function of PRRX1 in oesophageal squamous cancer metastasis.
MATERIALS AND METHODS
Patients and tissue samples
One hundred primary oesophageal squamous cancer tissue samples were collected from the Department of Thoracic Surgery of the First Affiliated Hospital of Chongqing Medical University (Chongqing,China).The patients did not receive preoperative chemotherapy or radiotherapy.These patients with oesophageal squamous cancer were performed on radical resection.This study was reviewed and ratified by the Research Ethics Committee of Chongqing Medical University.
Immunohistochemistry
To detect the expression of PRRX1,immunohistochemical staining was performed with the immunohistochemical SP kit (ZSGB-BIO,Beijing,China).The sections were deparaffinized,heated in a microwave oven at 90 °C for 20 min for antigen retrieval,incubated with 3% hydrogen peroxide for 25 min,then blocked by serum at 37 °C for 30 min.The sections were incubated with the primary antibody overnight at 4 °C and then incubated with the secondary antibody at 37 °C for 40 min.Next,the sections on the glass slide were incubated with streptavidin-HRP at 37 °C for 30 min and chromogen 3,3-diaminobenzidine for 15 min and then counterstained with haematoxylin.The staining for the target protein was observed under a microscope.
Two independent pathologists judged and scored staining in 10 random fields of view.As mentioned above,the staining was semi-quantitatively graded by determining the percentage of positively stained cells (0 points indicates 0%-5%,1 point indicates 6%-25%,2 points indicates 26%-50%,and 3 points indicates >50%) and expression intensity score (1 point=weak intensity,2 points=medium intensity,and 3 points=strong intensity).A total score >3 points was considered significant overexpression and was considered positive for data analysis.
Cell culture and antibodies
The human oesophageal squamous carcinoma cell lines EC109 and EC9706 were purchased from the Key Laboratory of General Surgery.The celllines were maintained in RPMI 1640 medium (Gibco,United States) containing 10% foetal bovine serum (HyClone,China) and cultured at 37 °C in a humidified atmosphere containing 5% CO2and 95% air.Cells were treated with 10 μmol/L XAV939 (a Wnt/β-catenin signalling inhibitor) or 20 mmol/L LiCl (a Wnt/β-catenin signalling activator) for 24 h to determine the effects of Wnt/β-catenin signalling on the function of PRRX1.
The antibodies against the following proteins were used: PRRX1 (OriGene,United States),N-cadherin,E-cadherin,Vimentin,and anti-lamin B1 (Sigma-Aldrich,United States),β-actin (Beyotime,China),HRP-conjugated goat anti-mouse IgG,Alexa Fluor 549-conjugated goat anti-rabbit IgG (H+L),and HRP-conjugated goat anti-rabbit IgG (ZSGB-BIO,Beijing,China).
Plasmid construction and transfection
The PRRX1 short hairpin RNA (shRNA) was constructed in a recombinant adenovirus gene delivery system by GeneChem Biomedical Co.,Ltd.(Shanghai,China),and the negative control was delivered by a blank vector lentiviral gene delivery system.The PRRX1 shRNA or blank vector lentiviral gene delivery system was transfected into cells,and then referred to as the PRRX1(i) or MOCK group,respectively.
Cell proliferation assay and soft agar colony formation assays
The cells were incubated in a 96-well plate at a density of 2 × 103cells per well.After 1,2,3,4,5,6 and 7 d,20 μL of MTT dye (Sigma-Aldrich) were added to each well and incubated at 37 °C for 4 h before dimethyl sulfoxide was added to each well.The absorbance was measured spectrophotometrically with a microplate reader (Bio-Rad,Hercules,CA,United States).
Soft agar colony formation assays were performed to evaluate the colony formation capacity.Cells were cultured in 6-well plates were cultured containing 0.35% agarose (RPMI medium 1640 mixed with agarose) at a density of 2 × 103cells per well.After 3 wk,the colonies were manually counted under a microscope.
Cell invasion and migration assays
Twenty-four-well Transwell chambers with an 8-μm pore size (Corning,New York,NY,United States) were coated with BD Matrigel Basement Membrane Matrix (BD Biosciences,San Diego,CA,United States),maintained overnight at 4 °C,and then polymerized at 37 °C.The transwell chambers used in the migration assay were not coated with Matrigel.Cells were plated in transwell chambers at a density of 1 × 105cells per well and incubated.After 20 h,the cells that passed through the transwell were stained with 4% paraformaldehyde and then stained with haematoxylin-eosin (HE).By counting the number of stained cells in the four quadrants from each insert and averaging the obtained triplicate values,the number of cells invaded or migrated through Matrigel was quantified.
Immunofluorescence staining
Cells were incubated in dishes.After 24 h,cells were fixed with 4% paraformaldehyde for 30 min,then permeabilized with 0.04% Triton X-100 for 10 min,and then blocked by 5% bovine serum albumin for 30 min.Cells were incubated with the primary antibody at 37 °C for 1 h,incubated with the appropriate Alexa Fluor 549-conjugated secondary antibody at 37 °C for 1 h,and counterstained with 4’,6-diamidino-2-phenylindole for 1 h.Immunofluorescence images were captured using a fluorescence microscope.
Animal studies
BALB/c nude mice were used to the role of PRRX1 on detect tumorigenicity and tumor formation in oesophageal tumour formation.Three groups (EC9706,EC9706 MOCK and EC9706 PRRX1(i) were expanded and subcutaneously inoculated into the flanks of nude mice (3 × 105cells).Tumour diameters were measured using Vernier callipers every week,and the tumour volumes were calculated using the following formula: V(mm3)=length × width2/2.After 6 wk,the mice were euthanized by Carbon Dioxide Euthanasis.
A total of 1 × 104cells was injected into the tail vein of each nude mouse to assay the effect of PRRX1 on oesophageal cancer cell metastasisin vivo.Necropsies were performed after 30 d,and the number of lung metastasis nodules in each mouse was counted.
Western blot analysis
Whole-cell extracts were prepared in lysis buffer (Beyotime,China),and the nuclear and cytoplasmic fractions were separated with a nuclear and cytoplasmic protein extraction kit (Beyotime,China).Then,40 μg of protein were loaded into each well,electrophoretically separated,and transferred to membranes.The membranes were then incubated with primary antibodies for 10 h at 4 °C and then incubated with secondary antibodies for 1 h at 37 °C.Finally,each band was analysed using an ECL chemiluminescence detection system (ChemiDoc™ XRS imager,Bio-Rad,United States).
Statistical analysis
Statistical analyses were performedby SPSS v.20 and GraphPad Prism 6 software.Student’sttest,was used to calculated significant differences.Pearson’s correlation coefficients and theχ2test,as appropriate.Overall survival was analysed using the logrank test and KaplanMeier analysis.Values ofP<0.05 were considered statistically significant.
RESULTS
PRRX1 expression is upregulated in oesophageal squamous cancer and related to shorter overall survival
We determined the PRRX1 expression level in oesophageal squamous tumour tissues and adjacent normal oesophageal tissues by performing immunohistochemistry.Compared with adjacent normal oesophageal tissues,PRRX1 was expressed at significantly higher levels in oesophageal squamous tumour tissues (Figures 1A-D).The expression level of PRRX1 in 100 oesophageal squamous cancer specimens was investigated.As shown in Figure 1E,during the 5-year follow-up,55.9% of patients with low PRRX1 expression survived,and only 37.9% of patients with high PRRX1 expression survived (P<0.05).As shown in Table 1,PRRX1 was expressed at significantly higher levels in oesophageal tumour tissues than in adjacent normal oesophageal tissues (P<0.05).The correlations between the expression levels of PRRX1 and the clinicopathological features of patients with oesophageal cancer were analysed (as shown in Table 2).Strong correlations were observed between the expression of PRRX1 and T-stage and lymph node metastasis and pTNM stage of tumour invasion (P<0.05).In addition,the expression of PRRX1 had no relationship with the age,gender,tumor location and histologic grade of oesophageal squamous cancer (P>0.05).

Figure 1 Paired-related homeobox 1 expression is upregulated in oesophageal cancer and related to shorter overall survival. A:Immunohistochemical detection of the expression of paired-related homeobox 1 (PRRX1) in oesophageal cancer specimens with a high (× 200);B: Middle (× 200);C:Low grade (× 200);D: Immunohistochemical detection of the expression of PRRX1 in adjacent normal oesophageal tissues (× 200);E: Logrank test and Kaplan-Meier analysis of the association of PRRX1 expression with the overall survival of patients with oesophageal cancer.aP <0.05.PRRX1: Paired-related homeobox 1.
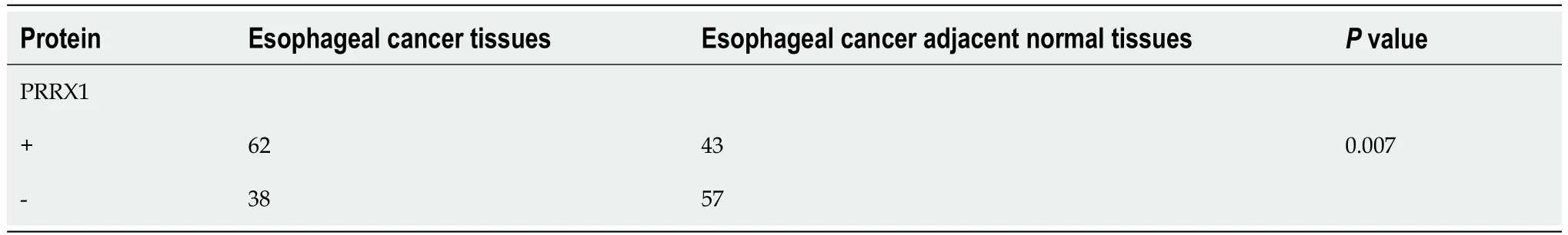
Table 1 Expression of paired-related homeobox 1 in 100 cases of esophageal cancer and adjacent normal esophageal tissues (λ2-test)
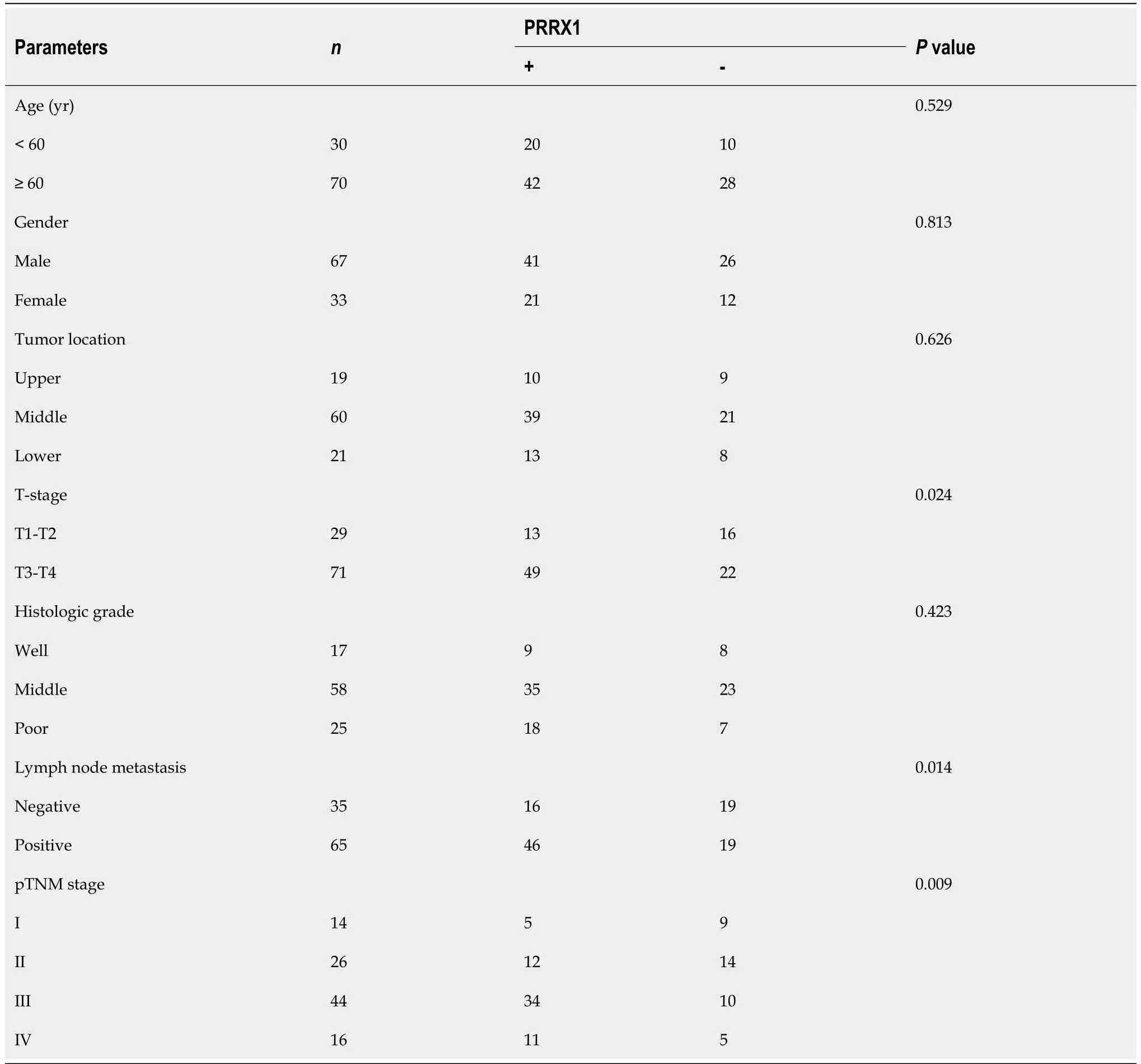
Table 2 Correlation between paired-related homeobox 1 immunostaining and clinicopathologic features in 100 cases of esophageal cancer tissues (λ2-test)
PRRX1 modulates cell proliferation,colony formation,invasion and migration in oesophageal cancer
High PRRX1 expression was detected in EC109 and EC9706 cells.After shRNA vector transfection,stable oesophageal cancer cell lines were established,in which the expression of PRRX1 was continuously inhibited.The PRRX1(i) groups were confirmed to express the PRRX1 protein at lower levels after transfection with the PRRX1 shRNA vector lentivirus.PRRX1 expression was more obviously inhibited in EC9706 PRRX1(i) cells than in EC109 PRRX1(i) cells (as shown in Figure 2),and thus EC9706 cells were used in follow-up biological experiments using oesophageal cancer cells.
Cell proliferation was assessed using the MTT assay.The proliferation of EC9706 cells was significantly decreased on days 4,5,6,and 7 after silencing endogenous PRRX1 (Figure 3A).Silencing the expression of PRRX1 inhibits the growth of oesophageal cancer cells.The soft agar colony formation assay revealed the effect of PRRX1 on the colony formation ability of oesophageal cancer cells.Smaller and fewer colonies were formed by EC9706 cells after PRRX1 was downregulated (Figure 3B).

Figure 3 Paired-related homeobox 1 modulates proliferation,colony formation,migration and invasion in oesophageal cancer cells. A:The MTT assay.Paired-related homeobox 1 (PRRX1) affected EC9706 cell proliferation;B: Soft agar colony formation assay.PRRX1 affected the ability of EC9706 cells to form colonies in soft agar,and the number of colonies was different in various groups of EC9706 cells;C: Cellular migration assays.PRRX1 affected the migration of EC9706 cells.The number of migrating EC9706 cells in various groups was different.Cells were stained with hematoxylin and eosin;D: Cellular invasion assays.PRRX1 affected the invasive properties of EC9706 cells.The number of invasive EC9706 cells in various groups was different.Cells were stained with hematoxylin and eosin.aP <0.05.PRRX1: Paired-related homeobox 1.
Cell migration and invasion assays were executed.In Figure 3C,compared with the EC9706 and EC9706 MOCK groups,the number of cells migrating through the Transwell was significantly reduced after PRRX1 silencing (P<0.05).In addition,as shown in Figure 3D,the number of cells passing through Matrigel was significantly reduced after PRRX1 was downregulated compared with the EC9706 and EC9706 MOCK groups (P<0.05).Based on these results,PRRX1 increases the proliferation,colony formation,invasion and migration of oesophageal cancer cells.
PRRX1 induces EMT in oesophageal cancer
In the EC109 PRRX1(i) and EC9706 PRRX1(i) groups,the level of the PRRX1 protein was obviously reduced,but no significant difference in the expression of PRRX1 was observed between the MOCK group and parental cell group.Then,the levels of EMT markers were also distinctly changed.The expression of N-cadherin and vimentin was significantly decreased;in contrast,E-cadherin expression was significantly increased in EC109 PRRX1(i) and EC9706 PRRX1(i) cells (Figure 2).Similar results were also obtained from the immunofluorescence staining experiment (Figure 4).The change in the level of the PRRX1 protein was accompanied by changes in the expression of EMT markers.These results reveal that PRRX1 induces EMT in oesophageal cancer cells.
PRRX1 regulates the Wnt/β-catenin pathway in oesophageal cancer
β-catenin asthe key protein in the Wnt/β-catenin pathway,and the expression of β-catenin was significantly decreased EC109 PRRX1(i) and EC9706 PRRX1(i) cells (Figure 2).Therefore,PRRX1 may affect the Wnt/β-catenin pathway in oesophageal cancer cells.PRRX1 silencing accompanied an obvious reduction in the total β-catenin level,especially the nuclear level of β-catenin (Figure 2).An obvious reduction in the nuclear level of β-catenin was also observed (Figure 4).Further research showed that the total and nuclear levels of the β-catenin protein were decreased after cells were treated with XAV939 (an inhibitor of Wnt/β-catenin signalling),meanwhile,significantly increased after cells were treated with LiCl (an activator of Wnt/β-catenin signalling) (Figure 5).This information suggests that PRRX1 is involved in regulating the Wnt/β-catenin pathway in oesophageal cancer cells.
PRRX1 induces EMT in oesophageal cancer via the Wnt/β-catenin pathway
After the level of the PRRX1 protein was obviously reduced in EC109 PRRX1(i) and EC9706 PRRX1(i) cells,a significant increase in E-cadherin levels and significant reductions in levels of the vimentin,N-cadherin,total and nuclear β-catenin proteins were examined (Figure 2).As shown in Figure 5,the activator of Wnt/β-catenin signalling LiCl increased the levels of the total and nuclear β-catenin proteins.Concomitantly,levels of the N-cadherin and vimentin proteins were significantly increased,the level of the E-cadherin protein was decreased,and the level of the PRRX1 protein was not changed in EC109 PRRX1(i) and EC9706 PRRX1(i) cells.At the same time,after the inhibition of Wnt/β-catenin signalling with XAV939,the total and nuclear levels of the β-catenin protein were decreased,levels of the vimentin and N-cadherin proteins were decreased,the expression of the E-cadherin protein was increased,and the level of the PRRX1 protein was not altered in EC109 and EC9706 cells.PRRX1 not only induced EMT but also altered the activity of the Wnt/β-catenin pathway.After further study,activation of the Wnt/β-catenin pathway potentially restores the effects of PRRX1 on inhibiting EMT,whereas inhibiting the activity of the Wnt/β-catenin pathway enhances the effect of the downregulation of PRRX1 on EMT.
PRRX1 promotes oesophageal cancer cell proliferation and metastasis in vivo
Xenografts of EC9706,EC9706 MOCK and EC9706 PRRX1(i) cells were established in nude mice to test the function of PRRX1 in tumorigenesis,and solid tumours developed after 6 wk.In the EC9706 PRRX1(i) group,the tumour volumes and weights were significantly smaller than those in the EC9706 and EC9706 MOCK groups (Figure 6A).
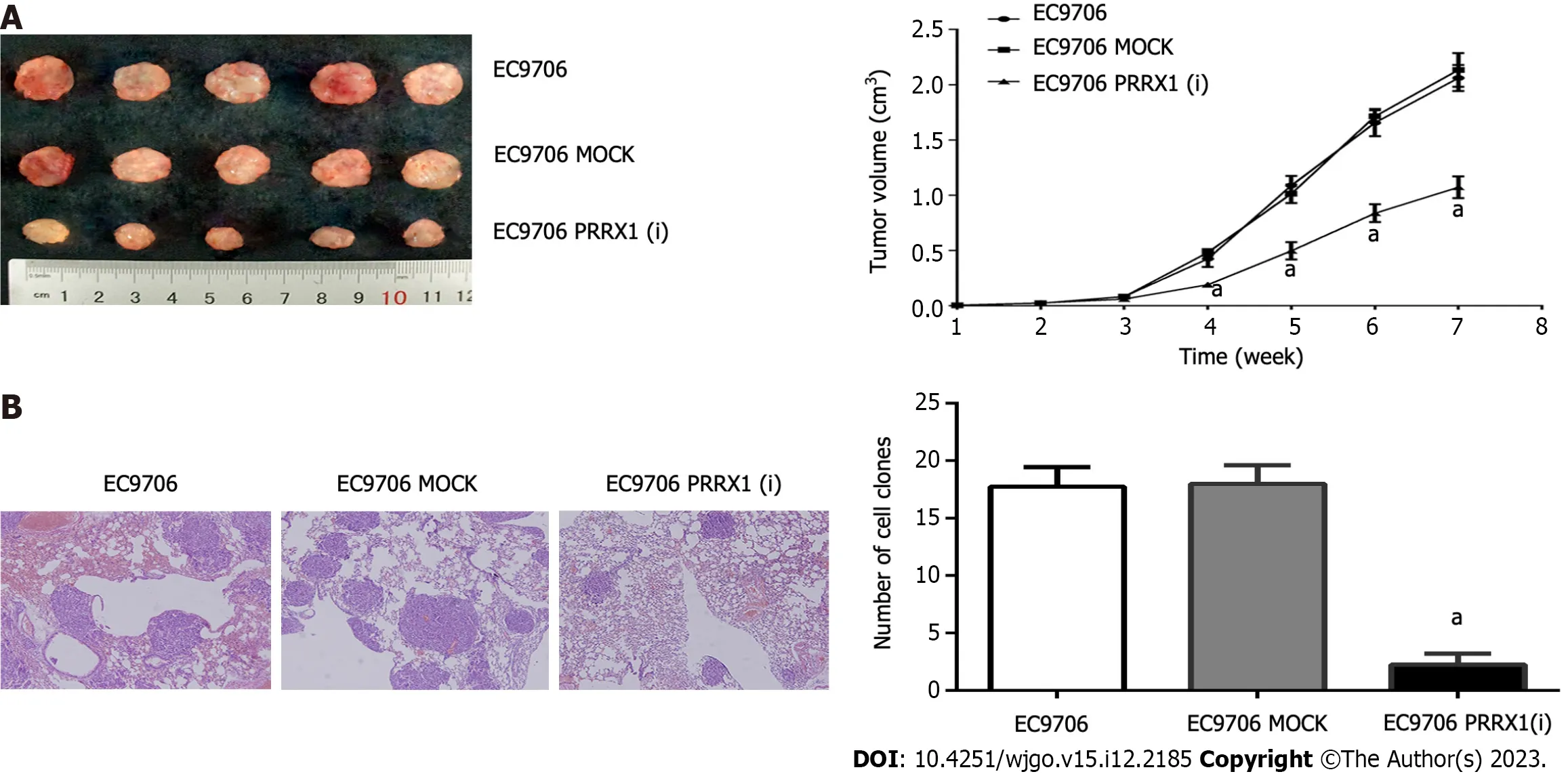
Figure 6 Paired-related homeobox 1 downregulation inhibits cell proliferation and lung metastasis in vivo. A: Paired-related homeobox 1(PRRX1) affected EC9706 cell proliferation in vivo.The tumour volume was measured weekly;B: The number of metastatic lung nodules was different in the various EC9706 groups.aP <0.05.PRRX1: Paired-related homeobox 1.
The number of lung metastatic foci was evaluated to explore the role of PRRX1 in oesophageal cancer cell metastasisin vivo,and a smaller number of lung metastatic nodules was observed in animals injected with PRRX1-silenced cells than in animals injected with control cells (Figure 6B).
DISCUSSION
EMT plays a key role in tumour progression[12].During EMT,epithelial cells obtain mesenchymal characteristics with the loss of epithelial cell markers (E-cadherin) and the acquisition of mesenchymal cell markers (vimentin and Ncadherin).EMT is activated or reversed (mesenchymal to epithelial transition,MET) by cells,revealing a form of plasticity that also leads to stemness and drug resistance[13].EMT is related to tumour metastasis,which is accompanied by the activation of signalling networks[14].EMT is reported to be important in the invasion and metastasis of cancer cells[3].
PRRX1 was reported for the first time to enhance the DNA binding activity of serum response factors[15].PRRX1 induces EMT by inhibiting the expression of E-cadherin[8,10].PRRX1 also plays a key role in the process of pancreatic regeneration and carcinogenesis[11].In oesophageal squamous cancer tumours,PRRX1 is expressed at higher levels than that in adjacent normal tissue,and the expression of PRRX1 is significantly correlated with the T-stage,lymph node metastasis and pTNM stage of oesophageal squamous cancer.High PRRX1 expression is related to a shorter overall survival.This study is a small sample size study,follow-up studies need a larger sample size to reduce bias.Oesophageal cancer cells (EC9706 and EC109) with high PRRX1 expression not only exhibit a spindle-like shape but also display a loss of E-cadherin expression and high vimentin and N-cadherin expression.After downregulating the expression of PRRX1,oesophageal cancer cells (EC9706 and EC109) upregulated the expression of E-cadherin and downregulated the expression of vimentin and N-cadherin,showing an oval-like shape.These results indicate that oesophageal cancer cells with downregulation of PRRX1 undergo the MET and that EMT can be reversed to the MET[16].At the same time,we also observed that PRRX1 silencing suppressed cell proliferation,colony formation,migration and invasionin vitro,and downregulating PRRX1 also inhibited proliferation and metastasisin vivo.During EMT,epithelial features of cancer cells are inhibited,and the expression of mesenchymal genes is upregulated.Then,an invasive and metastatic phenotype is acquired.EMT is presumed to play a central role in cancer metastasis[17].Based on these results,PRRX1 not only maintains EMT but also promotes cancer metastasis in oesophageal cancer.PRRX1 also induces EMT during carcinogenesis;therefore,EMT may play an important role in PRRX1-mediated tumour invasion and metastasis[10,18].
Where are you off to? You are in your Sunday-best clothes! We are going to Court, to woo the Princess! Don t you know what is known throughout all the country side? And they told him all about it
However,the molecular mechanism by which PRRX1 regulates EMT in oesophageal squamous cancer is unclear[10].In this study,it was found that the expression and localization of β-catenin in oesophageal cancer was related to the expression of PRRX1.And the aberrant expression of β-catenin may be a potential unfavourable factor contributing to the occurrence and development of oesophageal cancer[19].β-catenin functions as a key mediator in the Wnt/β-catenin signalling pathway.After activating Wnt/β-catenin signalling,β-catenin escapes proteasomal degradation and is transported to the nucleus,where it binds to its target genes and promotes a variety of carcinogenic pathways[20].The Wnt/β-catenin signalling pathway plays an important role in the progression,metastasis and invasion of oesophageal cancer[21].The Wnt/β-catenin signalling pathway regulates multiple biological processes,such as cardiac valve formation and cell proliferation,as well as EMT in gastrulation and cancer[22,23].When Wnt/β-catenin signalling is activated,the destruction complex is inhibited,β-catenin could gradually accumulate,and then translocate to the nucleus.The loss of E-cadherin expression may be caused by the translocation of β-catenin into the nucleus[23,24].High PRRX1 expression was accompanied by increased levels of β-catenin in the nucleus of oesophageal cancer cells and activation of the Wnt/β-catenin signalling pathway in the present study.Following PRRX1 downregulation,β-catenin expression was downregulated,and more obviously,β-catenin levels in the nucleus were decreased.In addition,XAV939,which as an inhibitor of Wnt/β-catenin signalling,inhibited the activity of Wnt/β-catenin signalling by preventing β-catenin translocation into the nucleus[25].High PRRX1 expression in oesophageal cancer cells induces EMT,and the effect on EMT was neutralized by XAV939.At the same time,LiCl-mediated activation of the Wnt/β-catenin pathway weakens the effect of PRRX1 downregulation on EMT.These results indicate that PRRX1 regulates Wnt/β-catenin signalling in oesophageal cancer and that Wnt/β-catenin signalling regulates the PRRX1-induced EMT.
In summary,the upregulation of PRRX1 is a common event in oesophageal cancer that is closely related to the metastasis of oesophageal squamous cancer.PRRX1 induces EMT by activating Wnt/β-catenin signalling and stimulates the invasion and metastasis of oesophageal cancer,which leads to poor prognosis of patients with oesophageal cancer.Our results are consistent with those observed in pancreatic cancer and colorectal and gastric cancer[9],but differ from the findings in breast cancer[8].Perhaps these data suggest that the function of PRRX1 is different in different cancers.More research is needed on PRRX1.PRRX1 is regulated by transforming growth factor-β and microRNAs[8,26].PRRX1 isoforms regulate the DNA damage response in pancreatic cancer cells in cooperation with FOXM1[27].Cellular phenotypic plasticity and dormancy of head and neck squamous cell carcinoma are regulated by PRRX1[28].In hepatocellular carcinoma,the downregulation of PRRX1 predicts a poor prognosis,and PRRX1 regulates the p53-dependent signalling pathway[29].
CONCLUSION
Given the importance of PRRX1 in EMT and the Wnt/β-catenin pathway in esophageal squamous cancer,our findings not only provide a better understanding of the molecular mechanisms of PRRX1 in esophageal squamous cancer metastasis,but also provide a better understanding of the role of PRRX1 in cancer metastasis,it also suggests some potential therapeutic intervention targets[11].
ARTICLE HIGHLIGHTS
Research background
Esophageal cancer is one of the most common cancers with high morbidity and mortality.It is unclear that paired-related homeobox 1 (PRRX1) induces epithelial-mesenchymal transition (EMT) in oesophageal cancer and the specific function of PRRX1 in oesophageal cancer metastasis.
Research motivation
This study is to assess the significance of PRRX1 expression and investigate the mechanism of EMT in oesophageal cancer metastasis.
Research objectives
One hundred primary oesophageal cancer tissue samples were collected from Thoracic Surgery and the human oesophageal squamous carcinoma cell lines EC109 and EC9706.
Research methods
The expression of PRRX1 by the chemical test of immunohistochemical tissue is in the esophageal tumor tissue.The PRRX1 short hair holder RNA (SHRNA) or blank virus gene delivery system is transfected into the cells.Cell proliferation measurement,the formation of soft agar colonies,cell invasion and migration measurement,and animal research are used to observe the cell biological characteristics of internal and internal body.Mouse experimental testing and metastasis of esophageal tumors in the body.Immunfluorescence staining and protein marks analysis is used to detect protein expression of EMT labeling and Wnt/β-catenin.XAV939 and LiCl are used to change the activity of Wnt/βcatenin pathway.
Research results
PRRX1 is expressed in high levels in the specimen of esophageal cancer and is closely related to the metastasis and prognosis of the tumor metastasis and prognosis of patients with esophageal cancer.The regulation of PRRX1 may have a significant impact on cell proliferation,especially the migration and invasion of esophageal cancer cells.The expression of PRRX1 is closely related to EMT and Wnt/β-catenin.Further experiments,using shRNA to silence the expression of PRRX1 have the opposite effect.In addition,when PRRX1 was expressed,the inhibitory effect of XAV939 on WNT/βcatenin’s inhibitory has denied the effect of PRRX1 on EMT.When the expression of PRRX1 was downregulated,the Wnt/β-catenin pathway with LiCl impaired the effect on EMT.
Research conclusions
Research perspectives
The expression of PRRX1 is closely related to the metastasis and prognosis of esophageal cancer,which also shows that PRRX1 may become a potential esophageal cancer treatment target.Our studies have confirmed that PRRX1 is regulating EMT through WNT/β-catenin.More PRRX1 regulation mechanisms and downstream target areas need to be more indepth research.
FOOTNOTES
Author contributions:Guo JB and Zhong L designed the research and wrote the paper;Wei JL performed the research and analyzed results;Du M,Wang B,and Fu ZX edited the manuscript and provided critical comments.
Institutional review board statement:All experimental protocols were approved by the Ethics Committee the First Affiliated Hospital of Chongqing Medical University (Approval number 2021-033).
Institutional animal care and use committee statement:All experimental protocols were approved by the Ethics Committee the First Affiliated Hospital of Chongqing Medical University (Approval number 2021-033).
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Data sharing statement:Technical appendix,statistical code,and dataset available from the corresponding author at zhongli401@126.com.
ARRIVE guidelines statement:The authors have read the ARRIVE guidelines,and the manuscript was prepared and revised according to the ARRIVE guidelines.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Jin-Bao Guo 0000-0002-4147-7708;Li Zhong 0009-0003-1440-6933.
S-Editor:Wang JJ
L-Editor:A
P-Editor:Zhang XD
 World Journal of Gastrointestinal Oncology2023年12期
World Journal of Gastrointestinal Oncology2023年12期
- World Journal of Gastrointestinal Oncology的其它文章
- Prognostic value of T cell immunoglobulin and mucin-domain containing-3 expression in upper gastrointestinal tract tumors: A meta-analysis
- Association between heat shock factor protein 4 methylation and colorectal cancer risk and potential molecular mechanisms: A bioinformatics study
- Evaluating the causal relationship between human blood metabolites and gastroesophageal reflux disease
- Intensive follow-up vs conventional follow-up for patients with nonmetastatic colorectal cancer treated with curative intent: A metaanalysis
- Combined TIM-3 and PD-1 blockade restrains hepatocellular carcinoma development by facilitating CD4+and CD8+T cellmediated antitumor immune responses
- Association of MBOAT7 rs641738 polymorphism with hepatocellular carcinoma susceptibility: A systematic review and meta-analysis
