Medaeops Guinot, 1967 (Brachyura, Xanthidae) from China seas, with the resurrection of Medaeops japonicus(Rathbun, 1898)*
Ziming YUAN, Wei JIANG, Zhongli SHA,**
1 Laboratory of Marine Organism Taxonomy and Phylogeny, Qingdao Key Laboratory of Marine Biodiversity and Conservation, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 Shandong Province Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences,Qingdao 266071, China
3 Laoshan Laboratory, Qingdao 266237, China
4 University of Chinese Academy of Sciences, Beijing 100049, China
Abstract The xanthid genus Medaeops Guinot, 1967 currently contains seven species reported from the Indo-West Pacific region.However, only one species, Medaeops granulosus (Haswell, 1882), has been recorded from China seas and was once thought to be widespread in the Indo-West Pacific region.In this study, two species of the Medaeops granulosus species-group were identified during the analysis on Medaeops crabs from China seas.Medaeops japonicus (Rathbun, 1898), which had previously been considered a junior synonym of M.granulosus, is shown to be a distinct species that can be distinguished from M.granulosus by the lower first anterolateral tooth, the larger and concentrated granules on carapace dorsally, and the different shape of the male’s first gonopod.In addition, a new recorded species,Medaeops edwardsi Guinot, 1967, was reported from China seas for the first time.A molecular analysis was conducted to determine the status of each species, and an updated key for species of the genus Medaeops was provided.
Keyword: euxanthine; Xanthoidea; crab; Decapoda; DNA barcode
1 INTRODUCTION
The genusMedaeopswas established by Guinot(1967) in a revision ofMedaeusDana, 1851, and initially contained three species:Medaeopsgranulosus(Haswell, 1882),M.neglectus(Balss, 1922a), andM.edwardsiGuinot, 1967, of whichM.granulosuswas selected as the type species.Currently, seven species have been reported in the genus:M.granulosus,M.neglectus,M.edwardsi,M.geminiDavie, 1997,M.merodontosDavie, 1997,M.sereneiNg & McLay, 2007, andM.potensMendoza, Chong &Ng, 2009.All the seven species were reported from the Indo-West Pacific region; however, onlyM.granulosushas been reported from China seas.In this study, two morphologically distinct species were found in the populations previously considered asM.granulosus.Materials collected from the South China Sea are here considered asM.granulosus,while individuals of the north coast of China seas are identified asMedaeopsjaponicus(Rathbun,1898).M.japonicuswas once considered a junior synonym ofM.granulosusby Guinot (1967), but herein was proved to be a valid species.In this study,we also found a new record ofM.edwardsifrom the South China Sea.
DNA barcoding based on mitochondrial cytochrome oxidase I (COI) sequences fromMedaeopscrabs were used for species delimitation in this genus.TheMedaeopsspecies key was also updated.
2 MATERIAL AND METHOD
Samples were collected from intertidal zones or by trawling from the shallow water area in Chinese waters from Guangxi to Shandong provinces.The specimens were stored in 70% ethanol and deposited at the Museum of Marine Biology, Chinese Academy of Sciences (MBMCAS), Qingdao, China.
Specimens were examined by ZEISS Stemi 2000-c and ZEISS Stemi SV 11 Apo stereomicroscopes and Nikon Eclipse Ci-L microscope.Photographs were taken using a Canon EOS 6D camera with Canon EF 100-mm and Canon MP-E 65-mm lenses or Nikon D800 camera with Nikon AF-S 105-mm lens.Male first gonopods were observed by scanning electron microscopy (SEM; model: Hitachi-S-3400N).
The morphological terminology used in this paper follows that of Dana (1852, 29, text fig), Serène(1984, 12–13, Fig.A, B) and Davie et al.(2015,Fig.71–2.6).The following abbreviations were used in the text: maximum carapace width (CW); median carapace length (CL); first gonopod of male (G1).
The sequence of COI (524 bp after alignment)was selected for phylogenetic analyses.COI sequences ofM.japonicus,M.granulosus,M.edwardsi, andM.neglectuswere analyzed, with two Eriphioidea species—EriphiasmithiiMacLeay, 1838 andMenippe rumphii(Fabricius, 1798)—selected as outgroups(Table 1).Genomic DNA ofMedaeopsspecies was extracted from muscle tissue using the OMEGA EZNA Tissue DNA Kit (USA).Sequences were amplified by polymerase chain reaction (PCR) with the primers dgLCO-1490 and dgHCO-2198 (Meyer,2003), jgLCO1490 and jgHCO2198 (Geller et al.,2013), and Pano-F and Pano-R (Thoma et al., 2014).A volume of 25 μL was used for the PCR and comprised 1-μL (2–200 ng) genomic DNA template,1 μL (10 pmol/L) of each primer, 12 μL of 2×PCR Mix (Guangzhou Dongsheng Biotechnology Co.,Ltd., China), and 10-μL ultrapure water.The PCR conditions involved an initial denaturation at 94 °C for 3 min; 35 cycles of denaturation at 94 °C for 30 s,annealing at 48 °C for 45 s, and extension at 72 °C for 45 s; and a final extension at 72 °C for 10 min.
The sequences were aligned by MEGA v6.06 using Muscle default settings (Tamura et al., 2013).Phylogenetic trees were reconstructed by Bayesian Inference (BI) and Maximum Likelihood (ML)methods as implemented in MrBayes v3.2.7(Huelsenbeck and Ronquist, 2001) and W-IQ-TREE(Trifinopoulos et al., 2016), respectively.Genetic divergences of COI between and within species were calculated by a Kimura 2-parameter distance model in MEGA v6.06 (Tamura et al., 2013).
3 TAXONOMY
Family Xanthidae MacLeay, 1838 Subfamily Euxanthinae Alcock, 1898 GenusMedaeopsGuinot, 1967
3.1 Medaeops japonicus (Rathbun, 1898) comb.nov.
Lophozozymus(Lophoxanthus)bellusvar.LeucomanusMiers, 1886: 115, Pl.11, Fig.1.[typelocality: Japan].

Table 1 Species and sequences used in the phylogenetic analysis with GenBank accession numbers and reference source
LophopanopeusjaponicusRathbun, 1898: 272;Balss, 1922 a: 125.[type locality: Japan].
LophoxanthuserosusParisi, 1916: 181, Fig.4;Menzies, 1948: 21, Pl.4, Fig.33.[type locality: Tokyo Bay].
MedaeusgranulosusSakai, 1939: 459, Pl.59,Fig.1, Pl.90, Fig.5; 1965: 135, Pl.69, Fig.2.
MedaeopsgranulosusGuinot, 1967: 366 (part);Kim, 1973: 382, Fig.145, Pl.82, Fig.110; Miyake,1983: 109, Pl.37, Fig.3; Lee, 2012: 123, Figs.92–94.(Not Haswell, 1882).
Material examined
Yellow Sea: MBM040675, 1♂, CW 16.4 mm, CL 11.2 mm, depth 29 m, 21 October 1958, Agassiz trawl; MBM040674, 1♀, CW 16.1 mm, CL 11.1 mm,depth 35 m, 24 April 1959, Agassiz trawl;MBM287005, 1 ♂, CW 14.2 mm, CL 9.6 mm, 1 ♀,CW 11.6 mm, CL 7.9 mm, Rizhao, Shandong,22 March 2019, coll.Juhao WANG; MBM287006,1♂, CW 16.1 mm, CL 11.2 mm, 1♀, CW 15.9 mm,CL 10.4 mm, Qingdao, Shandong, the first bathing beach, 15 December 2020, coll.Juhao WANG.
Jiaozhou Bay: MBM162986, 1 ♀, CW 8.7 mm CL 6.1 mm, depth 18 m, 4 August 1964, Agassiz trawl; MBM162985, 1♀, CW 12.0 mm, CL 8.3 mm,35 m, 4 August 1964, Agassiz trawl; MBM287004, 1♂,CW 8.3 mm, CL 5.9 mm, 12 November 2021, grab,coll.Dong DONG.
Zhoushan: MBM287014, 1♂, CW 12.5 mm, CL 9.0 mm, October 2020, coll.Jian CHEN;MBM287015, 1 ♀, CW 15.1 mm, CL 10.3 mm,October 2020, coll.Jian CHEN.
Description
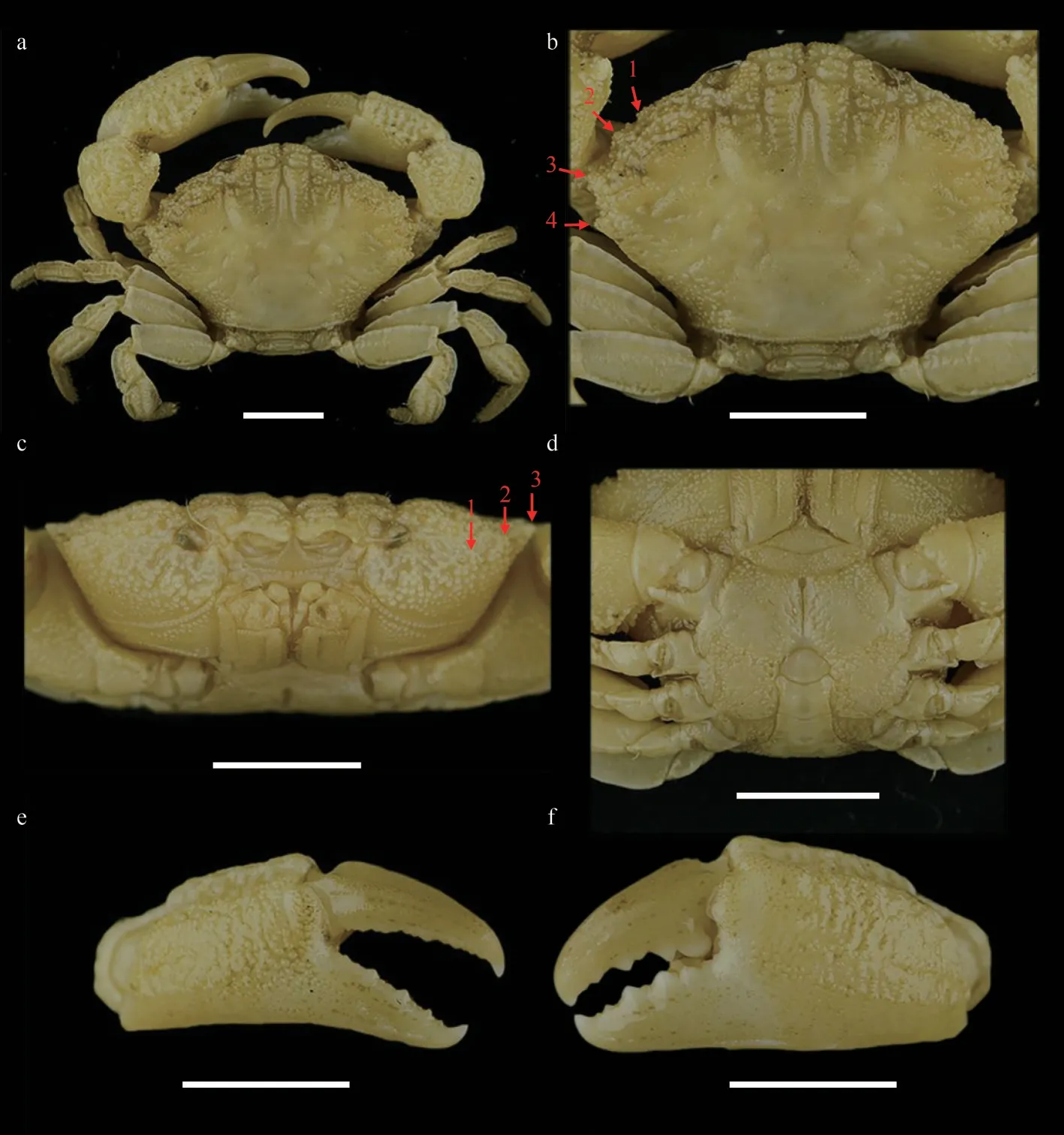
Fig.1 Medaeops japonicus (Rathbun, 1898), male, 16.4 mm×11.2 mm (MBM040675)
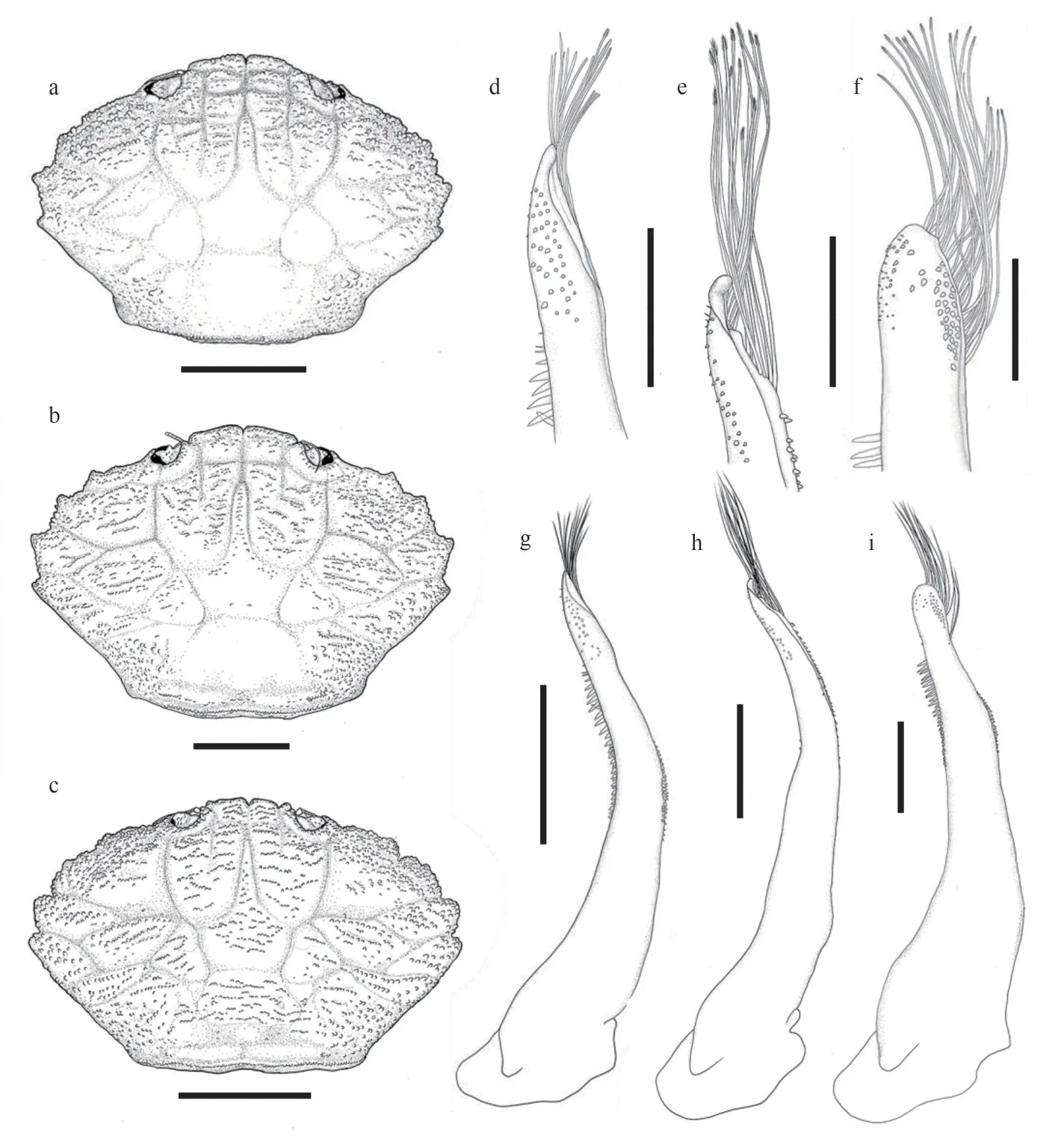
Fig.2 Carapace and G1 of species of the genus Medaeops
Carapace (Figs.1a, b, & 2a) hexagonal,approximately 1.5 times wider than long, regions well-defined anteriorly, covered with granules arranged in unclear strings, denser at anterolateral regions 1–5, medial regions 2–4; anterolateral region 6 and posterior regions flat.Frontal region approximately 1/4 of carapace width, divided into two lobes, margin granulated, slightly sinuous,separated from the dorsal margin of orbit by a deep notch.Anterolateral margin armed with four teeth except the outer orbital angle; the first tooth very small and weak, nearly invisible and covered by granules; second tooth wide and low; third tooth most prominent; fourth tooth small but evident.Posterolateral border subequal with anterolateral border; subhepatic covered with dense granules,pterygostomial region separated from subhepatic by a row of granules.Antennule (Fig.1c) situated transversely; antenna basal segment subrectangle,anterolateral angle produced, orbital hiatus filled by antennal flagellum.Third maxilliped (Fig.1c)completely covering buccal frame; ischium subquadrate, outer surface covered by granules, with submedian groove; merus subpentagonal, all the outer surface covered with granules.Thoracic sternites(Fig.1d) granular; the suture between thoracic sternites 1–2 and 2–3 distinct, backward convex, suture between thoracic sternites 3–4 visible, the median line of thoracic sternite 4 distinct and long.
Chelipeds (Fig.1e & f) slightly unequal; merus with granules on ventral and outer surface; carpus armed with a blunt tooth on inner angle, dorsal surface with granules forming a reticular relief; palm covered with granules, dorsal surface with irregular areoles, outer surface with a shallow groove near dorsal; dactylus armed with a central ridge and two longitudinal dorsal grooves; finger cutting edges with triangular teeth of different sizes, modified strong molar basal tooth on the large cheliped dactylus,finger tips sharp.
Ambulatory legs (Fig.1b) flat and merus, carpus,and propodus sculpted by sulcus in lateral and mesial faces; merus anterior edge cristate; carpus with three carina on anterior edge, more or less wavy;propodus slightly cristate; dactylus and subterminal of propodus covered with short setae, dactylus tip sharp, claw-shaped.
Pleonal somites (Fig.1d) 3–5 completely fused in male; distant angles of pleonite 6 prominent; telson short.First gonopod (Figs.2d, 2g, 3a, & 3d) stout,and inner and outer surface with small spines, small spines on outer surface transform into long spines on subterminal; ventral surface with small spines;distal lobe sharp and narrowed, rolled outward,outward-rolled region long; subdistal with long pinnate setae.
Live coloration
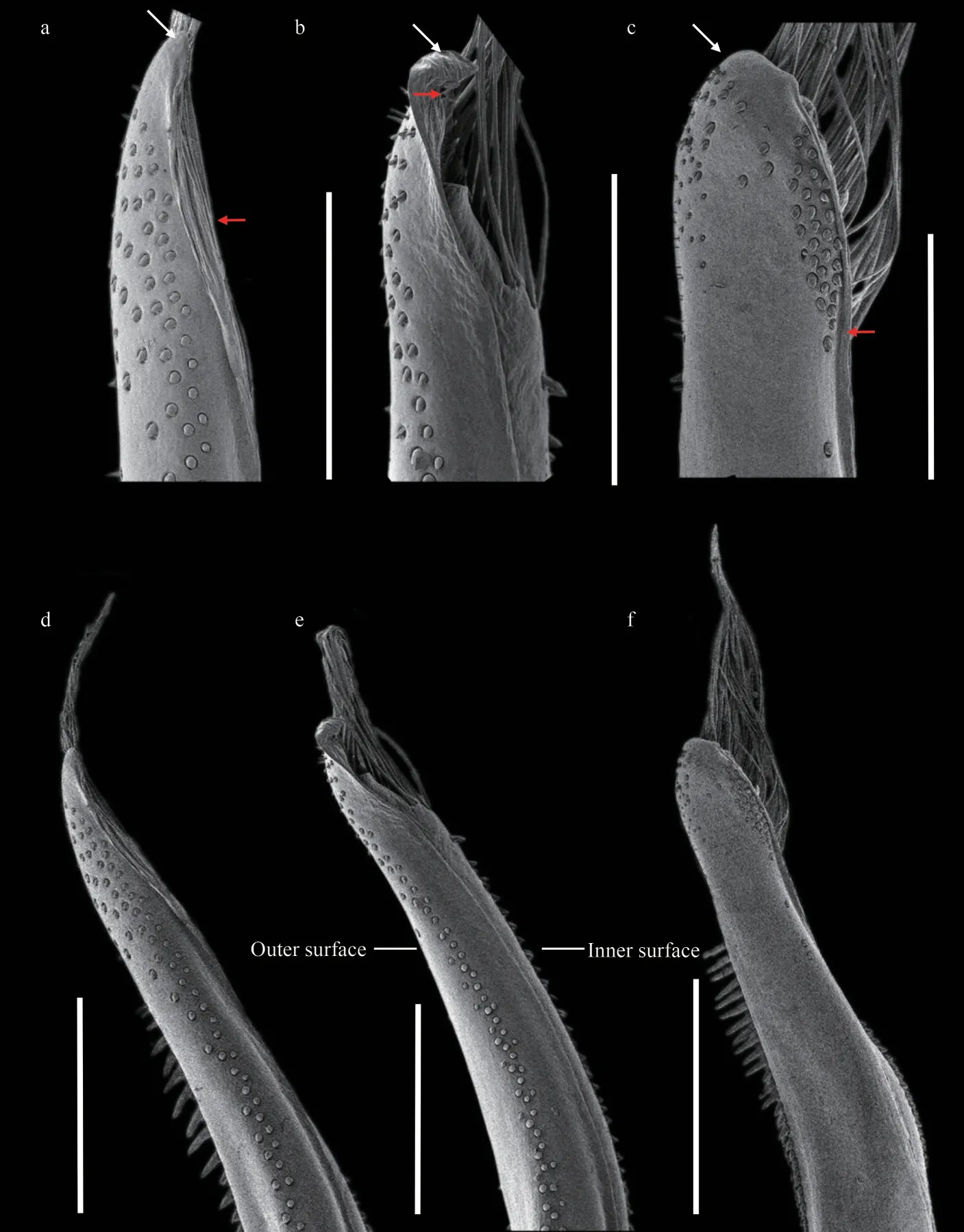
Fig.3 SEM of G1 of Medaeops species
Overall light yellow-brown, with dark lump and spots; carapace sometimes dark brown, with margins and posterior regions paler in color; fingers blackbrown, color somewhat expanding to palm; merus of ambulatory legs with brown bands or spots (Fig.4).
Distribution
The north coast of China seas: from Zhejiang to Shandong; west coast of Korean Peninsula, Kii Peninsula, Izu Peninsula, Sagami Bay, Tokyo Bay.Collected from intertidal to subtidal zones.
Remark

Fig.4 Live coloration and ecotope of Medaeops japonicus (Rathbun, 1898) from Qingdao
Medaeopsjaponicuswas first reported by Miers(1886) under the nameLophozozymus(Lophocxanthus)bellusvar.leucomanusbased on materials from Japan collected during the HMS Challenger expedition.Miers cast doubts on the identification because the specimens had the dactylus of chelipeds described as dull in color and not shining white as mentioned in the original description ofXanthodesleucomanusby Lockington (1877).Rathbun (1898) later transferred these Japanese materials to the genusLophopanopeusRathbun, 1898 and created a new name(LophopanopeusjaponicusRathbun, 1898) for them.Another species—LophoxanthuserosusParisi, 1916—had been described from material collected off Tokyo Bay.However, Balss (1922b) considered thatLophoxanthuserosuswas a junior synonym ofLophopanopeusjaponicusaccording to the description and illustration but did not elaborate further on this.Odhner (1925) listedLophoxanthuserosusandLophopanopeusjaponicusas synonyms ofLeptodius granulosusHaswell, 1882 without explanation, and transferred this species to the genusMedaeus.However, Menzie (1948) noticed that the male first pleopods ofLophoxanthuserosusare rather different from those ofMedaeusgranulosusand suggested that the species can be considered as valid.In a revision ofMedaeusby Guinot (1967),Leptodius granulosuswas transferred to the genusMedaeops,LophoxanthuserosusandLophopanopeusjaponicuswere treated as junior synonyms ofMedaeops granulosus, and the certain distribution ofM.granulosusin Australia, Japan, and China was recognized.Currently, this is the generally accepted view.
In the Chinese material of “M.granulosus”, we found two distinct species with constant morphological and molecular differences.The southern material was identified asM.granulosus(Haswell, 1882),while the northern form showed clearly different characteristics on their obvious reduced first anterolateral tooth, large granules concentrated on anterolateral regions, and the shape of male first gonopod.Although the Miers (1886) material from the Challenger expedition could not be found at the Natural History Museum (Paul F.Clark, personal communication), the material from Zhejiang to Shandong resembles the original description and figure ofLophozozymus(Lophocxanthus)bellusvar.Leucomanusand other reports based on materials from the coast of Japan and the Korean Peninsula(Parisi, 1916: Fig.4; Menzies, 1948: Pl.4, Fig.33;Sakai, 1939: Pl.59, Fig.1, Pl.90, Fig.5; Miyake,1983: Pl.37, Fig.3; Kim, 1973: Fig.145, Pl.82,Fig.110; Lee, 2012: Figs.92–94).In the molecular analyses based on COI DNA barcoding (Table 2),the southern and northern specimens showed distinct genetic differences (COI sequence divergences 7.1%).No significant genetic difference was found among the northern material (COI sequence similarities 99.9%among ON898603, ON898607, and ON898608).The southern specimens were also genetically almost identical to the sequences ofM.granulosusavailable in the GenBank database (COI sequence similarities 99.7% among the three Chinese specimens ON898601, ON898602, and ON898605, and specimens from Singapore HM751019 and Hawaii KF682805).Based on the above evidence, we resurrect the speciesLophopanopeusjaponicusRathbun, 1898 with a new combination nameMedaeopsjaponicus(Rathbun, 1898).
Medaeopsjaponicuscan be distinguished fromM.granulosusby: 1) first anterolateral tooth very small, nearly invisible and blended with granules(vs.first anterolateral tooth smaller than others but distinct, still visible even from anterior view inM.granulosus); 2) granules on carapace proportionally larger, more concentrated on anterolateral regions and subhepatic region (vs.granules proportionally smaller,forming more scattered lines inM.granulosus); 3) G1 outer surface with small spines, which transform into long spines on subterminal part (vs.only short and small spines on G1 outer surface inM.granulosus); 4) G1 distal lobe sharp and narrow,outward-rolled region long (vs.G1 distal lobe obtuse, outward-rolled region short inM.granulosus);5) the black-brown color of fingers slightly expanding to palm (vs.not expanding to palm inM.granulosus).
Medaeopsjaponicusis also similar toM.edwardsiGuinot, 1967 based on their blurry first anterolateral tooth and G1 with long spines on outer surface and distal lobe with longer outward-rolled region, and similar toM.neglectus(Balss, 1922a) mainly on the shape of G1.M.japonicuscan be easily distinguished fromM.edwardsiby: 1) second, third, and fourth anterolateral teeth sharp (vs.blunt and more vague inM.edwardsi); 2) carapace surface only with sporadic short setae or smooth (vs.densely covered by short setae on carapace, thoracic sternites, legs, and abdomen inM.edwardsi); 3) merus of ambulatory legs with anterior edge cristate (vs.merus of ambulatory legs with anterior edge not cristate inM.edwardsi); 4) G1 distal lobe sharp and narrowed (vs.distal lobe more obtuse inM.edwardsi).M.japonicuscan be distinguished fromM.neglectusby: 1) first anterolateral tooth very small, nearly invisible, and blended with granules (vs.first anterolateral tooth small but distinct inM.neglectus);2) carapace covered with larger granules (vs.carapace with smaller granules inM.neglectus); 3) ambulatory legs merus anterior edge distinct cristate (vs.ambulatory legs merus anterior edge not distinct cristate inM.neglectus) (Barnard, 1950; Forest and Guinot, 1961; Tirmizi and Ghani, 1996; Guinot, 1967;Serène, 1984; Mendoza et al., 2009; Naderloo, 2017).
To further delimit the species of genusMedaeops,molecular analyses based on COI DNA barcoding were performed.Interspecific divergences of COI sequences betweenM.japonicus,M.granulosus,M.edwardsi, andM.neglectuswere all greater than 7%2007: 48 (key); Mendoza et al., 2009: 51, Fig.2A–C,Fig.5A, B, Fig.6; Mendoza and Ng, 2010: 65; Dev Roy, 2013: 154 (list); Trivedi et al., 2018: 81 (list);Liu, 2008: 795 (list).
NonMedaeusgranulosusBalss, 1934: 507;Barnard, 1950: 219, Fig.41a, Fig.42a, b; Forest and Guinot, 1961: 56, Fig.45a, b, Pl.1, Fig.2 [=Medaeops neglectusBalss, 1922a].
NonMedaeusgranulosusSakai, 1939: 459,Pl.59, Fig.1, Pl.90, Fig.5; 1965: 135, Pl.69, Fig.2 [=Medaeopsjaponicus(Rathbun, 1898)].
NonMedaeopsgranulosusKensley, 1981: 44;Tirmizi and Ghani, 1996: 56–59, Figs.21, 22; Apel,2001: 87 [=MedaeopsneglectusBalss, 1922a].
NonMedaeopsgranulosusGuinot, 1967: 366(part); Kim, 1973: 382, Fig.145, Pl.82, Fig.110;Miyake, 1983: 109, Pl.37, Fig.3; Lee, 2012: 123,Figs.92–94.[=Medaeopsjaponicus(Rathbun, 1898)].
Material examined
Guangdong:MBM287011, 1 ♂, CW 11.2 mm,CL 7.8 mm, Dapeng, Shenzhen, September 2021;MBM287014, 1 ♂, CW 26.8 mm, CL 17.4 mm,(Table 2).Phylogenetic trees (Fig.5) based on COI sequences were reconstructed by BI and ML methods to reveal the interspecific relatedness.Individuals of the same species clustered together with high support values (100/99 forM.japonicus,100/88 forM.granulosus, and 100/98 forM.edwardsi).All the species were well supported.
3.2 Medaeops granulosus (Haswell, 1882)
LeptodiusgranulosusHaswell, 1882: 61.[type locality: Port Denison, Australia]
XanthomacgillivrayiMiers, 1884: 211, Pl.20,Fig.C.[type locality: Australia]
MedaeusgranulosusOdhner, 1925: 81; Gordon,1931: 543, Figs.19, 22A; Stephensen, 1946: 148,Fig.37A, B; Buitendijk, 1950: 75; Chhapgar, 1957:430, Pl.9, Figs.g–i.
MedaeopsgranulosusGuinot, 1967: 366, Fig.40(part); Serène and Umali, 1972: 65, Figs.58–59, Pl.7,Figs.1–2; Serène and Vadon, 1981: 122; Fig.55B(3),Pl.37(7); Serène, 1984: 91 (key); Dai and Yang, 1991:295, Fig.155B(3), Pl.37(7); Davie, 1997: 357 (key);Ng and Davie, 2002: 375 (list), 381; Ng and Mclay,Shenzhen, January 2022; MBM287009, 1 ♂, CW 28.1 mm, CL 18.5 mm, Dapeng, Shenzhen, depth 6 m, February 2021.
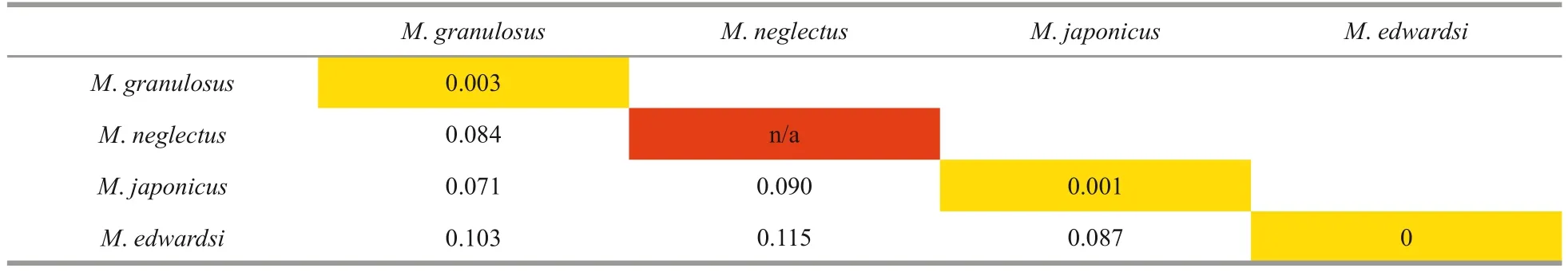
Table 2 Genetic divergence of COI gene among the four species of Medaeops calculated from Kimura 2-Parametercorrected calculations
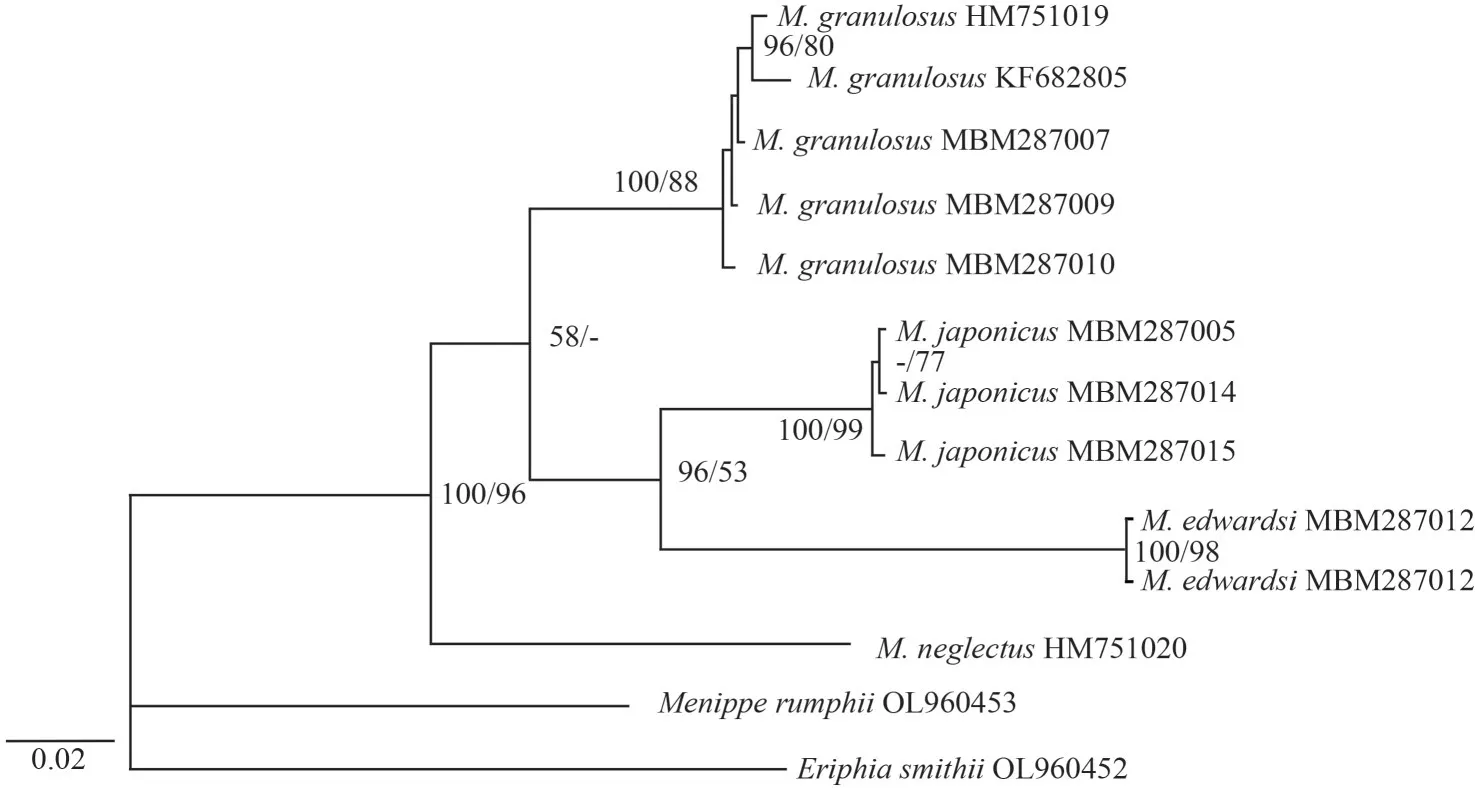
Fig.5 Phylogenetic relationships among species of the genus Medaeops based on COI sequences
Fujian:MBM287008, 1 ♂, CW 22.4 mm, CL 15.4 mm, Xiamen, Jinwan, 23 July 2021, coll.Xu ZHANG; MBM287010, 1 ♂, CW 25.7 mm, CL 17.3 mm, Xiamen, December 2021, coll.Xu ZHANG;MBM205016, 1♂, CW 31.1 mm, CL 20.4 mm, 1♀,CW 25.3 mm, CL 16.4 mm, 1♀, 20.0 mm×13.7 mm,Xiamen, December 2021, coll.Xu ZHANG.
Guangxi: MBM287007, 1♂, CW 19.55 mm, CL 13.32 mm, Weizhou Island, Beihai, December 2019,coll.Juhao WANG.
Description
Carapace (Figs.6a, 6b, & 2b) hexagonal,approximately 1.5 times wider than long, regions welldefined anteriorly, covered with granules arranged in unclear strings.Frontal region approximately 1/4 the breadth of carapace width, divided into two lobes,margins granulated, slightly sinuous, separated from the dorsal margin of orbit by a deep notch.Anterolateral margin armed with four teeth except the outer orbital angle; the first tooth small but distinct and sharp; second tooth wide, with a sharp tip; third tooth most prominent; fourth tooth small but evident.Posterolateral border subequal with anterolateral border; subhepatic covered with granules,pterygostomial region separated from subhepatic by a row of granules.Antennule (Fig.6c) situated transversely; antenna basal segment subrectangle,anterolateral angle produced, orbital hiatus filled by antennal flagellum.Third maxilliped (Fig.6c)completely covering buccal frame; ischium subquadrate, outer surface covered by granules, with submedian groove; merus subpentagonal, all the outer surface covered with granules.Thoracic sternites(Fig.6d) with granules on anterior part; the suture between thoracic sternites 1–2 and 2–3 distinct,backward convex, suture between thoracic sternites 3–4 visible, the median line of thoracic sternite 4 distinct and long.

Fig.6 Medaeops granulosus (Haswell, 1882), male, 22.4 mm×15.4 mm (MBM287008)
Chelipeds (Fig.6e & f) slightly unequal; merus with granules on ventral and outer surface; carpus armed with a blunt tooth on inner angle, dorsal surface with granules and tubercles; palm covered with granules, formed reticular relief on dorsal surface, outer surface with a shallow groove near dorsal; dactylus armed with a central ridge and two longitudinal dorsal grooves; finger cutting edges with blunt teeth in different sizes, modified strong molar basal tooth on the large cheliped dactylus finger tips sharp.
Ambulatory legs (Fig.6b) stout, covered with granules, merus anterior edge cristate, carpus anterior edge granular, sinuous; propodus and dactylus covered with short setae, dactylus tip sharp, claw-shaped.
Pleonal somites (Fig.6d) 3–5 completely fused in male; distant angles of pleonite 6 prominent; telson short.First gonopod (Figs.2e, 2h, 3b, & 3e) stout,and inner, outer, and ventral surface with small spines; distal lobe obtuse, rolled outward, outwardrolled region short; subdistal with long pinnate setae.
Live coloration
Carapace brownish, with margins and posterior regions paler in color; darker brown fingers not expanding to the palm; merus of ambulatory legs with bands or brown spots (Fig.7).
Distribution

Fig.7 Live coloration and ecotope of Medaeops granulosus (Haswell, 1882)
South China Sea: Guangxi, Hainan Island,Guangdong, Hongkong, Fujian, Taiwan Island; Gulf of Oman, west coast of Indian Subcontinent, Phuket Island, Singapore Strait, Philippine Islands, Australia,Hawaii Island.Collected from intertidal zone of rocky seashores.
Remark
Medaeopsgranulosus(Haswell, 1882) was originally described from Port Denison and Port Molle of eastern Australia.Miers described another species,XanthomacgillivrayiMiers 1884 based on Australian materials collected from Port Molle, Port Curtis, and Facing Island.Odhner (1925) listedX.macgillivrayias a synonym ofLeptodiusgranulosusHaswell, 1882.Guinot (1967) selectedM.granulosusas the type species ofMedaeops.
Owing to their very similar morphology,Medaeops granulosuswas confused withMedaeopsneglectus(Balss, 1922a) for a long time, andMedaeops neglectusis thought to be confined to the Red Sea and the western Indian Ocean (Guinot, 1967; Serène,1984; Naderloo and Türkay, 2012).According to the literature, the few diagnostic characters that distinguish these species are the anterior edge of the merus of the ambulatory legs has a distinct cristate inM.granulosus(contrast with the not distinct cristate on anterior edge of merus inM.neglectus), and the different shape of G1 (Guinot, 1967; Serène, 1984;Mendoza et al., 2009).The remaining records from the western Indian Ocean still need to be reexamined (Stephensen, 1946; Chhapgar, 1957; Dev Roy, 2013; Trivedi et al., 2018).
Present materials ofM.granulosusshow significant variations, predominantly on the crests on merus of ambulatory legs (from sharp and strongly protruding to only represented by a slightly extended edge), and the granularity of the carapace (from granules clear and closely packed to barely visible and hidden beneath wrinkles) (Fig.8).These variations are not accompanied by significant genetic differences (Table 2);consequently, they are regarded as intraspecific.
3.3 Medaeops edwardsi Guinot, 1967
MedaeopsedwardsiGuinot 1967: 369, Figs.33,42 [type locality: Malabar coast]; Serène, 1984: 91(in key), 92, Fig.53, Pl.12, Fig.E; Ghani and Tirmizi,1992: 43, Fig.4; Tirmizi and Ghani, 1996: 54-56,Fig.20; Naderloo et al., 2015: 407 (list); Naderloo,2017: 260, Figs.21.34; Mendoza, 2021: 467, Fig.4.
Material examined
MBM287012, 1 ♀, CW 10.1 mm, CL 7.7 mm,Mulan Bay, Wenchang, Hainan, 12 November 2016,coll.Junlong ZHANG et al.; MBM287013, 1♂, CW 32.3 mm, CW 20.5 mm, Weizhou Island, Beihai,Guangxi, December 2019, coll.Juhao WANG.

Fig.8 Medaeops granulosus (Haswell, 1882)
Diagnosis
Carapace (Figs.9a, 9b, & 2c) transversely ovate,approximately 1.5 times wider than long, dorsal flat,with granules arranged in lines, dense short setae in front of the granular lines; anterolateral margin with four blunt and vague teeth; merus of ambulatory legs with anterior edge not cristate (Fig.9a); G1(Figs.2f, 2i, 3c, & 3f) stout, and inner and outer surface with larger spines on subterminal part of outer surface; distal lobe round, rolled outward,outward-rolled region long and narrow, subdistal with long pinnate setae.
Distribution
South China Sea: Guangxi, Hainan Island;Madagascar Island, Gulf of Oman, west coast of Indian subcontinent, Singapore Strait, and Australia.Collected from intertidal zone.
Remark
This species was first reported on the Malabar Coast and the paratype is probably from Madagascar(see Guinot, 1967).Another record ofM.edwardsiwas found among the type material ofM.granulosus, which was collected from Queensland (Mendoza et al., 2009).
The original description of this species given by Guinot (1967) is detailed and accurate.This is the first report ofMedaeopsedwardsiin Chinese waters.The current materials are generally consistent with previous descriptions (Guinot, 1967; Serène, 1984;Ghani and Tirmizi, 1992; Naderloo, 2017), except for the larger male, who shows prominent and welldefined anterolateral teeth separated by deep v-shaped notches (Figs.9b & 2c).In previous descriptions, the anterolateral margin ofM.edwardsiis formed by four denticulate lobes separated by deep cracks but not formed by teeth (Guinot, 1967, Fig.33; Serène,1984, Pl.12, Fig.E; Ghani and Tirmizi, 1992, Fig.4;Tirmizi and Ghani, 1996, Fig.20; Naderloo, 2017,Figs.21, 34; Mendoza, 2021, Fig.4).In contrast, the anterolateral margin of the smaller female is consistent with previous descriptions.Considering there are no genetic differences between the two individuals (Table 2, COI sequence similarity is 100%between ON898604 and ON898606), such difference is considered as a sexual or ontogenetic difference.
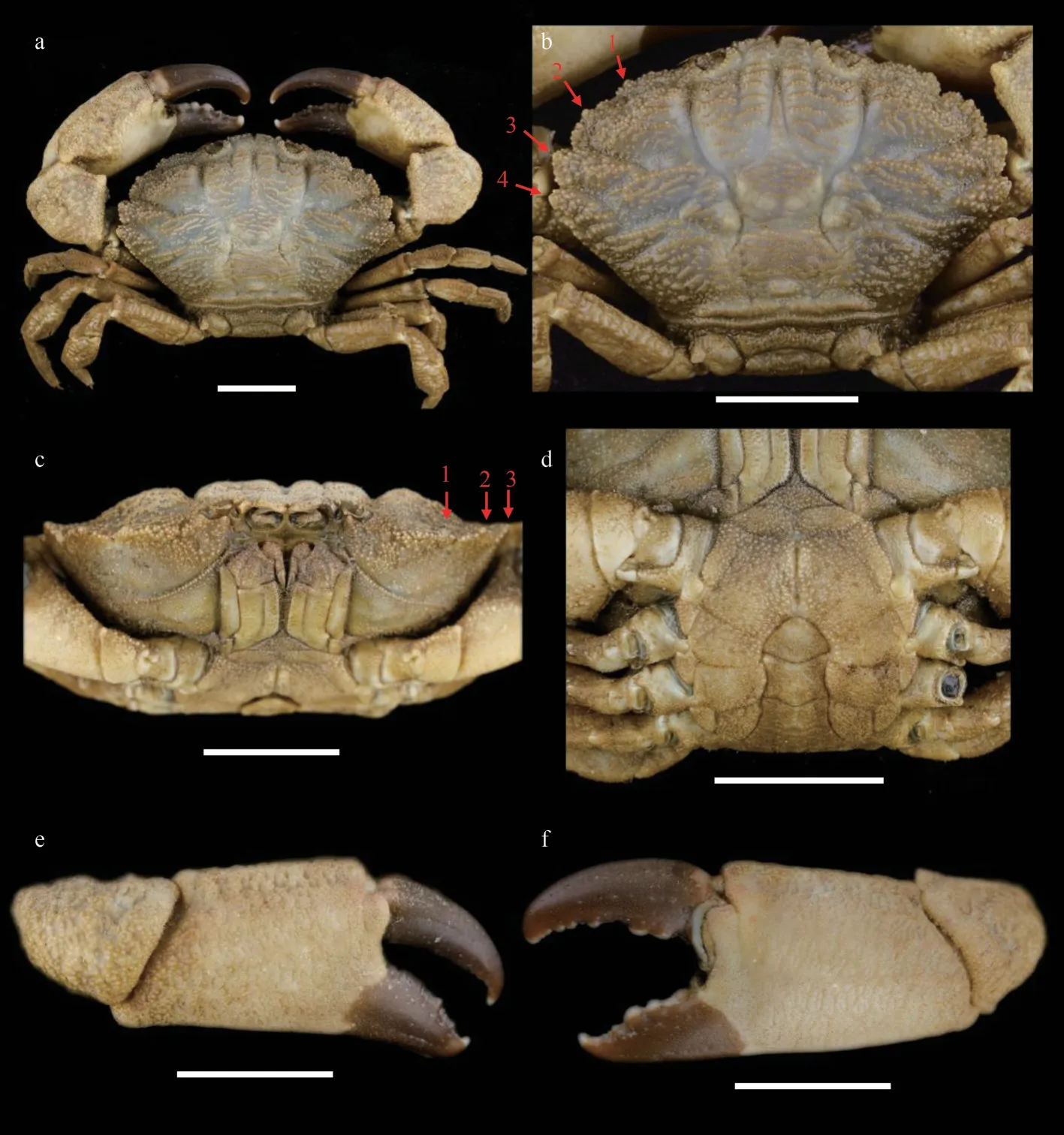
Fig.9 Medaeops edwardsi Guinot, 1967, male (32.3 mm×20.5 mm) (MBM287013)
Considering the many name changes in the genusMedaeops, the geographical distribution of the three species in the China seas are now updated (Fig.10)and a key to the species of the genusMedaeopsis provided below.
Map review No.GS(2016)1665.
4 KEY TO THE SPECIES OF MEDAEOPS(ADAPTED FROM NG & MCLAY, 2007)
1 Carapace anterolateral margin teeth only weakly developed on an evenly convex margin...............................M.edwardsiGuinot, 1967
-Carapace anterolateral teeth prominent,broad.........................................................................2
2 Ambulatory legs distinctly carinate along anterior margins.........................................................3
-Ambulatory legs not distinctly carinate along anterior margins.........................................................5
3 Front distinct prominent; anterior half of carapace is much shorter than posterior half; anterolateral teeth developed and prominent.......................M.potensMendoza, Chong & Ng, 2009
-Front not prominent, anterior half of carapace subequal with posterior half......................................4
4 First anterolateral tooth of carapace weak and small; G1 inner surface with long spines on subterminal part, distal lobe sharp and narrow,outward-rolled region long..........................................M.japonicus(Rathbun, 1898)
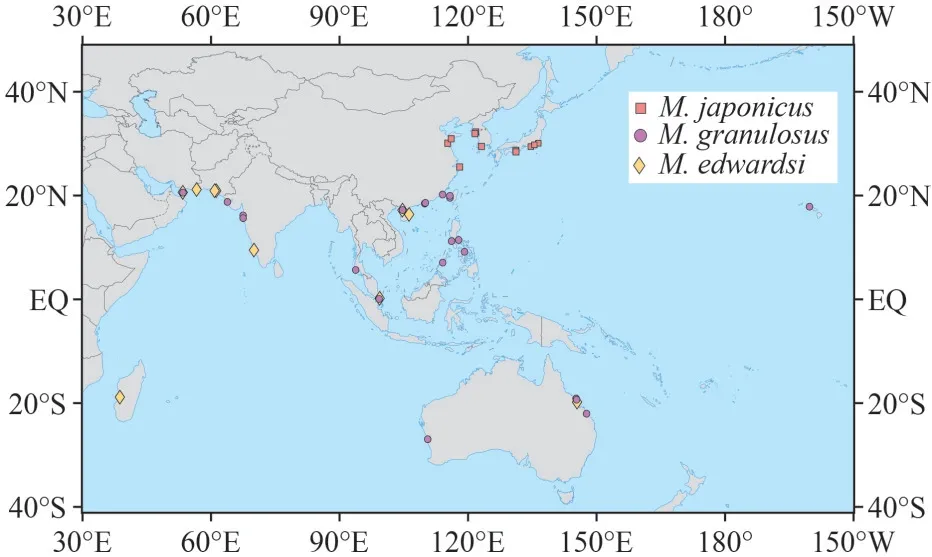
Fig.10 Worldwide distribution of M.granulosus, M.japonicus, and M.edwardsi
-First anterolateral tooth of carapace small but distinct; G1 inner surface only with short spines,distal lobe obtuse, outward-rolled region short...........................M.granulosus(Haswell, 1882)
5 Carapace medial regions 4 distinct; ambulatory legs with row of spaced teeth along anterior margins of merus..........................M.merodontosDavie, 1997
-Carapace medial regions 4 not distinct;ambulatory legs with anterior margins of merus smooth or with at most large granules......................6
6 Ambulatory legs relatively long; carapace evenly granular.Frontal lobes separated by a broad notch.......................................M.geminiDavie, 1997
-Ambulatory legs relatively short; carapace with granules arranged in clumps especially on anterior third; frontal lobes separated by a narrow slit or notch; relatively short ambulatory legs.....................7
7 Carapace regions well marked on anterior 2/3;pleonal segment 6 with concave margins; G1 stout..................................M.neglectus (Balss, 1922)
-Carapace regions well marked only on anterior 1/3; pleonal segment 6 with straight parallel margins; G1 slender.........................................................................................M.sereneiNg & McLay, 2007
5 DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
6 ACKNOWLEDGMENT
We would like to thank Dong DONG, Junlong ZHANG (Institute of Oceanology, Chinese Academy of Sciences), and Juhao WANG and Xu ZHANG for the specimen collection, and Jian CHEN and Xingle GUO (Zhejiang Ocean University) for kindly providing the Zhoushan specimens.We are also very grateful to Juhao WANG and Xu ZHANG from Institute of Zoology, Chinese Academy of Sciences for providing photos of live crabs.We would like to thank Wei LIU and Yuanyuan SUN (Institute of Oceanology, Chinese Academy of Sciences) for their help in the SEM observation.
 Journal of Oceanology and Limnology2023年6期
Journal of Oceanology and Limnology2023年6期
- Journal of Oceanology and Limnology的其它文章
- Trends of carbon and nutrient accumulation through time in the Andong salt marsh, Hangzhou Bay, China*
- Physical processes determining the distribution patterns of Nemopilema nomurai in the East China Sea*
- Comparison in structure and predicted function of epiphytic bacteria on Neopyropia yezoensis and Neopyropia katadae*
- Interaction between macroalgae and microplastics: Caulerpa lentillifera and Gracilaria tenuistipitata as microplastic bio-elimination vectors*
- Lake regime shift from submerged macrophyte to phytoplankton affected phosphorus speciation in sediment and eutrophic state in Caohai Lake, Guizhou, China*
- Temporal characteristics of algae-denitrifying bacteria co-occurrence patterns and denitrifier assembly in epiphytic biofilms on submerged macrophytes in Caohai Lake, SW China*
