Role of extracellular polymeric substances in resistance to allelochemical stress on Microcystis aeruginsosa and its mechanism*
Li YIN, Ying XU, Desheng KONG, Juan WANG, Kaipian SHI, Yong ZHANG,Huan HE, Shaogui YANG, Lixiao NI, Shiyin LI,4,**
1 School of Environment, Nanjing Normal University, Nanjing 210023, China
2 Department of Geological Sciences, University of Alabama, Tuscaloosa, AL 35487, USA
3 Key Laboratory of Integrated Regulation and Resource Development on Shallow Lakes of Ministry of Education; School of Environment, Hohai University, Nanjing 210098, China
4 Jiangsu Center for Collaborative Innovation in Geographical Information Resource Development and Application, Nanjing 210023, China
Abstract Using allelochemicals to suppress cyanobacteria growth is a prospective method for its high efficiency and ecological safety.However, the suppression efficiency is affected inevitably by the extracellular polymeric substances (EPS) produced by cyanobacteria, and the knowledge about the roles of EPS in resistance to allelochemical stress is scarce.For the study, two typical anti-cyanobacterial allelochemicals were adopted to investigate the role of EPS in resistance to allelochemical stress on Microcystis aeruginosa.Results show that EPS was crucial in alleviating the toxicity of allelochemicals to algae, especially in stabilizing the metabolism and photosynthetic activity of algal cells.The aggregation rate of algal cells increased with the increase of EPS secretion, which alleviated the stress of allelopathy.Tryptophan proteins and humic acids in EPS provided a binding site for allelochemicals, and the EPSallelochemicals complex were formed by chemical bonding.This study improved our comprehension of the role of EPS in algal inhibition by allelochemicals.
Keyword: allelochemicals; extracellular polymeric substances; cyanobacteria; Microcystis aeruginosa;allelopathy
1 INTRODUCTION
In recent years, water eutrophication becomes increasingly serious and develops rapidly, which have led to the excessive proliferation of harmful algal blooms (HABs), damaged aquatic ecosystems,and harmed the functions of water as fishery,landscape, and drinking water supply, etc.(Weber et al., 2020).Several associated negative impacts on the aquatic environment as well as human health caused by HABs are increasingly and frequently reported worldwide (Paerl and Otten, 2013; Guo et al., 2021).Current strategies against HABs include chemical, physical, and biological technologies.However, the deficiencies of those technologies are obvious, e.g., ephemeral, expensive, labor intensive,and side-effects on non-target species, including humans, which restricted the promotion of the technologies (Li et al., 2020; Pal et al., 2020; Zhu et al., 2021a).Therefore, it is important to propose alternative techniques that are high efficiency,economy, and safe to control HABs.
Allelopathy, one of the successful mechanisms by which exotic plants invade algae, provides a novel way for controlling HABs (Li et al., 2021a).In recent years, the utilization of allelochemicals for HABs control has attracted wide attention.Allelochemicals are viewed as eco-friendly algicides because of the trait of incredible degradability, high selectivity, and negligible toxicity (Zhu et al.,2021a).Among all allelochemicals, phenols and fatty acids are widespread in plants, have strong allelopathic activity, and are extracted, separated,and characterized as inhibitors for HABs (Liu et al.,2014; Lu et al., 2017; Ni et al., 2018).Research onto improving the algae inhibition efficiency of allelochemicals is of rising interest (Gao et al., 2015).Algae can secrete extracellular polymeric substances(EPS) against the toxicity of allelochemicals, which limits the improvement of allelochemical activation performance.
EPS is a macromolecular substance secreted by algae or other organisms under specific conditions(Xu et al., 2013a).It exhibits a double-layered structure consisting of soluble EPS (SEPS) and bound EPS (BEPS), which forms a protective layer outside the cell (Sheng et al., 2010; Zhao et al.,2019a; Wang et al., 2022).According to previous studies, EPS is able to resist the toxicity of exogenous substances such as photocatalyst algaecide,H2O2algaecide, nanoparticles, heavy metals, and organic pollutants (Ueshima et al., 2008; Gao et al.,2015; Xie et al., 2020; Fan et al., 2021; Zhu et al.,2021b).Findings of those studies emphasize that the role of EPS as a buffer against biocidal effects in algae removal is very important.Under the allelopathy stress, the “long-term” mode of allelopathic algal inhibition maintained the buffering effect of EPS.If the dosage of allelochemicals is insufficient, the inhibition efficiency of algae may be affected by the release of EPS.Therefore, the importance of EPS should be assessed in the process of using allelochemicals as algal inhibitors.However, the role of EPS composition variations in easing allelochemical stress and the corresponding mechanisms remains unclear.
In this study,Microcystisaeruginosa, a common bloom-forming cyanobacterium, was used as the subject, and two typical anti-cyanobacterial allelochemicals, pyrogallic acid (PA), and linoleic acid (LA) (representing phenolic acid and fatty acid allelochemicals), were used to study the role of EPS against allelochemical stress inM.aeruginosa.Following issues were discussed in this study.What are the differences in the composition and secretion of EPS under allelopathic stress induced by PA and LA? What are the differences in the toxic effects of allelochemicals on algal cells in the presence or absence of EPS? What is the main mechanism by which EPS protects algal cells in view of changes in cell surface characteristics and chemical combinations?The answers obtained will help comprehend the role of EPS in the process of algal inhibition with allelochemicals.
2 MATERIAL AND METHOD
2.1 Material and reagent
Microcystisaeruginosa(FACHB905) used in this study was procured from the Freshwater Algae Culture Collection of the Institute of Hydrobiology,Chinese Academy of Sciences.M.aeruginosawas cultured with a sterile BG11 medium.The details of the algae culture and other reagents used in this experiment are listed in Supplementary Text S1.
2.2 Sample preparation and EPS extraction
To determine the change in cell surface properties under allelopathic stress, various concentrations of LA(0, 0.1, 0.2, 0.3, 0.4, and 0.5 mg/L) and PA (0, 0.5,1, 2, 4, 8, and 16 mg/L) were added to conical flasks in initial algal cell density of 3.52×106cells/mL in the exponential growth phase.These concentrations were determined from the growth curves ofM.aeruginosa(Supplementary Fig.S1) in different concentrations of LA and PA.To maintain samples homogeneity and reduce the minor differences in photon irradiance caused by different positions of the samples in the light incubator, the samples were randomly rearranged each day during the experiment.The inhibition experiment lasted for 28 d.After 24 h and 20 d, algal cells were collected to measure the aggregation ofM.aeruginosa.After 4, 8, 12, 16, 20,24, and 28 d, EPS of algal cells were extracted for the following analyses.The extraction of EPS,SEPS, and BEPS was performed by following the method reported by Xu et al.(2013a) (Supplementary Text S2), which has been reported as an effective method for cyanobacterial EPS extraction due to its high extraction efficiency and low cell lysis.A certain time lag was ensured between different experimental series, and the samples were analyzed immediately after sampling to ensure the accuracy of non-independent data.
2.3 Toxicity test
To elaborate the role of EPS on the toxicity effect of allelochemicals on algae,M.aeruginosa-coated EPS naturally (herein defined as EPS-C) and removed EPS artificially (herein defined as EPS-F)cells were prepared by the centrifugation methods described by Zhao et al.(2019a) and Xie et al.(2020) (Supplementary Text S3).
To evaluate the impact of EPS extraction on the physiological status of cells, the viability ofM.aeruginosawas determined by double staining with fluorescein diacetate and propidium iodide (FDAPI), and the growth curve of EPS-C/EPS-FM.aeruginosawas also monitored.
EPS-C and EPS-F algae cells were cultured in the dark with PA (0, 0.5, 1, 2, 4, 8, and 16 mg/L)and LA (0, 0.1, 0.25, 0.5, 1, 2, and 4 mg/L),respectively.After 6 h, algal cells were taken to measure the cell density and physiological activity indexes ofM.aeruginosa.
2.4 Standard aggregation test
Determination of standard aggregation was performed by following the method reported in Tan et al.(2018) (Supplementary Text S4), and the aggregation rate (A) was calculated according to Eq.1.
where OD0is the optical density at 680 nm at the beginning of the test and ODtis the value measured at the end of the study after 6 h.
2.5 EPS and allelochemical binding tests
The binding experiments were performed in tipbottom centrifuge tubes.Firstly, a 5-mL EPS sample was added to the tip-bottom centrifuge tubes individually, and then the EPS samples were mixed with allelochemical solution to make a series of mixtures with concentrations of PA and LA in the range of 0–10 and 0–5 mg/L, respectively, according to the upper limit of their effective algal inhibition concentration.Finally, the samples were balanced under shaded condition for 4 h before analysis.The experiment was carried out under shaded condition to avoid the photodegradation of allelochemicals.The same procedure mentioned above was repeated at 0 °C and 35 °C to explore the thermodynamic process of the reactions between allelochemicals and EPS.
2.6 Analytical method
2.6.1 EPS component analysis
The contents of polysaccharide, protein, and humic acids in the SEPS and BEPS were then determined by the Coomassie brilliant blue, phenolsulfuric acid, and modified Folin Lowry methods,respectively (Frølund et al., 1995, 1996; Chen,2012; Chen et al., 2015).
2.6.2 Physiological activity index analysis of algal cells
The growth inhibition ratio (r) was calculated by the following equation (Yang et al., 2021):
whereN0andN(×105cells/mL) are the cell number in controls and treatments, respectively.
The relative esterase activity and chlorophyll autofluorescence of algal cells were analyzed by a molecular fluorescence photometer (LF-1301009,Thermo Fisher, USA), and the specific experimental details are listed in Supplementary Text S5.
2.6.3 Spectral analysis of the binding between allelochemicals and EPS
Excitation-emission matrix (EEM) fluorescence spectra of EPS were recorded by a molecular fluorescence photometer (LF-1301009, Thermo Fisher, USA) at 25 °C, and the specific parameter settings are listed in Supplementary Text S6.The EEM data of SEPS and BEPS were analyzed using parallel factor analysis (PARAFAC) with DOMFluor Toolbox v1.7 (Stedmon and Bro, 2008).Synchronous fluorescence (SF) spectra were obtained with constant offset (60 nm) (Δλ) between the Ex and Em wavelengths.
2.6.4 Other analysis
The hydrodynamic diameter (Dh) of the sample where SEPS and BEPS are mixed with different allelochemicals was measured in triplicate by a Malvern nanoparticle potential instrument (Nano-ZS90, Malvern Instruments Ltd, Britain) at 25 °C.
2.7 Theoretical fitting model
The Stern-Volmer equation (Eq.3) (Eftink and Ghiron, 1981) was used in this study to confirm the quenching mechanism (Yan et al., 2019a):
whereF0andFdenote the fluorescence intensities of EPS in the absence and presence of allelochemicals, respectively;Kqis the quenching rate constant;τ0is the average lifetime of EPS(10-8s) (Lakowicz and Weber, 1973); and [Q] is the concentration (mol/L) of allelochemicals added.
The static fluorescence quenching process can be analyzed by the modified Stern-Volmer equation(Eq.4) (Fu et al., 2012):
whereKAis the effective quenching constant andfis the proportion of fluorescent groups coordinated by the allelochemicals.
During the process of static quenching, the binding constant (logKb) and the binding site number(n) of the reaction between allelochemicals and EPS can be analyzed by the modified Hill equation (Eq.5)(Zhang et al., 2008) to quantify the binding affinity of SEPS and BEPS with TA, LA, and PA:
The thermodynamic parameters can be calculated according to the Van’t Hoff equation (Eqs.6 & 7)(Yan et al., 2019b):
The values of entropy change (ΔS) J/(K·mol),enthalpy change (ΔH) (KJ/mol), and Gibbs free energy change (ΔG) (KJ/mol) can be calculated by Eqs.6 & 7, whereRdenotes the universal gas constant andTdenotes the thermodynamic temperature.
2.8 Statistics
The data from EPS component determination tests, standard aggregation tests, and toxicity tests were expressed as means±standard deviations (n=3).The mean values of the experimental data of EPS and allelochemical binding tests were used for the subsequent model fitting (2.7).Data were analyzed using SPSS software (SPSS 20.0).Significant differences were analyzed by a one-way analysis of variance (ANOVA) with least significant difference(LSD) tests.P<0.05 was considered significantly different by LSD (*).P<0.01 was considered extreme significantly different by LSD (**).
3 RESULT
3.1 Effects of EPS on algae cells ease allelopathy stress
To assess the contribution of EPS secreted by algal cells to resistance to allelopathy stress, the toxic effects of allelochemicals on algal cells with or without EPS were investigated.Supplementary Figs.S2 & S3 show that no dead cells were observed, and there was little difference in the algal density ofM.aeruginosawith or without EPS within 28 days,suggesting that the extraction of EPS did not adversely affect the viability of algal cells.
After treatment with LA and PA, the comparisons between the relative esterase activity, chlorophyll autofluorescence, and the growth inhibition curve between EPS-C and EPS-FM.aeruginosaare shown in Fig.1.In the presence of LA (4 mg/L) and PA (16 mg/L), the relative esterase activities of EPSC algae were 1.90 times and 1.61 times as high as those of the EPS-F groups, respectively (Fig.1a &d).The changes in chlorophyll autofluorescence values ofM.aeruginosawith or without EPS under allelopathic stress are shown in Fig.1b & e.When the concentrations of LA was 4 mg/L and PA was 16 mg/L, the fluorescence intensity values of the EPS-F groups were 1.16 times (4 mg/L LA) and 2.79 times (16 mg/L PA) as high as those of EPS-C groups, respectively.Additionally, the growth inhibition ratio of EPS-F algal cells was higher than that in the EPS-C groups (Fig.1c & f).The differences between the 96 h growth inhibition ratio of algal cells with or without EPS were 24.57% (PA:16 mg/L) and 17.05% (LA: 0.5 mg/L), respectively.
3.2 Variation in EPS compositions under allelopathy stress
The variations in polysaccharide, proteins, and humic acid in EPS secreted byM.aeruginosaat different concentrations of LA and PA are shown in Fig.2.Polysaccharides in the LA treatment groups(0.2, 0.3, 0.4, and 0.5 mg/L) were significantly higher than those in the control group at 12, 16, 20,24, and 28 d.Polysaccharides in the PA treatment groups (0.50, 1.0, 2.0, 4.0, 8.0, and 16 mg/L) were significantly higher than those in the control group at 12 d.The polysaccharide concentrations under the 16-mg/L PA and 0.5-mg/L LA treatments after 16 d were 1.03 times (48.35±0.01 mg/L) and 1.81 times(84.69±0.02 mg/L) as high as that of the control group, respectively (Fig.2a & d).Proteins in EPS showed an increasing trend with time (4–28 d)under different treatments of LA (0.2, 0.4, and 0.5 mg/L) and PA (4, 8, and 16 mg/L) (Fig.2b & e).The protein concentrations of EPS reached a maximum of 41.87±2.13 mg/L and 22.12±0.35 mg/L after 28 d of culture with exposure to 0.5-mg/L LA and 16-mg/L PA, respectively.Humic acid in the EPS of the experimental group (0.2-mg/L LA, 1.0-,and 4.0-mg/L PA) showed a trend of increasing(4–20 d) and then decreasing (20–28 d) over time,reaching the maximum value at day 20 (Fig.2c & f).The humic acid concentrations under the 0.5-mg/L LA and 8-mg/L PA treatments after 20 d were 1.98 times (42.80±0.10 mg/L) and 1.08 times (23.30±0.13 mg/L) as high as that of the control group,respectively (Fig.2c & f).In addition, the treatments with 16-mg/L PA resulted in a higher increase in humic acid than the lower concentration treatments(0.5, 1.0, 2.0, 4.0, and 8.0 mg/L) at 4–28 d.
Additionally, the Pearson correlation coefficients between the components of EPS and allelochemicals are provided in Supplementary Tables S1 & S2.The correlation coefficients between LA and proteins,polysaccharides, and humic acid were 0.991(P<0.01), 0.987 (P<0.01), and 0.949 (P<0.05),respectively, at 16 d (Supplementary Table S1).The correlation coefficients between PA and proteins,polysaccharides, and humic acid were 0.830(P<0.05), 0.837 (P<0.05), and 0.847 (P<0.05),respectively, at 24 d (Supplementary Table S2).
3.3 Contribution of EPS to aggregation of cyanobacteria
The effects of LA and PA at different concentrations on the aggregation rate ofM.aeruginosain the short term (24 h) and long term(20 d) are shown in Fig.3a.In the presence of 0.5-mg/L LA and 10-mg/L PA, the aggregation rate increased from 25.97%±1.49% to 57.17%±5.42% and 12.90%±0.66% to 19.59%±0.61% during cultivation(24 h–20 d), respectively (Fig.3a).
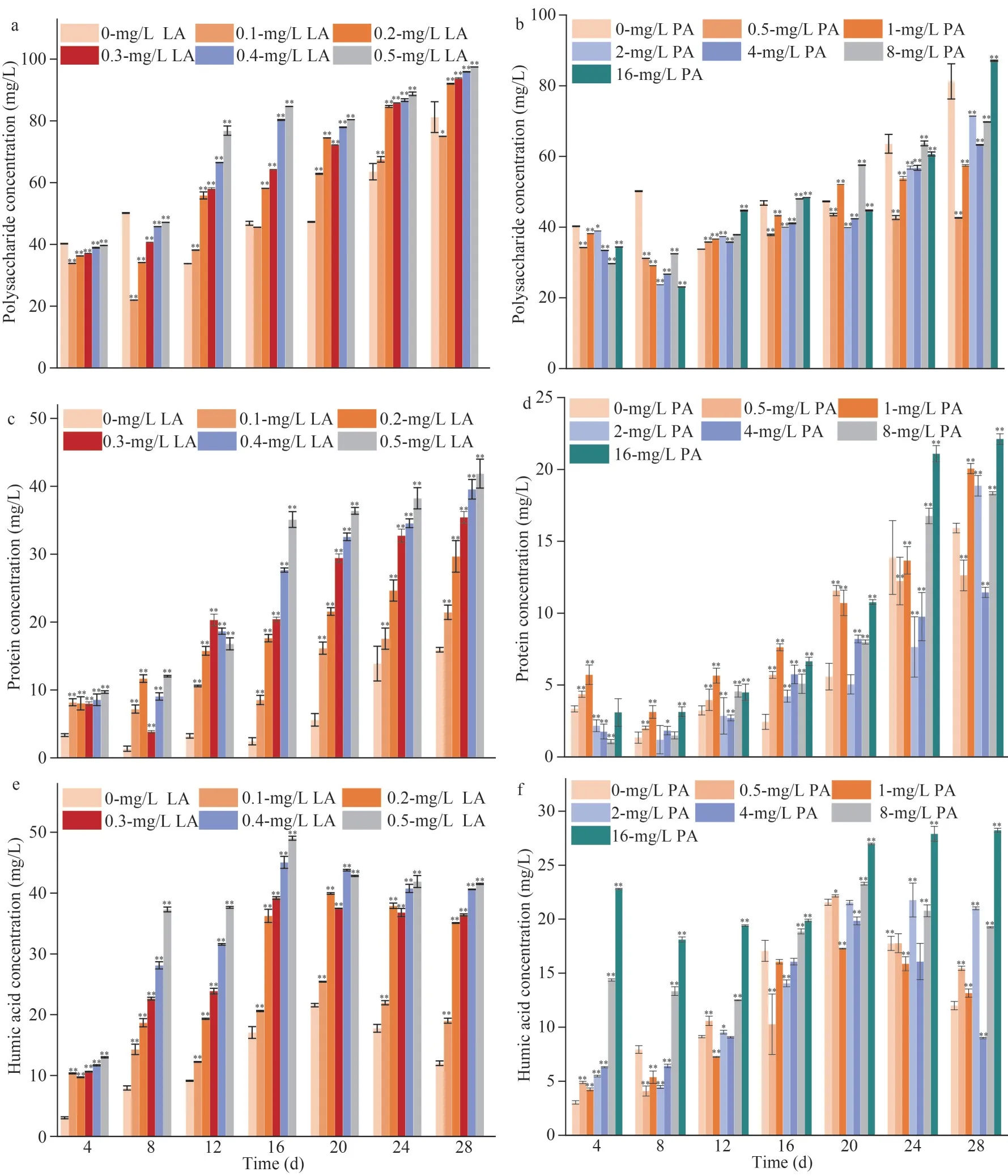
Fig.2 Variations in polysaccharide (a, b), proteins (c, d), and humic acid (e, f) in EPS excreted by Microcystis aeruginosa at different concentrations of LA (a, c, e) and PA (b, d, f)
The changes in protein/polysaccharide (PN/PS)in EPS secreted byM.aeruginosaunder allelopathy stress are shown in Fig.3b and Supplementary Fig.S4.The PN/PS values showed an increasing trend from 4 d to 28 d, and the increase was higher than that in the control group.The value of PN/PS in EPS without allelochemicals was 0.20 at day 28,while the value of PN/PS with 0.5-mg/L LA and 8-mg/L PA reached 0.43 and 0.26, respectively(Supplementary Fig.S4).

Fig.3 The aggregation rate of M.aeruginosa (a) and the changes in protein/polysaccharide (PN/PS) in EPS of M.aeruginosa (b) cultivated at different concentrations of LA and PA
3.4 Response mechanism of fluorescent groups in EPS with allelochemicals

Fig.4 EEM fluorescence spectra of the two main components, proteins (a, c) and humic acids (b, d), in the SEPS (a, b) and BEPS (c, d) extracted by PRAFAC analysis
To further explore the difference between the barrier function of SEPS and BEPS released by algal cells under allelopathy stress, the interaction mechanisms between SEPS/BEPS and two typical anti-cyanobacterial allelochemicals were determined using spectral methods.The extracted SEPS and BEPS were analyzed by compositional analysis(Supplementary Table S3 & Text S7) and fluorescence spectroscopy.The fluorescence spectra of SEPS and BEPS of cyanobacteria are shown in Fig.4.Two components (tryptophan-like proteins and humiclike substances) of SEPS and BEPS were identified by PARAFAC analysis (Supplementary Text S8 &Table S4) (Baker et al., 2001; Chen et al., 2003).The Stern-Volmer equation was applied to investigate the mechanism of EPS fluorescence quenching by allelochemicals.As listed in Table 1, all the experimental values forKqwere greater than 2.0×1010L/(mol·s).The modified Stern-Volmer equation was applied to further evaluate the static fluorescence quenching.During the process of static quenching,the fluorescence data were fitted with a modified Hill equation (Eq.5) to calculate the values of logKbandn.Table 1 shows that the values ofnwere all close to 1.In addition, the logKbof the fluorescence in BEPS were higher than those in SEPS.To evaluate the binding mode of allelochemicals to EPS, the thermodynamic parameters were calculated by Eqs.4 &5.As summarized in Table 2, the ΔGvalues for both fluorophore proteins and humic acids were negative and the ΔSand ΔHof PA or LA binding with fluorophore proteins and humic acids was positive.
3.5 Structural changes of EPS after binding with allelochemicals
SF could reflect the properties of tryptophan residues in proteins when Δλis stabilized at 60 nm(Xu et al., 2013b).Figure 5 shows that with an increase in the concentration of PA and LA, the SF spectrum both decreased gradually.Furthermore, the tryptophan-like proteins were also slightly redshifted from 375 nm to 378 nm in EPS-PA.For the LA treatment group, the polarity of the microenvironment around the EPS tryptophan residue did not change obviously.
4 DISCUSSION
In the present study, we found that the toxic effect of allelochemicals on algal cells was reduced due to EPS.As depicted in Fig.1a & d, both LA and PA significantly inhibited the fluorescence intensity representing esterase activity of algal cells, which was related to the metabolic activity of the algae.The results indicated that EPS has a buffering effect on the decrease in lipase activity, which protects the metabolic activity of algal cells.The results shown in Section 3.1 also show the higher chlorophyll autofluorescence values of EPS-F groups to EPS-C groups.Since the instrument detected only chloroplasts of cells not used for biotransformation as fluorescent, the higher the fluorescence intensity,the weaker the photosynthetic activity (Xie et al.,2020).This means EPS had a buffering effect on the decrease in cellular photosynthetic activity.Xie et al.(2020) also found that EPS resisted the damage of Cd(Ⅱ) on photosynthetic activities.
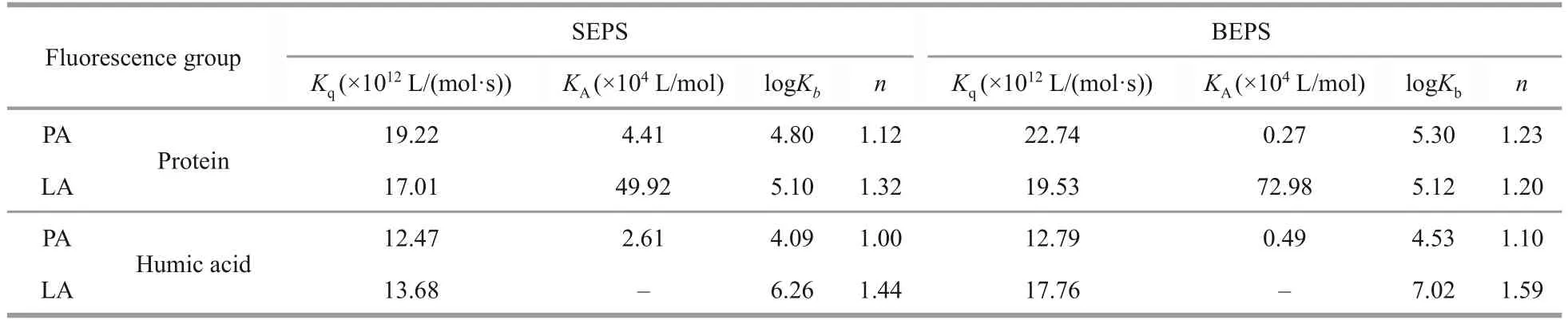
Table 1 The quenching constants (Kq, KA), binding constants (logKb), and binding sites (n) of the interactions between allelochemicals and proteins/humic acids in EPS at 25 °C

Table 2 Thermodynamic parameters of the interactions between proteins/humic acids in EPS and allelochemicals

Fig.5 The synchronous fluorescence spectra of EPS in the presence of LA/PA
Polysaccharides, proteins, and humic acids in the EPS of algal cells with allelochemicals were assayed (Section 3.2), as these three are the main components in EPS in this test (Sheng et al., 2010).Polysaccharides in all groups were found to be higher than proteins and humic acid, which was consistent with the result of Ni et al.(2017) that polysaccharides in EPS served as defenders when the algae suffered allelopathy stress.Proteins in EPS also showed a similar production trend as the polysaccharides, which indicated that proteins may play an important role in defending algal cells from damage.Previous studies also found that proteins are important components in EPS that restrain the intrusion of nano-ZnO (Zhao et al., 2019a), CeO2NPs (You et al., 2015), and Cd(Ⅱ) (Xie et al., 2020)into algal cells.The higher content of humic acid in the PA/LA treatment group (0–0.5-mg/L LA and 8-,16-mg/L PA) than the control group was also observed at 4–28 d.According to the research of Zhao et al.(2018), the presence of humic acid in the system containingM.aeruginosaand other organisms could reduce iron bioavailability and thus affect the organism’s growth.The results of the Pearson correlation coefficients between the components of EPS and LA demonstrated that proteins,polysaccharides, and humic acid in EPS were significantly increased with the increase of LA concentration at 16, 20, and 24 d, implying that the response of algal cells is enhanced from the perspective of increased EPS content under higher concentrations of LA stress.In addition, the content of proteins, polysaccharides, and humic acid in EPS was also positively correlated with the concentration of PA at 24 d, while the correlation coefficient between PA concentration and protein and polysaccharides content in EPS was less than 0.5 at 4, 8, and 20 d, which needs further exploration.
Continuous changes in the content of the main component in EPS secreted by algae under LA or PA stress was observed (Fig.2), and the difference in their composition ratios could affect surface properties such as surface charge and hydrophobicity,largely affecting aggregation, which is related to the resistance of algal cells to allelopathy stress adversity (Badawy et al., 2010; Tan et al., 2018).The results shown in Section 3.3 demonstrate that long-term allelopathy stress could induce aggregation ofM.aeruginosa.Previous studies have shown that aggregation among algal cells can make them more resistant to adversity and enhance their adaptation to environmental survival (Li et al., 2021b; Wang et al., 2021; Huang et al., 2022a, b).This means that algal cells may adapt to allelopathy stress by increasing the aggregation rate.In addition, the increase in the aggregation rate ofM.aeruginosaunder LA stress was higher than that under PA stress, which may be related to the increased secretion of EPS under different types of allelopathy stress.It has been reported that EPS plays a crucial role in the formation ofMicrocystisaggregates(Andreadakis, 1993; Liu et al., 2010; Xu et al.,2014).Previous studies have shown that the content of proteins and polysaccharides in EPS could affect cell surface properties such as hydrophobicity,which in turn affects cell aggregation (Zhao et al.,2018).The results shown in Section 3.3 suggested that the presence of LA or PA particularly accelerated the yield of PN in EPS.Previous studies have shown that PN/PS is positively correlated with aggregation (Tan et al., 2018).The increase in PN/PS will decrease the net surface charge of algal cells, which then decreases electrostatic repulsion and thus promotes intercellular aggregation.Additionally, the PN/PS and hydrophobic properties were positively correlated because the hydrophobic fraction in EPS is mainly composed of proteins and the hydrophilic fraction is mainly composed of polysaccharides (Liu and Fang, 2003; Xia et al.,2016).This suggests that algal cells may promote their aggregation behavior by inducing variations in polysaccharides and proteins in EPS to alleviate allelopathy stress.
Spectral methods were used to further explore the interaction between EPS and allelochemicals.The quenching mode between the main fluorescent components in EPS and allelochemicals can be determined by the value ofKq.Generally, ifKqwas larger than 2.0×1010L/(mol·s), the process was dominated by static quenching.The results shown in Section 3.4 indicate that static quenching was the major quenching process between the EPS and LA/PA (Ware, 1962), implying the static complex was formed.Two fluorescent substances of EPS had a large binding capacity to LA and PA with the formation of EPS-PA and EPS-LA complexes.The binding sites and bonding strength between fluorescent component in EPS and allelochemicals can be analyzed according to the fluorescence data.PA,LA, and the two fluorescence groups of EPS had a relatively independent binding site.The higher logKbof the fluorescence in BEPS than SEPS implied that the binding strength of PA-LA to the fluorescence in BEPS was higher than that in SEPS,which was consistent with the conclusion when allelochemical chosen to tannic acid in our previous study (Yin et al., 2022).Additionally, the negative ΔGvalues for both fluorescence proteins and humic acids indicated that the interaction between the fluorophores in EPS and PA or LA was spontaneous(Yan et al., 2019b).The positive ΔSvalues for both fluorescence proteins and humic acids indicated that the increased structural disorder with the formation of EPS-allelochemical complexes (Pan et al., 2010).The positive ΔHvalues proved that the reaction of EPS and LA/PA was endothermic.The driven force of the interactions between allelochemicals and EPS is associated with thermodynamic parameters.Generally, negative values of ΔHand ΔSrepresent hydrogen bond and electrostatic interaction, while positive values of ΔHand ΔSindicate hydrophobic interaction (Yan et al., 2017).In Table 2, the positive values of ΔHand ΔSindicated that the hydrophobic effect was the main binding force of proteins or humic acids in EPS with PA or LA.
The structural changes of EPS after binding with allelochemicals were explored by SF spectrum.The results shown in Section 3.5 indicate that tryptophan was the main reason for the fluorescence quenching of EPS.According to the research of Zhao et al.(2019b), the presence of tryptophan could promote the growth ofM.aeruginosa.Furthermore, the redshifted spectrum of EPS-PA was indicated that the presence of PA increased the polarity of the microenvironment near the tryptophan residue of EPS and that the hydrophobicity of the microenvironment was decreased.With a decrease in hydrophobicity,the degree of extension of the peptide chain in EPS proteins increases (Kragh-Hansen et al., 2001; Hu et al., 2004; Liu et al., 2019).This means that PA enters the hydrophobic region of the tryptophan residues in EPS, resulting in peptide chain extension and exposure of tryptophan to aqueous solutions(Xu et al., 2013b).As illustrated in Supplementary Fig.S5, the enhancement ofDhof EPS suggested that LA and PA interacted with EPS to form a complex with a larger particle size.The addition of allelochemicals leads to the volume expansion and morphological stretching of EPS.This was also in agreement with the SF spectra results (Fig.5), in which the extension of peptide chains in tryptophanlike proteins indicated a larger molecular volume.
The differences in the role of algal EPS in response to LA/PA were observed in this study.The change of each component content in EPS caused by LA/PA was different (Figs.2 & 3b).The content of polysaccharide and the ratio of PN/PS in EPS induced by LA were higher than that of PA when the concentration of LA and PA was 5 mg/L.The above differences were one of the reasons for the higher aggregation rate of algal cells in response to LA stress than PA stress (Fig.3a) due to the viscosity of polysaccharide itself and the increase of PN/PS was positively correlated with aggregation (Tan et al.,2018).Based on the research mentioned above, it can be reasonably supposed that the combination of agitation or other physical methods to prevent the aggregation of algal cells is conducive to the implementation of algal inhibition by LA.In addition, the binding capacity between LA/PA and the two fluorescent substances in EPS confirmed in Table 1 provides us with a new inspiration that the combination of allelopathic algae inhibition with the addition of microorganisms that easily decompose EPS might be more advisable as the superiority in high efficiency.
5 CONCLUSION
This study showed that EPS secreted byM.aeruginosaplayed a crucial role in protecting cells from the toxicity of allelochemicals.After the secretion of EPS fromM.aeruginosa, the aggregation rate of algal cells increased due to a large number of polysaccharide in EPS.In addition,tryptophan proteins and humic acids in EPS provided binding sites for allelochemicals to form EPS-allelochemical complexes by chemical binding.Furthermore, the differences in EPS secretion of algae in response to different types of allelochemicals were discovered for the first time in this study.Under LA stress, the viscosity of EPS caused by a large amount of polysaccharide secretion increased, which promoted algal aggregation from damage.However, under PA allelopathy stress,the increase in protein and humic acid in EPS was one of the important reasons to avoid cell damage.Therefore, future work should focused on the combination of allelopathic algae inhibition with other strategies, such as physical agitation and the addition of microorganisms that easily decompose EPS, which might be more advisable as the superiority in high efficiency.
6 DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the first author upon reasonable request.
 Journal of Oceanology and Limnology2023年6期
Journal of Oceanology and Limnology2023年6期
- Journal of Oceanology and Limnology的其它文章
- Trends of carbon and nutrient accumulation through time in the Andong salt marsh, Hangzhou Bay, China*
- Physical processes determining the distribution patterns of Nemopilema nomurai in the East China Sea*
- Comparison in structure and predicted function of epiphytic bacteria on Neopyropia yezoensis and Neopyropia katadae*
- Interaction between macroalgae and microplastics: Caulerpa lentillifera and Gracilaria tenuistipitata as microplastic bio-elimination vectors*
- Lake regime shift from submerged macrophyte to phytoplankton affected phosphorus speciation in sediment and eutrophic state in Caohai Lake, Guizhou, China*
- Temporal characteristics of algae-denitrifying bacteria co-occurrence patterns and denitrifier assembly in epiphytic biofilms on submerged macrophytes in Caohai Lake, SW China*
