Evaluating the causal relationship between human blood metabolites and gastroesophageal reflux disease
Jia-Yan Hu,Mi Lv,Kun-Li Zhang,Xi-Yun Qiao,Yu-Xi Wang,Feng-Yun Wang
Abstract BACKGROUND Gastroesophageal reflux disease (GERD) affects approximately 13% of the global population.However,the pathogenesis of GERD has not been fully elucidated.The development of metabolomics as a branch of systems biology in recent years has opened up new avenues for the investigation of disease processes.As a powerful statistical tool,Mendelian randomization (MR) is widely used to explore the causal relationship between exposure and outcome.AIM To analyze of the relationship between 486 blood metabolites and GERD.METHODS Two-sample MR analysis was used to assess the causal relationship between blood metabolites and GERD.A genome-wide association study (GWAS) of 486 metabolites was the exposure,and two different GWAS datasets of GERD were used as endpoints for the base analysis and replication and meta-analysis.Bonferroni correction is used to determine causal correlation features (P <1.03 × 10-4).The results were subjected to sensitivity analysis to assess heterogeneity and pleiotropy.Using the MR Steiger filtration method to detect whether there is a reverse causal relationship between metabolites and GERD.In addition,metabolic pathway analysis was conducted using the online database based MetaboAnalyst 5.0 software.RESULTS In MR analysis,four blood metabolites are negatively correlated with GERD: Levulinate (4-oxovalerate),stearate (18:0),adrenate (22:4n6) and p-acetamidophenylglucuronide.However,we also found a positive correlation between four blood metabolites and GERD: Kynurenine,1-linoleoylglycerophosphoethanolamine,butyrylcarnitine and guanosine.And bonferroni correction showed that butyrylcarnitine (odd ratio 1.10,95% confidence interval: 1.05-1.16,P=7.71 × 10-5) was the most reliable causal metabolite.In addition,one significant pathways,the “glycerophospholipid metabolism” pathway,can be involved in the pathogenesis of GERD.CONCLUSION Our study found through the integration of genomics and metabolomics that butyrylcarnitine may be a potential biomarker for GERD,which will help further elucidate the pathogenesis of GERD and better guide its treatment.At the same time,this also contributes to early screening and prevention of GERD.However,the results of this study require further confirmation from both basic and clinical real-world studies.
Key Words: Blood metabolites;Gastroesophageal reflux disease;Mendelian randomization;Causality;Pathogenesis;Biomarkers;Metabolic pathway
INTRODUCTION
Gastroesophageal reflux disease (GERD) refers to a disease in which gastric contents reflux into the esophagus,causing corresponding esophageal symptoms and/or complications.This condition affects approximately 13% of the global population[1].GERD is not life-threatening,but it impairs patients' quality of life and increases the risk of other esophageal complications such as esophagitis,Barrett's esophagus (BE),and esophageal adenocarcinoma[2].Previous epidemiological studies have identified several possible risk factors for GERD,including smoking,alcohol consumption and diabetes[3-5],which have played a role in the prevention of GERD.However,there are no studies on the blood metabolomics of GERD.
The development of metabolomics as a branch of systems biology in recent years has opened up new avenues for the investigation of disease processes.By identifying altered metabolites or metabolic pathways,metabolomics can specifically shed light on the molecular causes of disease[6].Unlike genomics,transcriptomics or proteomics,metabolomics describes the concentration and flux of low molecular metabolites present in biological fluids or tissues[7].It allows a global assessment of the cellular state in a real environment,taking into account gene expression,genetic regulation,changes in the kinetic activity and regulation of enzymes,and changes in metabolic responses[8].Due to metabolic fluctuations downstream of changes in DNA,RNA,and protein levels,metabolomics provides a sensitive and comprehensive interpretation of biological systems.Metabolomics has now been widely used to characterize specific metabolic phenotypes associated with digestive diseases and has identified many metabolites[9-11].To our knowledge,there are no thorough investigations that systematically examine the causative association between blood metabolites and GERD,although Liuet al[12] described 23 metabolic abnormalities related to GERD.Based on the information currently available,it is not possible to identify the metabolite profile that contributes to the development of GERD due to the inherent limitations of traditional observational research.
As a powerful statistical tool,Mendelian randomization (MR) is widely used to explore the causal relationship between exposure and outcome[13].In particular,MR was able to circumvent the drawbacks of randomized controlled experiments by choosing exposure-related single nucleotide polymorphisms (SNPs) as instrumental variables (IVs)[14].Because genetic variants are randomly assigned during meiosis,MR was able to largely avoid confounding factors by using this alternative to IVs that mimic randomized controlled trials[15].In addition,genotype formation occurs before the onset of the disease and is usually not affected by disease progression.Thus,reverse causality is unlikely.In this study,we used MR analysis to thoroughly investigate the causal relationships between 486 blood metabolites and GERD using data from a genome-wide association study (GWAS).Additionally,we identified the metabolic pathways that cause GERD.In addition to advancing our understanding of the pathophysiological mechanisms underlying GERD,the integration of metabolomics and genomics offers fresh perspectives on the early detection and management of the disease.
MATERIALS AND METHODS
Study design
An effective MR study should follow three assumptions: (1) IVs are closely related to exposure factors;(2) IVs are not related to confounding factors;and (3) IVs are not related to outcomes and affect outcome onlyviaexposures[16].Two independent GWAS alliances give the genetic information of GERD,subjected to preliminary and replication analyses,followed by meta-analysis .The overview of the study is showed in Figure 1.

Figure 1 Overview of this Mendelian randomization analysis. Assumption 1,genetic instruments are strongly associated with the exposures of interest;Assumption 2,genetic instruments are independent of confounding factors;Assumption 3,genetic instruments are not associated with outcome and affect outcome only via exposures.IVW: Inverse variance weighted;LD: Linkage disequilibrium;LOO analysis: Leave-one-out analysis;MR-PRESSO: MR-Pleiotropy RESidual sum and outlier;SNPs: Single nucleotide polymorphisms;WM: Weighted median;BMI: Body mass index;T2DM: Type 2 diabetes mellitus.
GWAS data for 486 blood metabolites and GERD
Genetic data for blood metabolites were obtained from the Metabolomics GWAS server (https://metabolomics.helmholtz-muenchen.de/gwas/).Shinet al[17] identified nearly 2.1 million SNPs of 486 metabolites related to human genetic variation through genome-wide association scanning and high-throughput metabolic analysis.Of the 486 metabolites,107 are defined as unknown because their chemical properties are still unclear.Another 309 metabolites were chemically identified and assigned to eight broad metabolomes,including amino acid,carbohydrate,cofactors and vitamin,energy,lipid,nucleotide,peptide,and xenobiotic metabolism.The detailed names of 486 metabolites are shown in Supplementary Table 1,among which the chemical properties of the metabolites named X -are unknown.
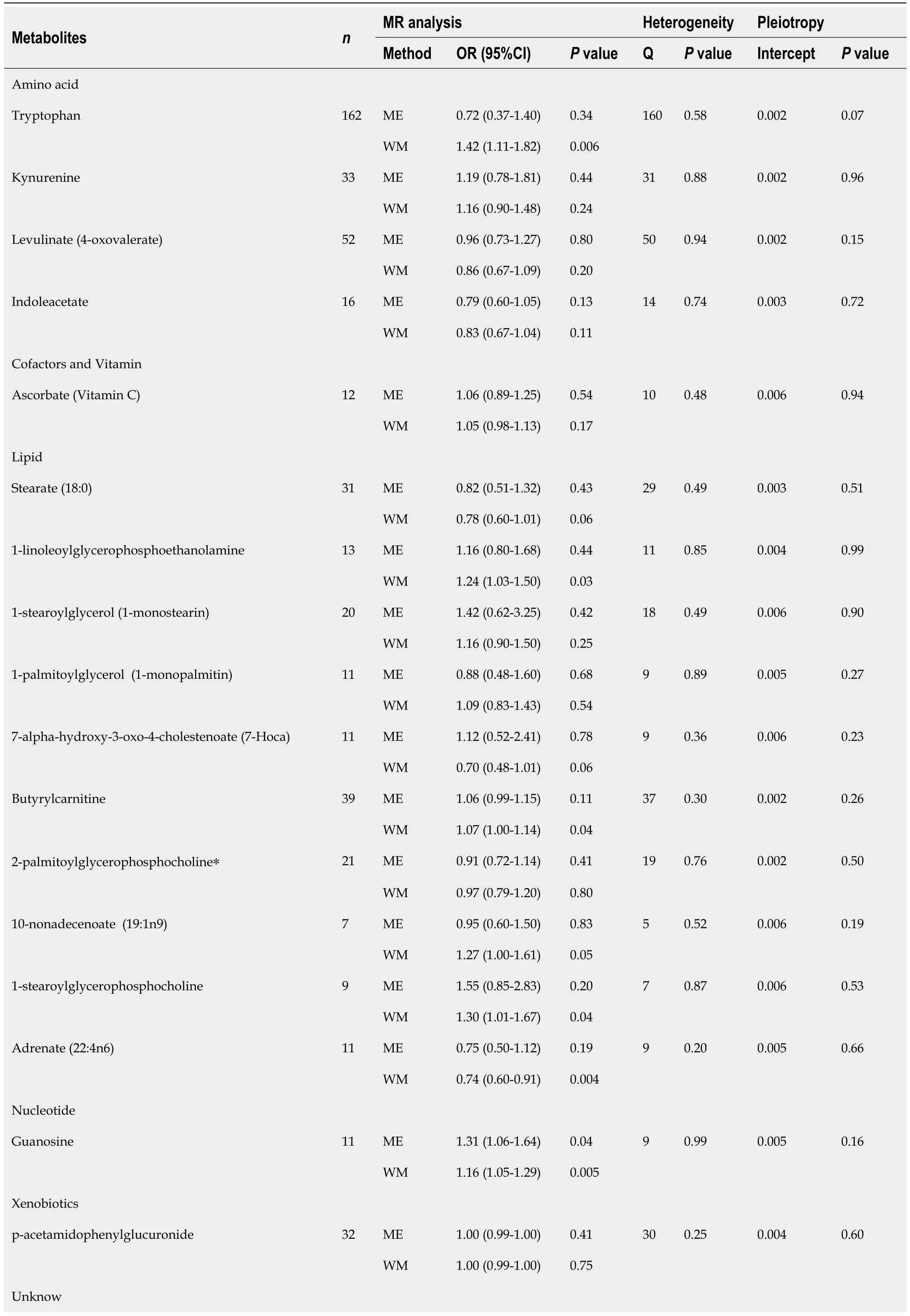
Table 1 Supplementary and sensitivity analyses for causality from blood metabolites on gastroesophageal reflux disease
Download GERD's GWAS summary data from IEU (https://gwas.mrcieu.ac.uk/).The GWAS directory login number is ebi-a-GCST9000514.Specifically,GWAS data containing 2320781 SNPs were obtained from a previous GERD-related GWAS study conducted by Onget al[18] and colleagues with a total sample size of 602604 Europeans containing 129080 cases and 473524 controls.The above GWAS data were used for the preliminary analysis of GERD.To validate our results by conducting replication analysis and meta-analysis,we repeated the MR analysis using the GERD data (54854 GERD patients and 401473 healthy controls) published by Wuet al[19].This data is publicly available on the website: https://cnsgenomics.com/content/data.
Instruments selection
We performed a series of steps to select eligible genetic variants associated with metabolites.Given the small number of metabolite-related SNPs,we relaxed the significance threshold ofP<1 × 10-5to select metabolite-related SNPs.We then clumped SNPs by removing linkage disequilibrium (R2 >0.1 and within 500kb).This criterion has been widely applied in previous studies[20-22].To satisfy hypothesis (3),we removed the SNPs associated with the results in the IVs (P<1 × 10-5).We eliminate bias caused by weak IVs by calculating the R2 and F statistics for each SNP to measure statistical strength.SNPs with F <10 were defined as weak genetic variants and were deleted.We further coordinated the SNPs of exposure and outcome,and removed the SNPs with palindromic effects and allele discordance (e.g.A/GvsA/C).Then,the final results were subjected to MR analysis.
Statistical analysis and sensitivity analysis
The causal relationship between blood metabolites and GERD was mainly assessed based on the results of random-effect inverse variance weighted (IVW).IVW is based on the hypothesis that there is no horizontal pleiotropy for all SNPs and the results from the pooled analysis of Walden ratios for all genetic variants,under the premise that IVW provides the most accurate assessment of causal effects[23].Therefore,we used IVW-based estimates to initially screen for blood metabolites that have a causal effect on GERD.To obtain more reliable results,we used two additional methods to further evaluate metabolites with significant estimates (IVW derivedP<0.05).The MR-Egger and weighted median (WM) methods are used as complementary analyses.These two methods can provide more robust estimates under the relaxed.WM assumes that at least half of the tools are valid[24] and MR-Egger provides horizontal pleiotropy and heterogeneity detection in the presence of horizontal pleiotropy for all SNPs[25].When consistent with the InSide hypothesis (IVs intensity independent of direct effects),MR-Egger regression can provide unbiased estimates[26].
For the initially determined significant estimates (IVWP<0.05),sensitivity analysis will be performed to assess any deviation from the MR hypothesis.Horizontal pleiotropy was observed when IVs affected the results through other pathways than exposure.Horizontal pleiotropy was assessed based on the Egger intercept.Cochran Q test was used to test for the presence of heterogeneity,.Heterogeneity was considered to exist whenP<0.05,I2>25%[27].For data with significant associations,Radial MR was used to identify heterogeneous values,and MR analysis was repeated after eliminating heterogeneous SNPs to obtain more accurate results[28].Finally,we used MR-PRESSO to check again for the presence of heterogeneous SNPs[29].We used leave-one-out (LOO) analysis to ensure the robustness of the results.By discarding each SNP in turn and then performing MR analysis to assess whether the results are heavily influenced by a single SNP.
In conclusion,we rigorously screened blood metabolites with potential causal relationship with GERD by multiple criteria: (1) SignificantP-values for preliminary analysis (IVW derivedP<0.05);(2) The direction and amplitude of the three MR methods were consistent;(3) There was no heterogeneity or level pleiotropy in Mr results;and (4) MR estimates are not significantly confounded by individual SNPs.
Replication and metaanalysis
To fully assess the robustness of candidate metabolites identified based on the above criteria,we repeated the IVW analysis in another GERD cohort.In brief,the data from IEU with code ebi-a-GCST90000514 was used for the preliminary analysis and the data with the title GORD_summary was used for the replication analysis.Meta-analyses were based on a random-effects IVW model and performed on Review Manager 5.4 software.
Evaluation of genetic directionality
We verified whether the observed causality was biased by reversal of causality using the Steiger test[30].Using the Steiger test,we determined whether the included SNPs explained the variability of GERD better than the detected metabolites.When the combination of SNPs was found to contribute more to the genetic risk of GERD than metabolites (SteigerP>0.05),it indicated that the direction of causal inference may be biased.
Confounding analysis
Although we evaluated the horizontal pleiotropy of Mr results through a series of sensitivity analyses to detect any SNPs that violated the MR hypothesis,there may also be a small number of residual confounding SNPs.Therefore,we examined the IVs of metabolites on the Phenoscanner V2 website (http://www.phenoscanner.medschl.cam.ac.uk/) to assess whether each SNP was associated with known risk factors for GERD,such as smoking,alcohol consumption,type 2 diabetes,and body mass index (BMI).If any SNP was observed to be associated with the above confounding factors (P<1 × 10-5),then MR analysis was repeated after removing these SNPs to verify the reliability of the results.
Metabolic pathway analysis
To clarify the biological mechanisms underlying the effects of blood metabolites on GERD,we further performed metabolic pathway analysis using MetaboAnalyst 5.0 (https://www.metaboanalyst.ca/)[29] to explore the potential pathogenesis of GERD.
RESULTS
Preliminary analysis
After strict control of the quality of IVs,SNPs of 25 metabolites were obtained.Filtered IVs contain 5 to 162 SNPs (X-11452 consists of 5 SNPs,while tryptophan sulfate consists of 162 SNPs).All metabolite-related SNPs had F-statistics greater than 10,which shows the strong power of the IVs.Supplementary Table 2 displays the specific IV data.Prior to MR analysis,radial MR locates and eliminates all outliers (Supplementary Table 3).IVW analysis initially identified 25 metabolites with a potential causal relationship with GERD,including 17 metabolites with known chemical identity and 8 metabolites with unknown chemical identity.These 17 known metabolites include amino acids,cofactors,vitamins,lipids,nucleotides,and xenobiotic metabolism factors (Figure 2).Among the 17 known metabolic traits,butyrylcarnitine was significantly associated with GERD after Bonferroni correction.Twenty-one metabolites that passed the strict screening requirements were used for the follow-up analysis by sensitivity analysis (Figure 3).In short,the MR estimates derived from WM and MR–Egger regression presented consistent directions and amplitudes,supporting the robustness of causality (Table 1).Pvalues andI2associated with Cochran Q indicated that no heterogeneity was found.In addition,the MR-Egger intercept term indicated a low risk of horizontal pleiotropy (Table 1).The LOO analysis did not find any high-impact SNPs biasing the pooled effect estimates (Supplementary Figure 1),and 21 metabolites that met the above criteria were included in the next study.

Table 2 Genetic predictors of butyrylcarnitine and their association with GERD
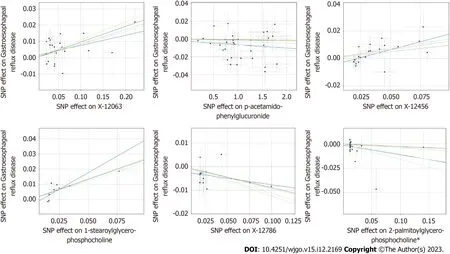
Figure 3 Scatterplot of significantly associated (inverse variance weighted derived P <0.05) and directionally consistent estimates. SNP:Single nucleotide polymorphisms.
Replication and meta-analysis
The meta-analysis further identified 14 metabolites (8 known and 6 unknown) that could affect GERD (Figure 4).In detail,levulinate (4-oxovalerate) [odd ratio (OR) 0.80,95% confidence interval (CI): 0.72-0.89,P<0.0001],stearate (18:0) (OR 0.77,95%CI: 0.64-0.92,P=0.004),adrenate (22:4n6) (OR 0.83,95%CI: 0.74-0.94,P=0.004),p-acetamidophenylglucuronide (OR 0.99,95%CI: 0.99-1.00,P=0.0002),X-11247 (OR 0.92,95%CI: 0.88-0.96,P=0.0006),X-12786 (OR 0.90,95%CI: 0.82-0.98,P=0.01) decreased risk of GERD,while kynurenine (OR 1.20,95%CI: 1.07-1.35,P=0.002),1-linoleoylglycerophosphoethanolamine (OR 1.17,95%CI: 1.05-1.31,P=0.004),butyrylcarnitine (OR 1.09,95%CI: 1.05-1.13,P<0.0001),guanosine (OR 1.10,95%CI: 1.03-1.18,P=0.003),X-09108 (OR 1.40,95%CI: 1.13-1.74,P=0.002),X-11452 (OR 1.14,95%CI: 1.02-1.29,P=0.03),X-12063 (OR 1.07,95%CI: 1.03-1.11,P=0.0008),and X-12456 (OR 1.12,95%CI: 1.06-1.19,P=0.0001) increased susceptibility to GERD.

Figure 4 Meta-analysis of significantly associated (inverse variance weighted derived P <0.05) between metabolites and gastroesophageal reflux disease. CI: Confidence interval;OR: Odd ratio.
Genetic basis for the causal association
We further investigated genetic variants affecting metabolite levels and GERD.The 39 SNPs of IVs for butyrylcarnitine are shown in Table 2.Among them,rs4767937 showed a strong correlation with butyrylcarnitine (β=0.1052;SE=0.0035,P=1.00 × 10-200).Notably,it had the strongest association with GERD (β=0.0142;SE=0.0048,P=0.0032).The effect of this SNP on butyrylcarnitine and GERD suggests that the relevant genetic loci may provide valuable information for the biological mechanism of GERD,and butyrylcarnitine may be an important functional mediator of the biological processes affecting GERD.
Direction validation
We performed the Steiger test to verify the direction of the effect from metabolites to GERD.The SteigerPvalue indicates that the identified causality is not biased by reverse causality.The results are shown in Supplementary Table 4.
Confounding analysis
We used Phenoscanner to examine all SNPs associated with the metabolites that were positive on initial screening (IVW <0.05) (including glycine,N-acetylglycine,and 1-palmitoylglycerol (1 monopalmitin)) to test the validity of Hypothesis 2 (IVs are independent of confounders).The data were disregarded since the exclusion of glycine and N-acetylglycine was meaningless because rs715 was related to BMI and rs1260326 in 1-palmitoylglycerol (1-monopalmitin) was connected with alcohol use.Supplementary Table 5 displays the findings for the remaining 25 metabolites.In total,14 SNPs were found to be associated with common GERD risk variables;however,even after eliminating these SNPs,the estimates were still significant.Two well-known metabolites,adrenate (22:4n6) and 1-linoylglycerophosphoethanolamine*,were unaffected by any confounding factors.
Metabolic pathway analysis
Unfortunately,we only found 1 metabolic pathway that may be involved in the etiology of GERD (Supplementary Table 6),despite the existence of 8 recognized metabolites.One possible metabolic pathway in the pathophysiology of GERD is the glycerolipid metabolism pathway,which includes 1-linoleoylglycerophosphoethanolamine.Many of the metabolites that we discovered have also not yet been attributed to any of the metabolic pathways that are already listed in the kyoto encyclopedia of genes and genomes databases or The Small Molecule Pathway databases.Therefore,further research is needed to explore whether these metabolites are involved in the biological processes related to GERD occurrence.
DISCUSSION
In this study,we integrated two large-scale GWAS datasets to explore the causal effects of 486 blood metabolites on GERD through a rigorous MR design.Our study found 14 blood metabolites associated with GERD,among which butyrylcarnitine showed a significant positive correlation with GERD.This relationship is not affected by confounding factors such as smoking,alcohol consumption,BMI,and can be well replicated using samples from other data sources.In addition,we identified a metabolic pathway that may be involved in the biological mechanisms of GERD.This may be the first study to explore the causal relationship between serum metabolites and GERD by combining metabolomics and genomics.Given the unclear pathogenesis of GERD and the lack of blood metabolomics research related to GERD,this study is of great significance.
The onset of GERD mainly consists of two mechanisms: the invasion of reflux and the destruction of the anti-reflux barrier at the esophageal junction.Usually,the anti reflux defense mechanism of the esophagus is in balance with the erosive effect of reflux substances on the esophageal mucosa.When the person's defense mechanism decreases or the damaging effect increases,the balance is disrupted,which may lead to the occurrence of GERD[31].At present,the exploration of the pathogenesis of GERD is still at the macro level,and multiple studies suggest that abnormal esophageal sphincter function,esophageal hiatal hernia,and esophageal motility disorders play important roles[32-34].However,the specific mechanism by which esophageal hiatal hernia participates in GERD is unclear,and a series of issues such as the causal relationship between esophageal peristaltic dysfunction and GERD and whether it is involved in the occurrence of esophagitis,remain poorly understood[35].Therefore,more research is needed to help better elucidate the pathogenesis of GERD.Twenty-four-hour pH impedance monitoring,gastrointestinal endoscopy,and PPI testing are the gold standards for the clinical diagnosis and treatment of GERD,but the first two diagnostic methods are invasive and may cause a series of adverse reactions,such as headache and nausea.Therefore,there is an urgent need for accurate and effective biomarkers for the clinical diagnosis and early prevention of GERD.Metabolomics technology reveals the changes that have occurred in the body,serving as a bridge between genotype and phenotype,enabling researchers to understand diseases from a microscopic perspective.It is worth noting that blood metabolites reflect both endogenous and exogenous processes of disease occurrence[36].For example,Matthew's study found differences in serum metabolites among GERD,BE,and high grade dysplasia/esophageadicarcinoma (EA) patients that could help distinguish patients at different stages of EA progression[37].At present,there is still a significant lack of blood metabolomics research on GERD.Therefore,we conducted a key MR study to clarify the causal relationship between blood metabolites and GERD and the metabolic pathways involved,thus providing reference directions for further elucidating the pathogenesis of GERD and early screening and treatment.
A key clinical contribution of this study is the discovery of biomarkers.Our study supports a positive correlation between butyrylcarnitine and the risk of GERD from a causal perspective by combining genetics and metabolomics.Butyrylcarnitine belongs to the acyl carnitine group,which is composed of incomplete fatty acids β prooxidant compounds produced by oxidation.At present,there are no reports on the relationship between butyrylcarnitine and GERD,and we cannot accurately explain this relationship either.However,the carnitine shuttle pathway carries longchain fatty acids from the cytoplasm to the mitochondria for later βoxidation,which necessitates acetyl-CoA and results in the esterification of L-carnitine to produce acyl carnitine derivatives[38].The disturbance of the carnitine shuttle may lead to impaired mitochondrial function,which may reduce the ability of cells to process reactive oxygen species and increase the levels of inflammatory cytokines,leading to increased cell dysfunction and cell death[39].This change may trigger GERD.On the other hand,weakened antioxidant capacity leads to a poor ability to prevent esophageal mucosal damage,which can also increase the severity of GERD[40,41].Second,butyrylcarnitine is closely related to common GERD risk factors such as diabetes,obesity,anxiety and depression and other mental diseases.Studies have shown that butyrylcarnitine is involved in diet-induced insulin resistance,which in turn is related to the oxidation rate of fatty acids exceeding that of tricarboxylic acids and respiratory chains,leading to the accumulation of FAO intermediates such as acyl carnitine in mitochondria,and abnormal insulin signaling[42].This indicates that butyryl carnitine plays an important role in the occurrence and development of diabetes.In addition,previous studies have found a positivecorrelation between butyrylcarnitine and obesity,showing similar results in both children and adults[43,44].This result has been reported in individuals of Asian and European ancestry[45].Obesity can lead to an increase in the number of brief relaxations of the lower esophageal sphincter,esophageal motility disorders,hiatal hernia,and elevated intraabdominal pressure and is associated with complications such as BE and EA in GERD[46].In addition,butyrylcarnitine has been found to be involved in the development of depression.Duet al[47] found that increasing neuronal differentiation is associated with symptoms of depression in later years,and the increase in neuronal differentiation is jointly regulated by an increase in butyrylcarnitine levels and a decrease in the levels of the glycerophospholipid PC35:1 (16:0/199:1).Zhao's study found cognitive improvement and decreased levels of butyrylcarnitine in schizophrenia patients treated with olanzapine[48].These findings provide strong evidence for the involvement of butyrilcarnitine in the occurrence of mental illness.Therefore,we speculate that butyrylcarnitine may participate in the occurrence of GERD by increasing the risk factors for GERD.Finally,an increase in butyrylcarnitine is related to an increase in visceral fat content[49].For example,research has found that compared to healthy controls,patients with steatosis and steatohepatitis have significantly higher levels of butyrylcarnitine[50].Metabolically active visceral adipose tissue secretes adipokines and inflammatory cytokines,which may induce GERD and its complications.A recent MR study also confirmed a positive correlation between visceral adipose tissue accumulation and an increased risk of GERD[51].In summary,butyrylcarnitine may participate in the occurrence of GERD through multiple pathways.Unfortunately,there is currently a lack of direct evidence linking butyrylcarnitine with GERD,including the pathogenesis of the latter.Our research for the first time discovered a relationship between genetics and metabolomics,which is also a key focus of our future research.Metabolomics has a noninvasive advantage compared to gastroscopy and pathological tissue biopsy,as it can determine the material basis for the occurrence and development of GERD and further speculate on the metabolic pathways involved.This research method combining microdetection and macroanalysis has strong technical support and extensive practical significance.
Genetic factors played a central role in our study of the relationship between metabolites and GERD,and SNP rs4767937 (corresponding to the sppl3 gene) was most significantly associated with butyrylcarnitine and GERD.SPPL3 is widely expressed in the human gastrointestinal tract,most notably in the esophagus[52].Its main function is the intramembrane cleavage of aspartic proteases and it acts as an exonuclease by mediating the release and secretion of protein hydrolysis from the active site ectodomain of glycan-modified glycosidases and glycosyltransferases[53,54].Unfortunately,there have been no reports on SPPL3 and GERD or its risk factors and complications,and the specific pathological mechanisms of SPPL3 in GERD remain to be explored.
The only metabolic pathway identified in our study was that of glycerolipid metabolism,which involves 1-linoleoylglycerophosphoethanolamine.1-Linoleoylglycerophosphoethanolamine is an important component of phosphatidylethanolamine (PE),which is composed of fatty acids,ethanolamine,phosphoric acid and glycerol[55].As a lipid chaperone,PE participates in the folding of some membrane proteins and is considered to be closely related to anxiety.Reichel's study found that alcohol dependent patients often have anxiety when abstaining from alcohol,and their plasma PE concentration is also higher after abstaining from alcohol[56].In addition,Yanget al[21] explored the relationship between metabolites and some psychiatric disorders and found that 1-linoleoylglycerophosphoethanolamine was associated with the risk of major depression,which was further confirmed in a later study[57].Therefore,it is probable that 1-linoleoylglycerophosphoethanolamine,similar to butyrylcarnitine,can influence depression to mediate the onset of GERD.In addition,an early study found that lansoprazole,one of the drugs of choice for GERD treatment,inhibited cytosolic PHOSPHO1 (a phosphatase that breaks down phosphocholine and phosphoethanolamine) in a noncompetitive manner[58].This provides some reference for revealing the relationship between the two.Regarding glycerolipid metabolism,the signaling properties of glycerolipids have been elucidated in many fields from neuroscience and cancer to diabetes and obesity.The triglyceride metabolic pathway is the main route of action for several drugs.For example,LLKL,a new traditional Chinese medicine formulation,is able to exert hypoglycemic and gut microbiota-regulating effects by inhibiting triglyceride metabolism[59].Unfortunately,however,no abnormalities in lipid metabolism have been reported in GERD.Given the lack of previous studies,the specific effects of GERD-related metabolites on GERD derived from this study need to be explored in detail under experimental conditions.
This MR analysis has several advantages.First,this is the most systematic and complete study to date on exploring the causal relationship between blood metabolites and GERD.Second,our results are convincing.The three MR estimates are highly consistent in direction and sensitivity analysis.The strict MR analysis allows us to avoid the rigorous MR analyses allow us to avoid the pitfalls of previous studies,such as reverse causality and confounding disturbances,and ensure the robustness of our results.Third,the reliability of the results was further verified by replication analysis and meta-analysis of additional GWAS data.Fourth,our study offers fresh insights into the molecular pathways underlying the pathogenesis of GERD by combining genomics and metabolomics.
There are also some limitations to this study.First,given the small number of metabolite-related SNPs,our MR analysis set a slightly relaxed threshold.However,the F statistic of all SNPs associated with metabolites was greater than 10,indicating that IVS have a strong power.Furthermore,the Steiger test results' consistent causal direction support lends credence to our lenient threshold choice.Second,for the MR analysis,we solely used GWAS data from people with European ancestry in order to reduce the impact of ethnic differences.Therefore,it merits further investigation and validation to determine whether our findings hold true for other populations.Third,our study did not perform subgroup analysis on GERD.Because the existing dataset does not distinguish between GERD subtypes,which could be further subdivided when the data are more complete,there may be differences between subtypes.Moreover,although MR analysis provides valuable insights into the etiology,our findings should be rigorously confirmed by randomized controlled trials and basic research before clinical application.
CONCLUSION
In summary,this MR study revealed that eight known blood metabolites are causally associated with GERD,with butyrylcarnitine showing a significant association signal after Bonferroni correction.Our study also highlights the extent to which genetic factors (such as SPPL3) contribute to changes in metabolic levels and the development of GERD.Glycerolipid metabolism has also been found to be possibly related to the biological processes behind GERD.Although further validation of experimental data is needed,the discovery of these serum metabolites provides valuable insights into the early screening,prevention and treatment of GERD and the design of future clinical studies.This combined genomic and metabolomic MR analysis also provides a reference direction for exploring the etiology and pathogenesis of GERD.Future research should also include genetic and metabolomic data related to GERD related diseases.For example,non erosive reflux disease,reflux esophagitis,BE,and hiatal hernia.At the same time,it is necessary to compare the genetic and metabolomic differences among various diseases,which will help clarify the relationship between diseases and better explain GERD.
ARTICLE HIGHLIGHTS
Research background
Gastroesophageal reflux disease (GERD) affects approximately 13% of the global population.However,the pathogenesis of GERD has not been fully elucidated.The development of metabolomics as a branch of systems biology in recent years has opened up new avenues for the investigation of disease processes.As a powerful statistical tool,Mendelian randomization (MR) is widely used to explore the causal relationship between exposure and outcome.
Research motivation
At present,there is still a significant lack of blood metabolomics research on GERD.
Research objectives
We used MR analysis to thoroughly investigate the causal relationships between 486 blood metabolites and GERD using data from a genome-wide association study (GWAS).Additionally,we identified the metabolic pathways that cause GERD.In addition to advancing our understanding of the pathophysiological mechanisms underlying GERD,the integration of metabolomics and genomics offers fresh perspectives on the early detection and management of the disease.
Research methods
Two-sample MR analysis was used to assess the causal relationship between blood metabolites and GERD.A GWAS of 486 metabolites was the exposure,and two different GWAS datasets of GERD were used as endpoints for the base analysis and replication and meta-analysis.Using the MR Steiger filtration method to detect whether there is a reverse causal relationship between metabolites and GERD.In addition,metabolic pathway analysis was conducted using the online database based MetaboAnalyst 5.0 software.
Research results
The results of this study indicated significant associations between eight metabolites,levulinate (4-oxovalerate) [odd ratio (OR) 0.80,95% confidence interval (CI): 0.72-0.89,P<0.0001],stearate (18:0) (OR 0.77,95%CI: 0.64-0.92,P=0.004),adrenate (22:4n6) (OR 0.83,95%CI: 0.74-0.94,P=0.004),p-acetamidophenylglucuronide (OR 0.99,95%CI: 0.99-1.00,P=0.0002),kynurenine (OR 1.20,95%CI: 1.07-1.35,P=0.002),1-linoleoylglycerophosphoethanolamine (OR 1.17,95%CI: 1.05-1.31,P=0.004),butyrylcarnitine (OR 1.09,95%CI: 1.05-1.13,P<0.0001),and guanosine (OR 1.10,95%CI: 1.03-1.18,P=0.003),and GERD.Bonferroni correction showed that butyrylcarnitine (OR 1.10,95%CI: 1.05-1.16,P=7.71 × 10-5) was the most reliable causal metabolite.Glycerophospholipid metabolism may be involved in the pathogenesis of GERD.
Research conclusions
Through the integration of genomics and metabolomics,we found that butyrylcarnitine may be a potential biomarker for GERD.
Research perspectives
The relationship between GERD and butyrilcarnitine needs further confirmation from basic and clinical real-world studies.Future research should also include genetic and metabolomic data related to GERD related diseases.For example,non erosive reflux disease,reflux esophagitis,BE,and hiatal hernia.At the same time,it is necessary to compare the genetic and metabolomic differences among various diseases,which will help clarify the relationship between diseases and better explain GERD.
ACKNOWLEDGEMENTS
We first appreciate the original GWAS data provided by So-Youn Shinet aland Jue-Sheng Onget aland Yeda Wuet aland the GWAS Catalog database.
FOOTNOTES
Author contributions:Hu JY and Lv M designed the research;Hu JY,Zhang KL,Qiao XY collected and analyzed the data;Hu JY,Lv M and Zhang KL drafted the manuscript;Qiao XY,Wang YX and Wang FY revised the manuscript;Wang FY for the entire text;all authors contributed to the article and approved the submitted version.
Supported byNational Natural Science Foundation of China,No.82174363.
Institutional review board statement:Only publicly available genome-wide association study (GWAS) data were used in this study,and the Ethics approval and consent to participate could be available in the original GWAS study.
Conflict-of-interest statement:The authors report no conflicts of interest.
Data sharing statement:All data generated or during this study are included in this published article and the supplementary materials.genome-wide association study (GWAS) summary statistics for human blood metabolites were publicly available at https://metabolomics.helmholtz-muenchen.de/gwas/.GWAS summary statistics for gastroesophageal reflux disease can be found here: https://cnsgenomics.com/content/data and https://gwas.mrcieu.ac.uk/.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Jia-Yan Hu 0000-0001-6525-6371;Mi Lv 0000-0002-1715-8643;Feng-Yun Wang 0000-0002-4058-7905.
S-Editor:Qu XL
L-Editor:A
P-Editor:Xu ZH
 World Journal of Gastrointestinal Oncology2023年12期
World Journal of Gastrointestinal Oncology2023年12期
- World Journal of Gastrointestinal Oncology的其它文章
- Prognostic value of T cell immunoglobulin and mucin-domain containing-3 expression in upper gastrointestinal tract tumors: A meta-analysis
- Association between heat shock factor protein 4 methylation and colorectal cancer risk and potential molecular mechanisms: A bioinformatics study
- Paired-related homeobox 1 induces epithelial-mesenchymal transition in oesophageal squamous cancer
- Intensive follow-up vs conventional follow-up for patients with nonmetastatic colorectal cancer treated with curative intent: A metaanalysis
- Combined TIM-3 and PD-1 blockade restrains hepatocellular carcinoma development by facilitating CD4+and CD8+T cellmediated antitumor immune responses
- Association of MBOAT7 rs641738 polymorphism with hepatocellular carcinoma susceptibility: A systematic review and meta-analysis
