Temporal characteristics of algae-denitrifying bacteria co-occurrence patterns and denitrifier assembly in epiphytic biofilms on submerged macrophytes in Caohai Lake, SW China*
Pinhua XIA , Guoqing LI, Xianfei HUANG, Lei SHI, Xin DU, Tao LIN
Guizhou Province Key Laboratory for Information System of Mountainous Areas and Protection of Ecological Environment,Guizhou Normal University, Guiyang 550001, China
Abstract Denitrifying bacteria in epiphytic biofilms play a crucial role in nitrogen cycle in aquatic habitats.However, little is known about the connection between algae and denitrifying bacteria and their assembly processes in epiphytic biofilms.Epiphytic biofilms were collected from submerged macrophytes(Patamogeton lucens and Najas marina L.) in the Caohai Lake, Guizhou, SW China, from July to November 2020 to: (1) investigate the impact of abiotic and biotic variables on denitrifying bacterial communities; (2) investigate the temporal variation of the algae-denitrifying bacteria co-occurrence networks; and (3) determine the contribution of deterministic and stochastic processes to the formation of denitrifying bacterial communities.Abiotic and biotic factors influenced the variation in the denitrifying bacterial community, as shown in the Mantel test.The co-occurrence network analysis unveiled intricate interactions among algae to denitrifying bacteria.Denitrifying bacterial community co-occurrence network complexity (larger average degrees representing stronger network complexity) increased continuously from July to September and decreased in October before increasing in November.The co-occurrence network complexity of the algae and nirS-encoding denitrifying bacteria tended to increase from July to November.The co-occurrence network complexity of the algal and denitrifying bacterial communities was modified by ammonia nitrogen (NH+4-N) and total phosphorus (TP), pH, and water temperature (WT), according to the ordinary least-squares (OLS) model.The modified stochasticity ratio(MST) results reveal that deterministic selection dominated the assembly of denitrifying bacterial communities.The influence of environmental variables to denitrifying bacterial communities, as well as characteristics of algal-bacterial co-occurrence networks and the assembly process of denitrifying bacterial communities, were discovered in epiphytic biofilms in this study.The findings could aid in the appropriate understanding and use of epiphytic biofilms denitrification function, as well as the enhancement of water quality.
Keyword: denitrifying bacteria; epiphytic biofilms; co-occurrence networks; submerged macrophytes;community assembly
1 INTRODUCTION
Biofilms are a complex community of microbiota(algae, bacteria, fungi, animals, and inorganic and organic detritus) that are attached to substrata (Wetzel,1983; Gubelit and Grossart, 2020).Biofilms are widespread in soils, sediments, submerged plants, and surfaces of natural and artificial substrates and are essential for the absorption, decomposition and transformation to water pollutants (Pang et al., 2016;Han et al., 2018).The submerged plant-biofilm system absorbs approximately 30% of the ammonia nitrogen and removes more than 50% of the ammonia nitrogen by denitrification in constructed wetland mesocosms (Mu et al., 2020).Exceptional capacity for denitrification of epiphytic bacterial communities in natural aquatic ecosystems uses functional predictive analysis (Yan et al., 2019).Epiphytic biofilms therefore play an important function within the nitrogen cycle in aquatic ecological systems (Mu et al., 2020; Li et al., 2022).
Denitrifying functional microorganisms drive denitrification in ecosystems.Microorganisms release enzymes such as nitrate reductase (Nar), nitrite reductase (Nir), nitric oxide reductase (Nor), nitrite reductase (Nir), nitric oxide reductase (Nor), and nitrous oxide reductase (Nos) that catalyze the denitrification process (Fozia et al., 2020).Nitrite reductase (nirSandnirK) are functionally equivalent but structurally separate restriction enzymes in denitrification that mediate the conversion of nitrite(NO-2) to nitric oxide (NO) (Kraft et al., 2011;Shrewsbury et al., 2016; Chen et al., 2017).ThenirSandnirKfunctional genes have been widely used to research denitrifying bacteria in sediments (Zheng et al., 2015; Hong et al., 2019), soil (Wang et al., 2019),compost (Zhong et al., 2020), wastewater (Lu et al.,2014), and biofilms (Vila-Costa et al., 2014; Zhang et al., 2020).Temperature (Zhou et al., 2016), pH (Liu et al., 2010; Yang et al., 2017), nitrate-nitrogen (NO-3-N)(Shi et al., 2019; Dai et al., 2020), carbon to nitrogen ratio (C꞉N) (Dai et al., 2020), total nitrogen (TN)(Zhou et al., 2016), dissolved oxygen (DO) (Shi et al.,2019), and ammonia-nitrogen (NH+4-N) (Zhou et al.,2016; Shi et al., 2019; Dai et al., 2020) all affect the community composition, abundance, and diversity ofnirS- andnirK-type denitrifying bacteria.In addition,adding algae can be very effective in enhancing the effectiveness of denitrification in artificial wetlands(Cheng et al., 2021).The genes of thenirSand thenirKand their relationship with environmental factors in submerged plant biofilms were investigated(Lyautey et al., 2013; Vila-Costa et al., 2014; Yan et al., 2018; Zhang et al., 2020).However, previous research has overlooked the association with algal and denitrifiers in epiphytic biofilms.
There are intricate relationships among the algae and bacteria, such as metabolism, competition, and parasitism (Natrah et al., 2014).Algae can release oxygen and secrete organic matter or dead residues as a nutrient source to promote bacterial growth(Natrah et al., 2014).Algae may also secrete antagonistic substances to kill bacteria or compete for nutrient deficiencies (Desbois et al., 2008; Natrah et al., 2014).Moreover, algae can secrete carbon source substances to promote denitrification (Ishida et al.,2008; Lu et al., 2014), and there may be complex interactions between algae and denitrifying bacteria.Co-occurrence network analysis, in which nodes represent species and links represent interactions between species, is widely used to study the interactions between microbial communities and their responses on environmental variations (Wang et al.,2021; Yu et al., 2021; Liu et al., 2022).As a result, cooccurrence network analysis opens up new avenues for research into the interaction between algal and denitrifying bacterial communities.
The primary factors of microbial community assembly in aquatic habitats are deterministic and stochastic processes.Non-random, ecological nichebased mechanisms such as environmental filtering and diverse biological interactions between community species are examples of deterministic processes (e.g.,competition, facilitation, metabolism, and predation)(Niederdorfer et al., 2021).Birth, death, dispersal, and migration are the key factors that drive microbial community assembly in stochastic processes (Zhou and Ning, 2017).The construction of microbial communities appears to be regulated by both deterministic and stochastic mechanisms (Zhou and Ning, 2017).Environmental filtration, interspecies interactions, and dispersion limitation all influence microbial co-occurrence patterns (D’Amen et al.,2018; Liu et al., 2022).Thus, to study on the mechanism of denitrifying bacterial community assembly is helpful to understand the algaedenitrifying bacteria co-occurrence patterns and correlation in epiphytic biofilms.
We studied the biofilm attached to typical submerged plants (PatamogetonlucensandNajas marinaL.) using high-throughput sequencing technologies and statistical analysis to: (1) determine the effect of abiotic and biotic variables on the denitrifying bacterial communities; (2) investigate the temporal variation of the algae-denitrifying bacteria cooccurrence networks; and (3) identify the contribution of deterministic and stochastic processes to the formation of denitrifying bacterial communities.
2 MATERIAL AND METHOD
2.1 Study area and sampling
The research site is located on the Caohai Lake(104°12′E–104°18′E, 26°49′N–26°53′N) in Guizhou Province, SW China (Supplementary Fig.S1).This area has a subtropical humid monsoon climate with an average annual temperature of 10.5 °C and 1 000 mm of precipitation (Xia et al., 2020).Submerged macrophytes (Patamogetonlucens,NajasmarinaL.) and water were collected at six sites in July,August, September, October, and November of 2020.
All plant samples were taken in triplicates from three to five plants of each submerged macrophyte and transferred to sterile 500-mL polyethylene bottles containing 400 mL of 50-mmol/L phosphatebuffered saline (PES, pH=7.4) solution, stored with ice bags, and returned to the laboratory rapidly(Zhang et al., 2016; Xia et al., 2020).For water chemistry analysis, water samples (1.5 L) were taken from the surface water in the sampling region.
2.2 Sample pretreatment
After 3 min of ultrasonic treatment in a KQ5200 DE ultrasonic cleaner bath (Kunshan Ultrasonic Instruments Manufacture Co.Ltd., Kunshan,China), 30 min in a SHZ-82A thermostatic shaking water bath (225 r/min) (Jintan Ronghua Instrument Manufacture Co.Ltd., Jiangsu, China), and another 3 min of ultrasonic treatment (Zhang et al., 2016;Xia et al., 2020).100 mL of mixed liquor was filtered through 0.22-μm membrane filters (Millipore Ireland Ltd., Ireland) after complete detachment for gather epiphytic denitrifiers (Yan et al., 2019).The filters were gathered and kept at 20 °C until the DNA was extracted.
Take out the leaves and lay them flat on a graduated glass plate, place them in the light for photography and finally calculate the leaf area(Song et al., 2019).The eluate was kept in 100 mL of formaldehyde solution (3%), then algae were accounted for and identified using phytoplankton atlas under the light microscope (Yan et al., 2019).The algal density was then calculated from the above data.The pH and water temperature (WT) of the overlying water were measured by a potable meter.Standard methods in China for assessing surface water were used to quantify total nitrogen(TN), total phosphorus (TP), ammonia (NH+4-N),chemical oxygen demand (CODMn, using the potassium permanganate method), and chlorophylla(Chla).Secchi disc was used to test water transparency (SD).
2.3 DNA extraction and Illumina MiSep highthroughput sequencing
According to the manufacturer’s instructions,microbial DNA was extracted from filters using a FastDNA®Spin Kit for Soil (MPBiomedicals, Santa Ana, CA, USA).DNA concentration was checked using a NanoDrop2000 and (Thermo Fisher Scientific,Waltham, MA, USA), and DNA quality was assessed by performing 1% agarose gel electrophoresis according to the manufacturer’s instructions.For PCR amplification analysis, DNA samples were kept at-80 °C.To investigate the microbial community composition ofnirK- andnirS-encoding denitrifiers in epiphytic biofilms on submerged macrophytes,thenirKgene was amplified using primers (F1aCu: 5′-ATCATGGTSCTGCCGCG-3′/R3Cu: 5′-GCCTCGA TCAGRTTGTGGTT-3′) and thenirSgene was amplified using primers (the 20-L PCR reaction mixture contains 4 L of 5X FastPfu Buffer, 0.8 mL of each primer (5 μm), 10 ng of template DNA,0.4 mL of FastPfu polymerase, 0.2 mL of BSA, 2 mL of 2.5 mmol/L dNTPs, and 20 mL of ddH2O).After that, the PCR products were purified, and libraries were created.An Illumina MiSeq platform was used for sequencing (Illumina, San Diego, USA).
2.4 Statistical analysis
ArcGIS was used to create the sampling diagram(version 10.6).In this work, we used “Hellinger” to transform microbial community data and log(x+1) to transform physicochemical data (excluding pH).R(version 4.0.1,https://www.r-project.org/) was used to examine physicochemical data for the water column from different month in significance of differences using nonparametric tests (Kruskal-Wallis test and Wilcoxon test).Boxplots were used to depict the physico-chemical properties of the surface water, which were created in R using the“ggplot2” package (Xia et al., 2020).
The algal composition and algal density data in the epiphytic biofilm were processed with Excel software and visualized with Hiplot (https://hiplot.com.cn/).The community’s alpha diversity was measured using the Chao 1 index, Shannon index,Simpson index, and phylogenetic diversity (PD)index, all of which were calculated using the R package “vegan”.The “aov” function in R was used to examine the substantial differences (P<0.05) in alpha diversity index of the denitrifying bacterial communities between different months using oneway analysis of variance (one-way ANOVA).
The principal coordinate analysis (PCoA) and permutation multivariate analysis of variance(PERMANOVA) based on Bray-Curtis distances at the denitrifying bacteria OTU level were implemented in“vegan” installation package in R, and in “ggplot2”package in R for visualization, to study the differences in denitrifying bacteria community in different months.Bray-Curtis distances at the denitrifying bacteria OTU level were implemented in the “vegan” installation package in R, and the“ggplot2” package in R for visualization, to study the differences in denitrifying bacteria community in different months.The “core” function in the“corrplot” package in R was used to compute and plot the Spearman correlation between physicochemical data and algal density in epiphytic biofilms.Using the“vegan” package in R, the Mantel test was used to investigate the association between denitrifying bacterial community composition and abiotic and biotic variables.
The co-occurrence network analysis was used to evaluate the interaction between denitrifiers and algae in epiphytic biofilms, and it was processed using the “WGCNA” package in R based on relative abundance of denitrifying bacteria and algal data, and then visualized with Gephi (V0.9.2)software.For the analysis, the best ordinary least squares (OLS) multiple regression models of variance in the complexity of the co-occurrence network of algae withnirS-type denitrifier and the network of the co-occurrence network of algae withnirK-type denitrifier were chosen.Using “MASS”packages in R, the adequacy of the models was assessed using Akaike’s information criterion(AIC), and the variance inflation factor (VIF) was determined using “CAR” packages in R.The niche breadth index and Modified stochasticity ratio(MST), which were generated using the “spaa” and“NST” packages in R, respectively, were used to analyze the relative importance of stochasticity and determinism for denitrifying community assembly.
3 RESULT
3.1 Physicochemical property
In different months, the physicochemical properties of the water body were examined(Supplementary Fig.S2).The water temperature gradually decreased from July to November, and there were significant differences in different months (P<0.05).From July to September, total phosphorus (TP), ammonia nitrogen (NH+4-N), and pH values decreased, then increased in November.The Chl-acontent and transparency (SD) were not significantly different between months.
3.2 Algae and denitrifying bacteria community composition and diversity
The algae in the epiphytic biofilms were identified, and 6 phylum and 77 genera of algae were obtained.The dominant phylum of algae in epiphytic biofilms were Chlorophyta (51.37%),Bacillariophyta (20.67%), and Cyanophyta (20.56%)(Fig.1).The density of algae in the epiphytic biofilms was 1.33×107cells/cm2(August), 3.54×106cells/cm2(October), 2.80×106cells/cm2(July),2.19×106cells/cm2(November), and 1.62×106cells/cm2(September) in descending order (Supplementary Fig.S3).
The dominate phylum ofnirS-type andnirK-type denitrifying bacteria were Proteobacteria (39.98%±19.64% and 51.50%±14.29%, respectively) and unclassified bacteria (51.86%±17.14% and 45.36%±13.74%, respectively) (Fig.2).In different months,the Shannon, Simpson, Chao1, and PD indices of thenirS-type denitrifying bacteria community varied significantly (P<0.05) (Table 1).From July through November, the Shannon index exhibited an upward trend (Table 1).At different month, the Simpson, Chao1, and PD indices of thenirK-type denitrifying bacterial community were not significantly different (Table 1).
3.3 Relationships between denitrifying bacteria and abiotic and biotic factors
ThenirS-type denitrifying bacteria differed significantly (R2=0.134,P=0.02) during different months, according to PCoA and PERMANOVA analyses based on the Bray-Curtis distances(Fig.3).ThenirS-type denitrifying bacteria’s beta diversity was highest in August, and significantly higher (P<0.05) in July–September than in October–November (Fig.3c).The Mantel test demonstrated a significantly positive association with the CODMn, Chla, SD, WT, Cyanophyta density, and total algal density (R>0.1,P<0.05) and a strong link with thenirS-type denitrifying bacteria and Euglenophyta density (R=0.215,P=0.002)(Fig.3d).
The PCoA and PERMANOVA analyses showed that thenirK-type denitrifying bacteria differed significantly (R2=0.160,P=0.01) at the different month (Fig.3).The largest beta diversity was found innirK-type denitrifying bacteria in September,which was significantly greater than in August,October, and November (P<0.05) (Fig.3c).The Mantel test found a significant correlation between thenirK-type denitrifying bacteria and Chla(R=0.158,P<0.05) (Fig.3d), and no significant correlation with other environmental factors.
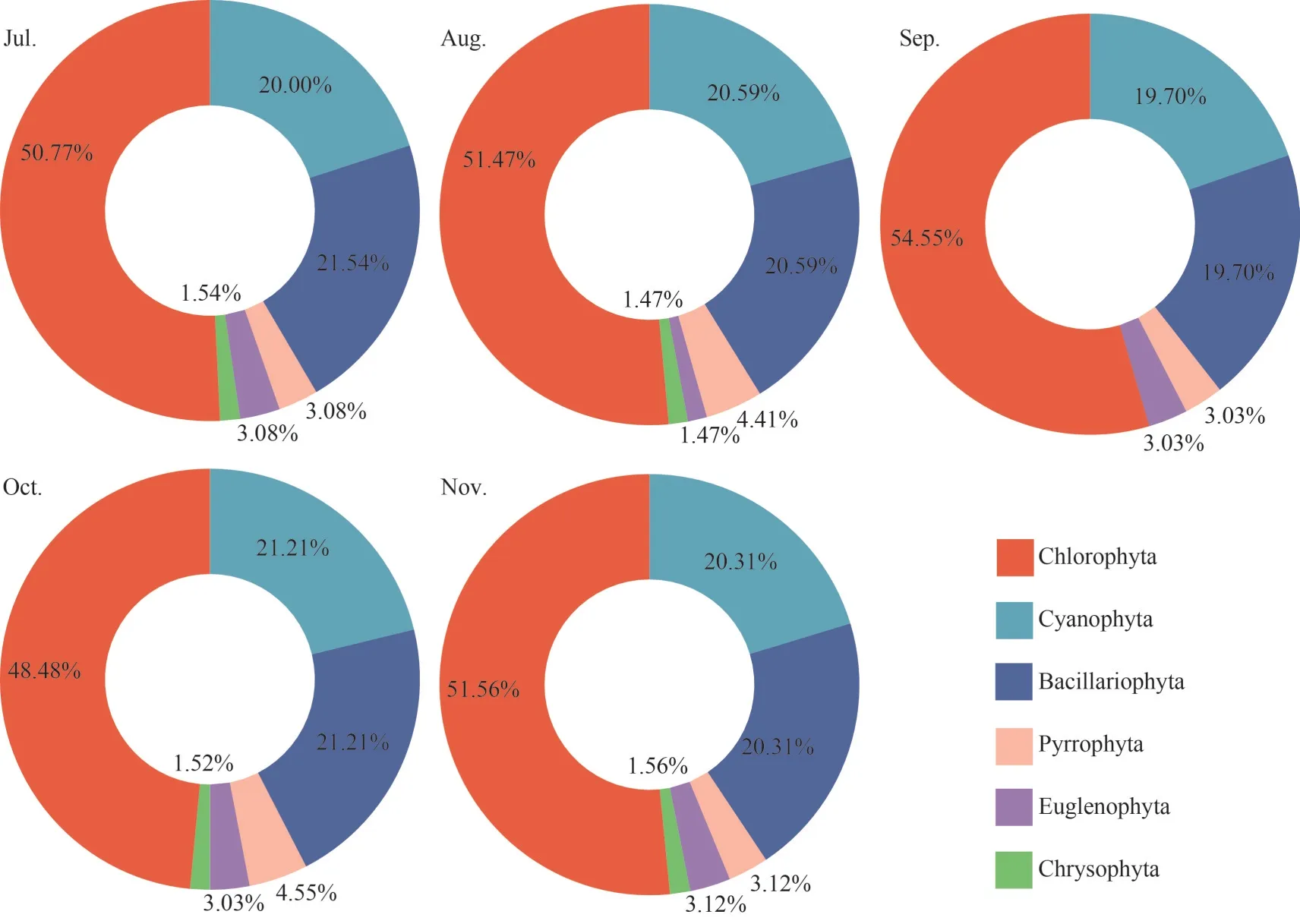
Fig.1 The relative abundance of algae in epiphytic biofilms at the phylum
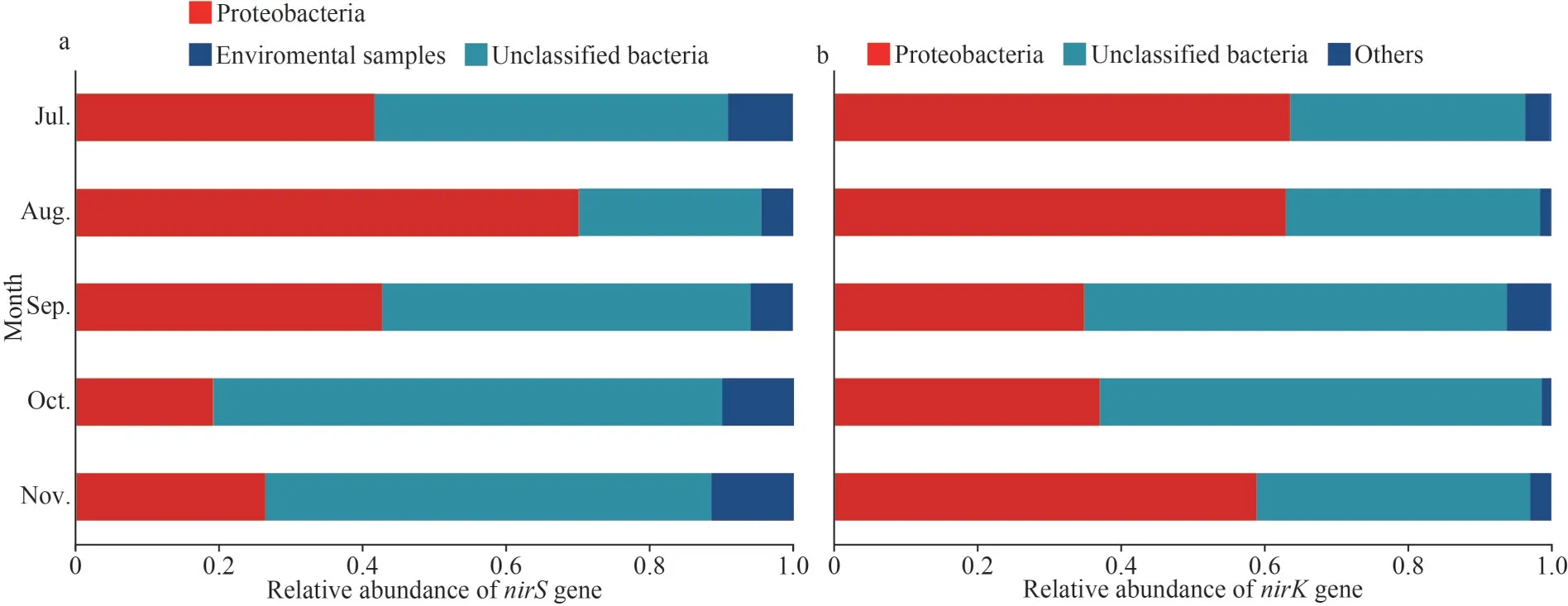
Fig.2 The relative abundance of denitrifying community in epiphytic biofilms at the phylum
3.4 Co-occurrence network
The denitrifying bacterial community characteristics in epiphytic biofilms were assessed using cooccurrence network analysis at different months(Supplementary Fig.S4).From July to September,thenirS-type denitrifying bacterial community cooccurrence network had increasing links and an average degree, then decreased in October before reaching its maximum nodes, links, and average degree in November (Supplementary Table S1).From July to September, the nodes, links, and average degree of thenirK-type denitrifying bacterial community co-occurrence network risen substantially and then decreased in October, with the links and average degree increasing in November, but the maximum number of nodes, links, and average degree appearing in September (Supplementary Table S1).Although the co-occurrence patterns ofnirS- andnirK-type denitrifying bacteria showed similar temporal variation, the periods when the highest average degree occurred were different.The average degree of co-occurrence network of thenirKtype denitrifying bacterial community was higher than thenirS-type denitrifying bacteria community except in November.
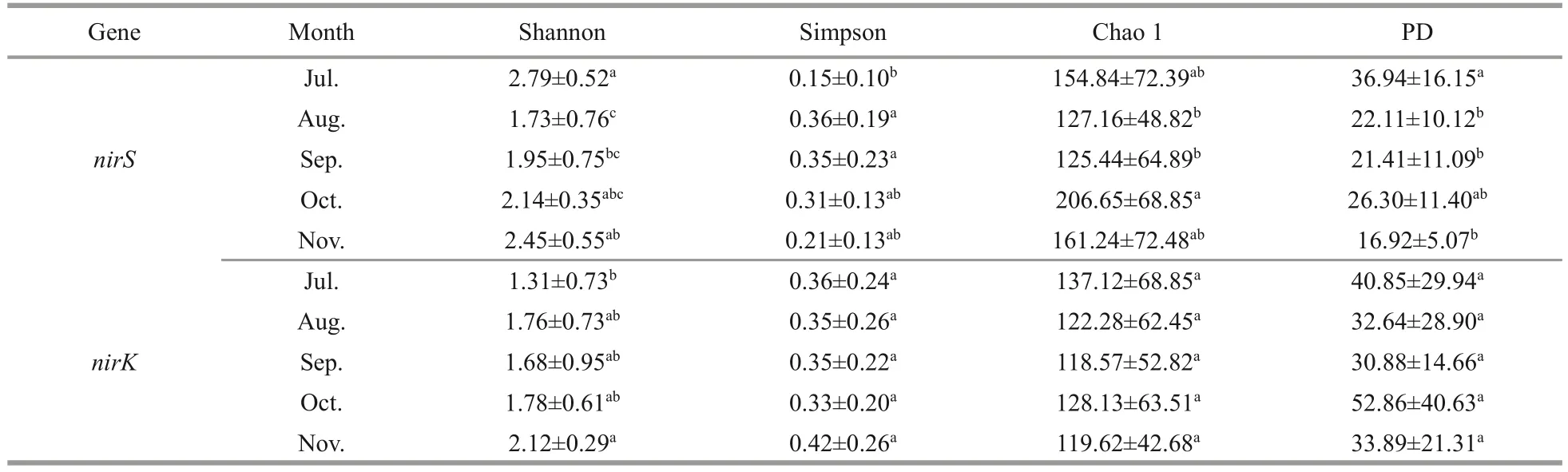
Table 1 The alpha diversity of nirS- and nirK-encoding bacteria in the epiphytic biofilms
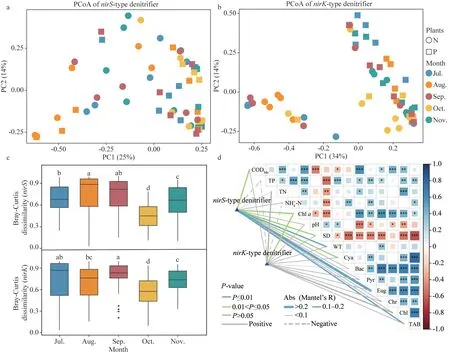
Fig.3 The principal coordinate analysis (PCoA) of nirS- and nirK-encoding denitrifier at OTU level (calculated using Bray-Curtis) (a & b); the beta-diversity were estimated based on a Bray-Curtis distance matrix (c); correlations among physicochemical properties, algae (phyla) density and the composition of the denitrifying community in epiphytic biofilms (d)
ThenirS- andnirK-type denitrifying bacteria(OTU level) and algae (genus level) from epiphytic biofilms at different months were used to create cooccurrence networks (Figs.4–5).The links of thenirS-type denitrifying bacteria and algae cooccurrence network in the biofilm from the July to the November showed an increasing trend and reached a maximum in the November (Fig.4).The average degree of thenirK-type denitrifying bacteria and algae co-occurrence network tended to increase from the July to the October, reaching a maximum in the October and decreasing to a minimum in the November (Fig.5).From July to October, thenirKtype denitrifying bacteria and algae co-occurrence network had a higher average degree than thenirStype denitrifying bacteria and algae co-occurrence network (Table 2).
The WT, TP, CODMn, NH+4-N, and pH explained 95.2% of the variation in the complexity of thenirStype denitrifying bacteria and algae co-occurrence network (Supplementary Table S2), while the WT,TP, TN, CODMn, NH+4-N, and pH explained 70.8%to the complexity by thenirK-type denitrifying bacteria with algae co-occurrence network(Supplementary Table S2), according to the ordinary least-squares (OLS) model.
3.5 Microbial community assembly process
Niche breadths were used to investigate the contributions of deterministic and stochastic processes within denitrifying bacterial community.In July, thenirS-type denitrifying bacteria’s niche breadth was significantly higher than in August,September, and October (P<0.05) (Fig.6).ThenirKtype denitrifying bacteria’s niche breadth was significantly higher in November than in July(P<0.05), and the niche breadth of thenirK-type denitrifying bacteria was increasing trend by month(Fig.6).The MST was used to analyze the dominant processes in microbial community construction.The MST mean values for thenirS- andnirK-type denitrifying bacterial communities were both below the 50% cut-off value, indicating a greater contribution from deterministic processes (Fig.7).Furthermore, the mean MST ofnirS-type denitrifying bacteria andnirK-type denitrifying bacteria has been increasing trend by month (Fig.7).
4 DISCUSSION
4.1 Effect of abiotic and biotic factors on denitrifying bacterial communities
Previous research has suggested that temperature can affect bacterial and algal dynamics in aquatic ecosystems either directly or indirectly (Wang et al.,2015; Xia et al., 2020).Because different species are subject different suitable growth and reproduction temperature.In our study, the abiotic factors(CODMn, SD, and WT) and biotic factors(Cyanophyta density, Euglenophyta density, total algae density, and Chla) and were significantly associated withnirS-type denitrifying bacteria communities (Fig.3).ThenirK-type denitrifying bacteria were only significantly correlated with Chla, and no other environmental factors were found to be significant (Fig.3).This indicates that the change of denitrifying bacteria community is closely related to the change of environmental factors.In addition, it was found that the variation of denitrifying bacteria community was not only related to the changes of physical and chemical factors, but also significantly related to the density of algae.Carbon sources, which are thought to be a key factor influencing the function and structure of denitrifying bacterial community (Lu et al., 2014;Zhou et al., 2016) and have a greater impact on denitrifying bacterial community structure compared to those of other factors (e.g., carbon to nitrogen ratio, C꞉N) (Lu et al., 2014).Denitrifying bacteria require organic carbon as an electron donor during cell growth and denitrification, and the addition of carbon sources can effectively promote cell growth and improve denitrification efficiency(Rajta et al., 2020).Different carbon sources such as glycerol, glucose, succinate, ethanol, sucrose,methanol, formic acid, and sodium pyruvate have been tested for their effects on denitrification rates(Rajta et al., 2020).Thus, the growth of denitrifying bacteria in aquatic ecosystems may be extremely dependent on the carbon sources secreted by algae.This may be the reason why the denitrifying bacteria community is significantly correlated with algal density.
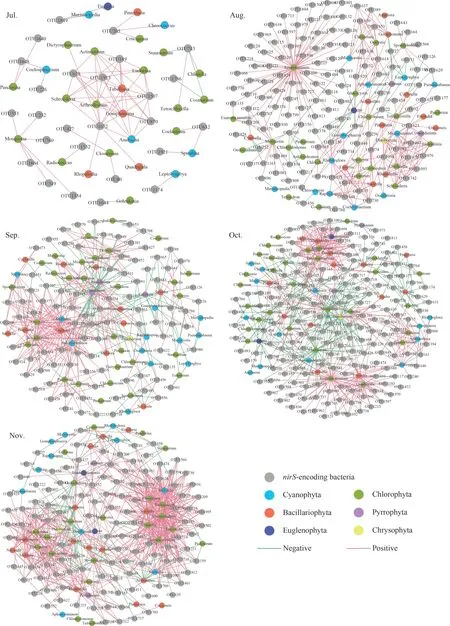
Fig.4 The association network of nirS-encoding denitrifiers and algae
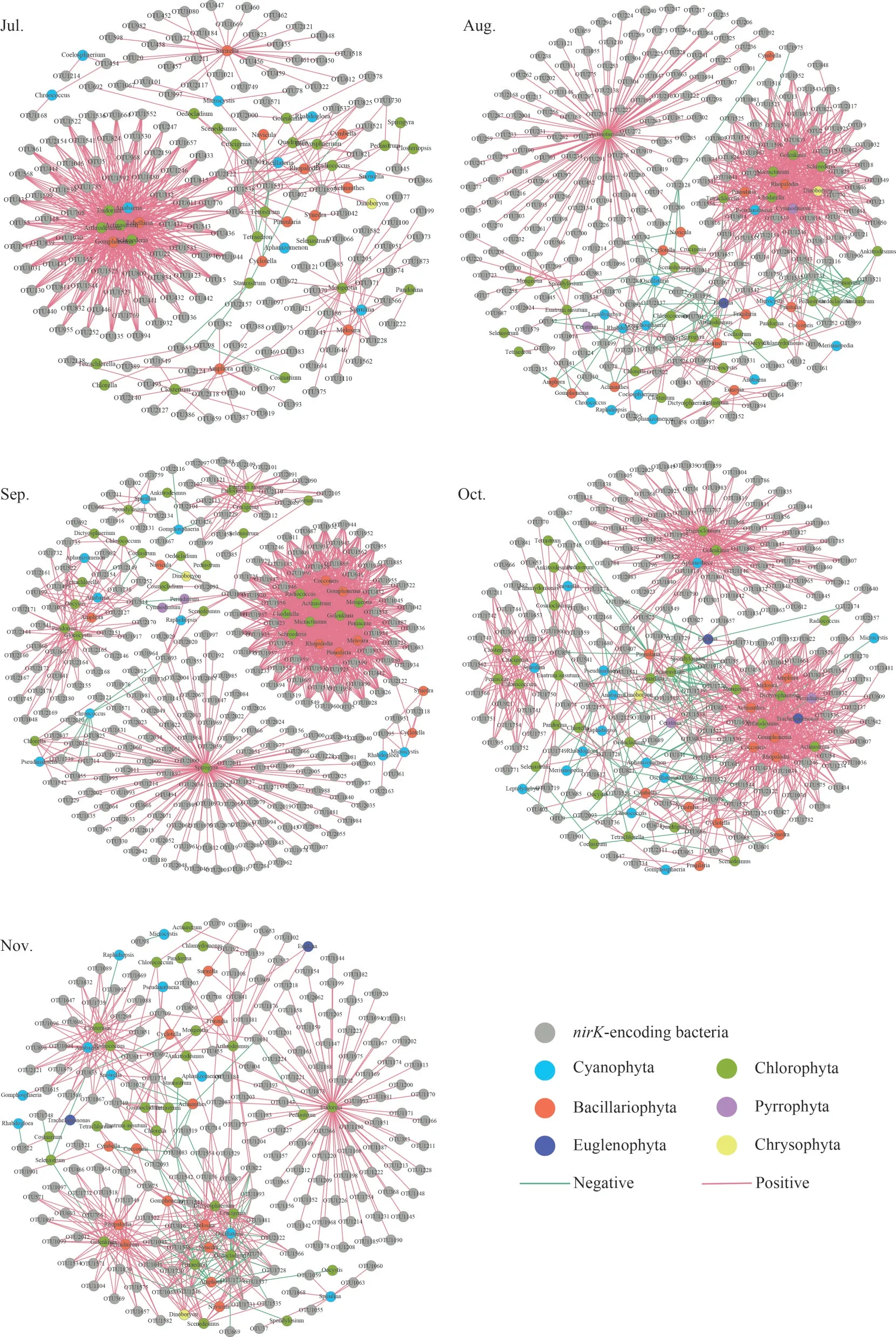
Fig.5 The association network of nirK-encoding denitrifiers and algae
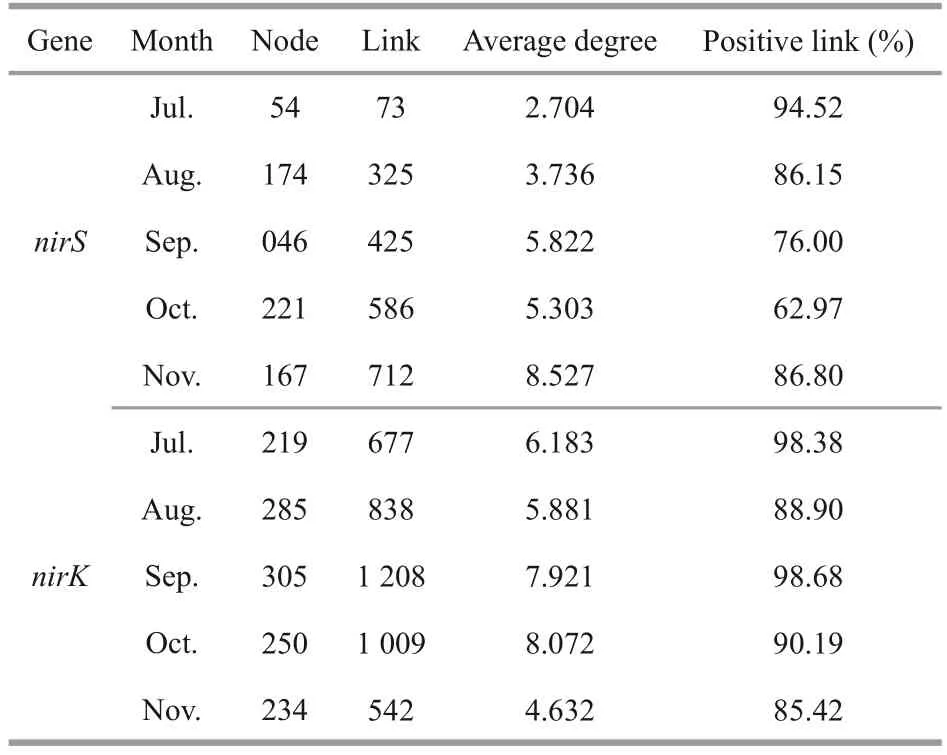
Table 2 The properties of the network co-occurrence of nirS-type and nirK-type denitrifying bacteria with algae in different months
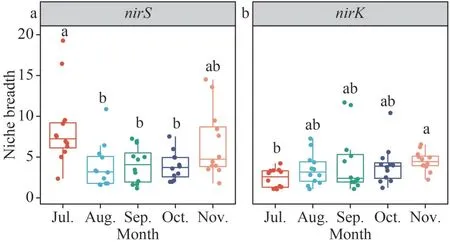
Fig.6 Boxplots showing the mean habitat niche breadth nirS- and nirK-type denitrifier in epiphytic biofilms
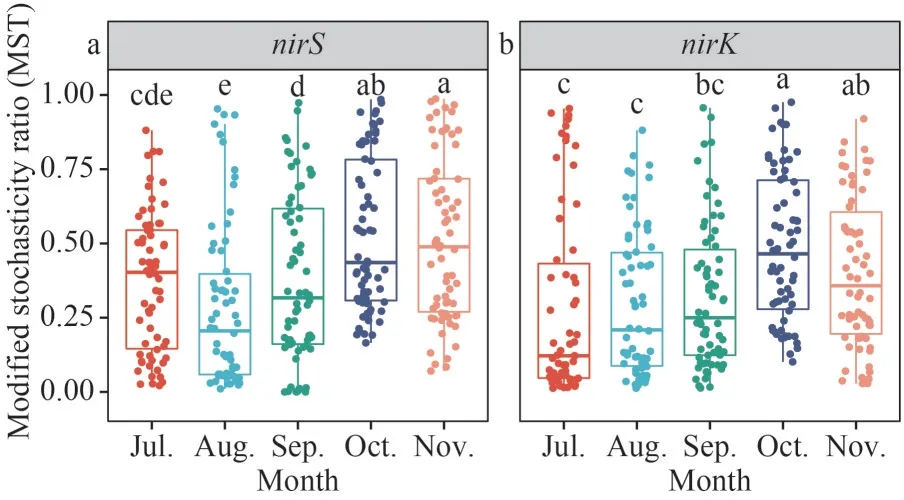
Fig.7 Barplots showing the comparison of modified stochasticity ratio (MST) of nirS- and nirK-type denitrifier in epiphytic biofilms
In aquatic ecosystems, Cyanophyta fix organic carbon and synthesize and secrete extracellular organic matter (EOM) such as polysaccharides,proteins, and nucleic acids through photosynthesis,and EOM can be used directly as a carbon source to promote heterotrophic bacteria’s growth (Henderson et al., 2008; Ramanan et al., 2015).The abundance of denitrifying bacteria increased significantly under moderate cyanobacterial blooms, and denitrification rates increased (Henderson et al., 2008; Deng et al.,2020), producing large amounts of EOM to supply denitrifying bacteria to facilitate the denitrification process.Cyanophyta carbon secretion is affected by external environmental changes; for example, elevated light intensity can significantly increase extracellular polysaccharide production inAnabaena(Moreno et al.,1998).Enhancing the proportion of polysaccharide substances in EOM can effectively promote denitrification (Chen et al., 2018).Furthermore,apoptotic Cyanophyta can be employed as a heterotrophic bacterial carbon source to encourage heterotrophic bacterial development (Natrah et al.,2014; Deng et al., 2020).Bacterial enzyme production is promoted by algae in unidentified bacterial biofilms under light (Espeland et al., 2001;Natrah et al., 2014).Chlorophyllahas good biocatalytic and electron transfer effects and can effectively promote nitrite reduction during denitrification (Lu et al., 2020).The lack of carbon source material in artificial wetlands can lead to reduced denitrification, and the addition of algae can be very effective in improving denitrification efficiency in artificial wetlands (Cheng et al., 2021).
In conclusion, the abiotic and biotic factors in our study had an important influence on the structure of denitrifying bacterial communities.In epiphytic biofilms on submerged macrophytes, there may be complicated interactions between algae and denitrifying bacteria.
4.2 Temporal variation of algae-denitrifying bacteria co-occurrence network
Microbial interactions and microbial community succession are frequently studied using cooccurrence network analysis (Jeong et al., 2016; Liu et al., 2020).Network analysis reveals the complex relationship between algae and bacteria and is dominated by positive correlations (Xia et al.,2020).In our study, the denitrifying bacteria community variation is closely related to abiotic and biotic factors, especially denitrifying bacteria and algae may have complex interactions.As a result, cooccurrence network analysis was utilized to investigate the interaction between algae and denitrifying bacteria in epiphytic biofilms, as well as their temporal alterations.
Saikia et al.(2013) concluded that biofilm formation consists of four stages: (1) nutrients are adsorbed on the solid surface following physicochemical processes;(2) bacteria begin to colonize and produce extracellular organic matter (EPS); (3) protozoa,algae, and other microorganisms colonize; and(4) eukaryotes begin to colonize and have nutritional and grazing functions.The second stage is where it starts to be defined as a biofilm.We collected algae and denitrifying bacteria in July, indicating that the biofilm has formed.The early stage of biofilm (July)in our study showed less microbial growth and weaker species interactions as compared those of the other month.The network complexity of the July to September of thenirS- andnirK-type denitrifying bacteria in the biofilm had an increasing trend.It was indicated a continuous colonization of the biofilm by microorganisms, an increase in species competition relationships, and the beginning of a large number of intra- and interspecific cooccurrence relationships (Niederdorfer et al., 2021).The network complexity decreased in the October,which was probably because the core community was gradually forming, with only transient new species attaching and migrating (Niederdorfer et al.,2021).The increase in network complexity during the November indicates that stable microbial communities were formed in the biofilm, and microbial interactions were enhanced.In our study,the co-occurrence network of thenirS-type denitrifying bacterial communities during the biofilm from July to November was less complex and more modular compared to the co-occurrence network of thenirK-type denitrifying bacterial communities (Table 2).ThenirS-type denitrifying bacteria associated more closely with each other than thenirK-type denitrifying bacteria, implying that higher co-occurrence network complexity indicated greater microbial interactions.
The difference in modularity may be due to ecological niche differentiation, habitat heterogeneity,or diversity selection (Li et al., 2021).Ecological studies have found the coexistence of specific species of algae and bacteria with specific interactions between them (Kouzuma and Watanabe, 2015).Algae and bacteria have complicated relationships that include metabolism, competition, and parasitism(Natrah et al., 2014).Organic matter secreted by algae sustains bacterial growth, whereas bacteria consume oxygen to release carbon dioxide to promote algal growth (Liu et al., 2017).Bacteria degrade organic matter while producing vitamin B12,an extracellular secretion necessary for algal growth(Natrah et al., 2014).In addition, some microalgal metabolites have bactericidal effects, such as green algal proteins, against both Gram-positive and Gram-negative bacteria (Gonçalves et al., 2017).Cooccurrence networks can be effective in uncovering potential algal-bacterial relationships, and biofilms as new life forms are ideal materials for studying temporal changes in algal-bacterial relationships.The links of the algae andnirS-type denitrifying bacteria co-occurrence network in our study from the July to the November increased, and the network complexity showed an increasing trend (Fig.4).Planktonic microorganisms constantly colonize biofilms in aquatic ecosystems, and some microorganisms return to the aquatic environment because of unsuitable growth (He et al., 2014).Along with the enhanced interaction between biofilm-growing algae and denitrifying bacteria, more microorganisms colonize the biofilm.Positive and negative interactions between microbial species in co-occurrence networks are considered to be reciprocal and antagonistic relationships (Miao et al., 2021).In our study, there was a tendency for a positive correlation between algae and denitrifying bacteria (positive links>60%),which was probably because algae secrete carbon sources to promote the growth of denitrifying bacteria, forming a reciprocal relationship (Lu et al.,2014; Natrah et al., 2014).In the October, the highest percentage of negative correlation edges between thenirS-type denitrifying bacteria and algal interaction networks indicated a large number of antagonistic relationships.This is because algae compete with bacteria for nutrient deficiency or secrete antagonistic substances to inhibit bacterial growth (Natrah et al., 2014).The complexity of thenirK-type denitrifying bacteria and algal cooccurrence network increased continuously from the July phase to the October, with enhanced microbial interactions.The lowest network complexity in the November period may be because algae associated with thenirK-type denitrifying bacteria are affected by seasonal changes.
Interaction patterns between algae and bacteria are not constant and are influenced by a variety of environmental factors and multiple interactions(Zhu et al., 2021).The algal-bacterial relationships and co-occurrence network complexity in our study were dynamic with the biofilm growth and were influenced by the external environment.Contaminants such as heavy metals, nanomaterials, and pesticides can affect algal-bacterial interactions (You et al.,2021).Under nutrient-limited conditions, heterotrophic bacteria may compete with Cyanophyta for nutrient salts such as nitrogen and phosphorus.Seasonal changes in algal community composition and biomass in epiphytic biofilms are characterized and significantly influenced by the WT, pH, and nutrients (Feng et al., 2011), thus affecting changes in the algal-bacterial network relationships.Our results suggest that changes in the nitrogen and phosphorus nutrients, pH, and temperature can affect changes in the complexity of the denitrifying bacteria-algae co-occurrence network.
4.3 Denitrifying community assembly in biofilms is dominated by deterministic processes
The niche-based theory and neutral theory are two significant complimentary methods for understanding microbial community building, and they are widely applied in diverse environments(Chen and Wen, 2021; Xiong et al., 2021).The sediment community in hot spring sediments had a stronger phylogenetic clustering and was predominantly directed by heterogeneous selection,whereas the water community was primarily governed by undominated stochastic processes and dispersal constraints (He et al., 2021).The formation of bacterial and archaeal community in subtropical riverine systems is strongly affected by deterministic and stochastic processes, but the stochastic process of bacterial communities is much more pronounced than that of archaeal communities (Wang et al., 2020).In the surface soil of a typical red soil critical zone,stochastic mechanisms dominate the bacterial community assembly process, but deterministic processes dominate in the deeper layers (Wu et al.,2020).UnderSpartinaalterniflorainvasion,stochastic processes drive the formation of archaeal and bacterial communities in sediments, with bacteria having a higher relative stochastic influence than archaea (Chen and Wen, 2021).In the Hanhe River,spatial factors (stochastic processes) explain more of the composition of the bacterioplankton community than environmental factors, with neutral community models explaining most of the fall and spring community variation, and the structure of the bacterioplankton community is largely influenced by stochastic processes (Sun et al., 2021).Microorganisms in these environments are not only controlled by deterministic factors and environmental conditions that control community construction but also by stochastic processes such as birth, death,colonization, and species formation.Deterministic processes dominate bacterial community development in epiphytic biofilms, although stochastic processes’ effects rose significantly in months later than June (He et al., 2020).
Based on niche breadth and MST, we investigated the assembly process ofnirS- andnirK-type denitrifying bacteria in biofilms.As a metric of stochasticity, the MST value reflects the contribution of the stochastic assembly relative to the deterministic assembly.The mean MST of thenirS- andnirK-type denitrifying bacterial communities was found to be below the 50% cut-off limit, showing that deterministic processes dominated thenirS- andnirKtype denitrifying bacteria.The deterministic process of thenirS-type denitrifying bacterial communities contributed the most in August, followed by the stochasticity process, which steadily grew.The contribution of the stochasticity process of thenirKtype denitrifying bacterial communities in July to October has increased the trend and decreased in November.This shows that the ecological process of the denitrifying bacteria in epiphytic biofilms is driven by the season.
In microorganisms, thenirSandnirKgenes are mutually exclusive, and bacteria with one or the other gene may have ecological niche differentiation(Jones and Hallin, 2010).We evaluated the niche breadth of the denitrifying bacterial communities and discovered that thenirS-type denitrifying bacteria had much more niche breadth than thenirKtype denitrifying bacteria.Organisms having a wider niche range may have more metabolic flexibility and are less impacted by deterministic processes(Chen et al., 2021), implying that thenirK-type bacteria are more susceptible to environmental filtering than thenirS-type denitrifying bacteria (Li et al., 2021).Deterministic processes based on the traditional ecological niche theory suggest that deterministic factors (e.g., species traits and interspecific interactions such as competition,predation, mutually beneficial symbiosis, and tradeoffs) and environmental conditions (e.g., pH,temperature, salinity, and moisture) control community construction (Zhou and Ning, 2017).The contribution of deterministic processes to the community assembly ofnirK-type denitrifying bacteria was higher than that ofnirS-type denitrifying bacteria (Supplementary Fig.S5); thus, interspecific interactions and interactions with algae were stronger in thenirK-type denitrifying bacteria than in thenirStype denitrifying bacteria.The assembly processes of thenirS-type andnirK-type denitrifying bacteria in the biofilms were dominated by deterministic mechanisms, which were driven by the season.
5 CONCLUSION
The community structure and co-occurrence network ofnirS- andnirK-type denitrifying bacteria epiphytic biofilms were studied in this study and found to be different in different months (July to November).The organization and composition of denitrifying bacterial communities are influenced by algal density and physicochemical variables.The cooccurrence network complexity ofnirS- andnirKtype denitrifying bacteria changed in a similar pattern.The algae andnirS-type denitrifying bacteria co-occurrence network complexity in epiphytic biofilms tended to increase from the July to the November, while the algae andnirK-type denitrifying bacteria co-occurrence network complexity tended to increase, and then decrease.Both genotypes were dominated by deterministic processes in community assembly, with thenirStype denitrifying bacteria having a larger ecological niche, larger MST, and higher migration rate than thenirK-type denitrifying bacteria.Our results provide new perspectives on the influence of algae on denitrifying bacterial communities, algalbacterial relationship succession, and denitrifying bacterial community assembly.Moreover, findings from this study could be used for the proper understanding and utilization of the denitrification function of epiphytic biofilms and the improvement of water environment quality.
6 DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
 Journal of Oceanology and Limnology2023年6期
Journal of Oceanology and Limnology2023年6期
- Journal of Oceanology and Limnology的其它文章
- Trends of carbon and nutrient accumulation through time in the Andong salt marsh, Hangzhou Bay, China*
- Physical processes determining the distribution patterns of Nemopilema nomurai in the East China Sea*
- Comparison in structure and predicted function of epiphytic bacteria on Neopyropia yezoensis and Neopyropia katadae*
- Interaction between macroalgae and microplastics: Caulerpa lentillifera and Gracilaria tenuistipitata as microplastic bio-elimination vectors*
- Lake regime shift from submerged macrophyte to phytoplankton affected phosphorus speciation in sediment and eutrophic state in Caohai Lake, Guizhou, China*
- Environment drives the co-occurrence of bacteria and microeukaryotes in a typical subtropical bay*
