Effect of extracellular polymeric substances on Dolichospermum aggregation during temperature rise*
Dailan DENG, Han MENG, You MA, Yongqi GUO, Zixuan WANG,Huan HE, Khan WAQAS, Jin’e LIU,**, Limin ZHANG
1 School of Environment, Jiangsu Center for Collaborative Innovation in Geographical Information Resource Development and Application, Nanjing Normal University, Nanjing 210023, China
2 Green Economy Development Institute, Nanjing University of Finance and Economics, Nanjing 210023, China
3 Jiangsu Engineering Lab of Water and Soil Eco-remediation, Nanjing 210023, China
Abstract Dolichospermum, a typical model filamentous of cyanobacteria, has the potential to cause severely bloom.Extracellular polymeric substances (EPSs) are considered to influence the aggregation of the algae, and temperature is a significant factor affecting EPSs secretion.However, the mechanism of how EPSs affects the aggregation of Dolichospermum is still unclear because the structure and composition of EPSs are complex.In this study, the effects of EPSs on the aggregation of Dolichospermum during the rise of temperature (7–37 °C) were determined.The results showed that the concentration of extracellular polysaccharides and proteins changed significantly with increasing temperature (P<0.01).Firstly, during the increasing temperature, the polysaccharide content of EPSs increased from 20.34 to 54.64 mg/L, and the polysaccharides in the soluble EPS (S-EPS) layer changed significantly.The protein content reached maximum value at 21 °C (14.52 mg/L) and varied significantly in S-EPS and loosely bound EPS (LB-EPS).In the EPSs matrix, humus substances and protein were main components of S-EPS and LB-EPS, and protein was the main component of tightly bound EPS (TB-EPS).Secondly, the cell density of Dolichospermum increased during the temperature rise while the aggregation ratio decreased.Moreover,zeta potential and surface thermodynamic analysis of Dolichospermum revealed that the interfacial free energy and electrostatic repulsion increased gradually with increasing temperature, which further reduced the aggregation of Dolichospermum.Finally, principal component analysis (PCA) analysis showed the aggregation of Dolichospermum was directly related to the changes of protein in EPSs (especially S-EPS and LB-EPS) and zeta potential, and polysaccharides in EPSs inhibited the aggregation of Dolichospermum.Based on these results, it was illustrated that the composition and concentration of EPSs affected the cell surface properties of Dolichospermum with the change of temperature and thus affected the aggregation of Dolichospermum.
Keyword: temperature; Dolichospermum; extracellular polymeric substances; aggregation
1 INTRODUCTION
With the increasing eutrophication in freshwater lakes, cyanobacteria blooms have become a global environmental problem, which occurred commonly in eutrophic lakes, ponds, rivers besides threatened the sustainability of freshwater ecosystems (Paerl and Paul, 2012).Temperature is considered as an important factor that directly controls the distribution and productivity of algae (Holsinger, 1955; Sakharova et al., 2020).Since the 20thcentury global atmospheric temperature has increased and cyanobacterial blooms have broken out widely (Carey et al., 2012; Maheaux et al., 2016).
In recent years, filamentous cyanobacteria blooms have occurred recurrently around the world, for example, Poyang Lake in China (Qian et al., 2019),the Baltic Sea in Northern Europe (Olofsson et al.,2019), Lake Balaton in Central Europe (Kovács et al.,2016), and Lake Sonachi and Lake Simbi in Africa(Ndlela et al., 2016).There are many environmental factors affecting the growth of filamentous cyanobacteria, among which, temperature is one of the main environmental factors.The fact that cyanobacteria preferred warm temperatures, many studies have been conducted to investigate cyanobacteria expansion might be changed by global warming (Paerl et al., 2011; Carey et al., 2012;Maheaux et al., 2016).Consequently, it remains to note that the duration, distribution, and intensity of cyanobacterial blooms are probably increased with increasing temperatures (Paerl and Huisman, 2009).
Dolichospermumis a photoautotrophic filamentous cyanobacterium capable of fixing atmospheric nitrogen (Zhang et al., 2021).In appropriate conditions, hundreds ofDolichospermumcells directly connect (Arévalo et al., 2021) and form filaments.These filaments could rapidly response to adapt to environmental changes (Weiss et al., 2019).For example,Dolichospermumcells form spore-like resting cells in cold winters (Garg and Maldener,2021).They are dormant cells which different from vegetative cells, helping bacteria to survive under severely cold environments (Sukenik et al., 2019).When the temperature is appropriate, the dormant organism recovers, reproduces and form aggregates(Garg and Maldener, 2021; Srivastava et al., 2021).It is reported cyanobacteria quickly resume their metabolism and photosynthetic rates when lake ice melted (Olson et al., 1998; Paerl et al., 1998).Furthermore, Reddy et al.(2019) discovered thatDolichospermumis sensitive to high temperature.Elevated temperature could inhibit growth and photosynthesis ofDolichospermum, produce reactive oxygen species, and damage RNA and protein (Wen et al., 2005).However, a large number of studies on the growth ofDolichospermumfocused on the culture at a single temperature, and few studies conducted at a continuous temperature.
In addition, the direct cause of algal blooms is supposed to be the accumulation of algal cells, which promotes vertical migration and rises to the lake surface to form algal collections (Chen et al., 2019).The microbial collection is largely influenced by surface properties, including zeta potential and hydrophobicity/hydrophilicity (Sheng et al., 2010).As the extracellular polymeric substances (EPSs) directly covered with the surface of algae cells, it is a kind of polymer organic matter produced through adsorption,excretion, secretion, and cell lysis.EPSs are composed of polysaccharides, proteins, lipids, and humus (Xu et al., 2010, 2013a; Liu et al., 2017).The polysaccharides were initially considered the main components of cyanobacteria EPSs (Forni et al., 1997).More recently,protein-corresponding substances have been found in EPSs (Xu et al., 2013a; Liu et al., 2017).The content and composition of EPSs were supposed to enhance cyanobacteria proliferation through cell adhesion and other processes (Yang et al., 2008), and can also alter the surface properties of algae cells to promote algae cells aggregation (Liu and Fang, 2002; Vogelaar et al.,2005; Yu et al., 2009).
Furthermore, EPSs were divided into three fractions,including soluble EPS (S-EPS), loosely bound EPS(LB-EPS), and tightly bound EPS (TB-EPS) (Xu et al., 2013a).Xu et al.(2013b) found that most organic matter was dispersed in TB-EPS, and only a small amount was dispersed in S-EPS and LB-EPS.Among them, TB-EPS played role in the process of cyanobacteria combination and stimulates the formation of algal cell collections, while LB-EPS contributes to the development of the combinations towards the direction of bloom (Xu et al., 2014).
Despite numerous researches followed on EPSs components and found soluble and binding EPSs secreted by cyanobacteria at certain growth stages have effects on algae growth (Yang et al., 2008;Qu et al., 2012b).But most of them focused onMicrocystis(Xu et al., 2013a, b, 2014), and less attention has been paid to filamentous cyanobacteria, for example,Dolichospermum.Therefore, the dynamic of EPSs separation process, response to the growth and aggregation of filamentous cyanobacteria cells remain unclear.
Dolichospermumis a harmful alga that produces anatoxins, which are more harmful thanmicrocystin(Swe et al., 2021).Moreover,Dolichospermum,the heterotrophic nitrogen-fixing microorganism,adverse environments (Zhang et al., 2021).Thus,clarifying the growth and aggregation mechanism ofAnabaenawill contribute to preventing algal blooms, and protecting the health of the environment.
In this study,Dolichospermumwas studied in laboratory.Dolichospermumwas cultivated under temperature rise treatments, and its growth dynamics and aggregation characteristics were determined.The EPSs released and aggregation characteristics, including contact angles and zeta potential, were systematically determined.The surface thermodynamic analysis was applied to interpret the results and the roles of EPSs, and explored its subfractions inDolichospermumaggregation.The results can help explain the aggregation change ofDolichospermumin aquatic environment under temperature change, and provide a basis for the management of filamentous cyanobacteria.
2 MATERIAL AND METHOD
2.1 Cultivation of Dolichospermum
The cyanobacterial strainDolichospermum(PCC 7120), was provided by the Institute of Hydrobiology,Chinese Academy of Sciences, and it was cultured in conical flasks with batch mode.Before cultivation,all experimental utensils and mediums were put in autoclaved at 121 °C for 30 min.Dolichospermumin exponentially growing stage was injected into a conical flask containing 1 200-mL BG-11 medium,and the initial absorbance was adjusted to 0.1, then evenly divided into three conical flasks and cultured at a light꞉dark cycle of 12 h꞉12 h at a light intensity of 30 μmol photons/(m2·s).Dolichospermumgrowth was determined through taking absorbance ofDolichospermumcultures at 680 nm using a spectrophotometer (Tecan SPARK, Austria).To simulate a temperature change gradient,Dolichospermumwere first acclimated at 7 °C for a week to adapt the low-temperature environment,and then the culture temperature increased by 2 °C every 3 days from 7 °C to 37 °C.The same volumes of algal fluid samples were collected to analyze on the last day of each temperature setting.All the flasks were shaken by hand three times a day at regular intervals during the experimental period.Each treatment was conducted in triplicate.
2.2 EPSs extraction and determination of fluorescence EEM
EPSs consist of S-EPS, LB-EPS, and TB-EPS(Li and Yang, 2007; Sheng et al., 2010; Xu et al.,2010).In this study, EPSs were extracted according to Xu et al.(2013a).All cuvettes were rinsed before being analyzed.The three-dimensional excitationemission matrix fluorescence spectrum (3D-EEM)was measured using a fluorescence spectrometer(LF-1301009, Thermo Fisher), and the measurement was carried out in accordance with Xu et al.(2013b).The difference is that the emission (Em)and excitation (Ex) wavelengths range from 250 nm to 450 nm at 5-nm increments and from 200 nm to 550 nm at 10-nm increments, respectively.Further,Rayleigh and Raman scattering were filtered using interpolation adopted from Bahram et al.(2006).
2.3 Measurement of zeta potential and contact angle
The zeta potential represents the electrostatic force on the cell surface, with which the stability of dispersions can be evaluated (Tan et al., 2020).In this study, the zeta potential was measured at 25 °C using a Zeta sizer (Nano ZS90, Malvern Instruments Ltd., UK) according to Hadjoudja et al.(2010).Before the test, the collected 50-μL sample was diluted 100 times with PBS buffer solution.All measurements were conducted in triplicate.
The contact angle (θ) was measured using a contact angle analyzer (JC2000D, Powereach,Shanghai, China) according to the standard sessile drop method (Funke, 1995).Pure water, glycerol,and diiodomethane were dropped onto the membrane containingDolichospermum, separately.Each sample was tested at least seven times to obtain the arithmetic mean.
2.4 Interfacial thermodynamic characteristics of Dolichospermum
The changes in the interfacial thermodynamics ofDolichospermumsurface in different temperatures were estimated by contact angle measurement.The interfacial free energy (ΔGslIFE), where sl means sample and liquid, between the algae and water is the sum of van der Waals interaction free energy(ΔGslLW) and Lewis acid-base interaction free energy(ΔGslAB) (Florence, 2007).The value of ΔGslIFEcan characterize the interaction between algae and water(Hou et al., 2015).When ΔGslIFE>0, the binding force between algae and water is stronger than that between water molecules, and algae cells are more inclined to combine with water molecules than with each other, so the algae exhibit hydrophilicity.The detailed calculation process is described in Supplementary equations 1–11 and the relevant parameters involved were shown in Supplementary Table S1.
2.5 Dolichospermum growth detection and determination of aggregation ability
Cell density was measured at absorbance of 680 nm (OD680).Furthermore, the maximum photoelectron production (Fv/Fm) and actual photoelectron production (Fv′/Fm′) were measured using a pulse amplitude modulated fluorometer(AquaPen, PSI, Czech Republic).
The aggregation ability of the algal cells is usually evaluated by the aggregation rate (Tan et al.,2018).The aggregation rate was determined according to Xu et al.(2014).At 680 nm, the optical density ofDolichospermumsample was determined(A0).After 6 h of standing in a clean test tube, the algal liquid from 2 cm of the upper layer was carefully removed and the optical density was measured again at 680 nm (At).The aggregation ratio ofDolichospermumsamples was calculated by using the following equation:
2.6 Statistical analysis and chemical measurement
All chemicals used in this work were of analytical grade.Data are presented as the means±standard deviations.Significant differences were analyzed by one-way analysis of variance (ANOVA).All statistical analyses were performed by SPSS 22.0.Pearson correlation and principal component analysis (PCA) were performed to describe the relationships among the EPS content, aggregation ability, and zeta potential.
The polysaccharide content was determined using the phenol sulfuric acid with glucose as a standard (Dubois et al., 1956).Protein content was measured according to Bistgani et al.(2017) using bovine serum albumin as standard.
3 RESULT
3.1 Changes of growth and aggregation ability of Dolichospermum
At 7–11 °C, photosynthetic activity entered resting status.Fv/Fmdid not increase significantly, and the biomass (OD680valua) remained unchanged at 7–13 °C.With the recovery of the photosynthetic activity at 11 °C (Fig.1a),Dolichospermumbiomass began to increase significantly at 13 °C (Fig.1b).Thus,the recruitment temperature ofDolichospermumwas determined as 11 °C in this study.In addition, the lower limit of temperature tolerance was 11 °C forDolichospermumgrowth.
Then, when the cultivation temperature was improved to 21 °C, the maximum photoelectron production (Fv/Fm), actual photoelectron production(Fv′/Fm′) and the growth rate reached the maximum.Therefore, the optimum temperature forDolichospermumgrowth was considered 21 °C in this experiment.
When the cultivation temperature kept increasing from 21 °C to 37 °C, the photosynthetic activity and the growth rate decreased.Finally, the biomass stopped increasing at 35 °C, which was considered the upper limit of temperature tolerance forDolichospermumgrowth.
At 7–11 °C, the aggregation rate increased rapidly, while the aggregation rate decreased significantly at 11–37 °C (Fig.1c).It was observed that the aggregating ofDolichospermumoccurred at the stage of rapid growth when the photosynthetic activity and the growth rate increased rapidly.

Fig.1 Effects of temperature on the growth of Dolichospermum and aggregation variations
It may be the protein that promotedDolichospermumaggregation in EPSs components because the significant increase of the protein concentration in the EPSs was observed at 11–21 °C(Fig.2b).It was proved that most polysaccharides exhibited hydrophilicity and proteins exhibited hydrophobicity (Liu et al., 2010).When the protein proportion increased,Dolichospermumtended to be hydrophobic and easier to gather.As the temperature continued to increase, the protein proportion decreased.Consequently, the algal cells tended to be hydrophilic and difficult to aggregate.
3.2 Physicochemical of the EPSs matrix for Dolichospermum with increasing of temperature
3.2.1 Composition variations among EPSs fractions at different temperatures
Extracellular polymers enhance the ability of algae to resist external stress (Dang et al., 2018; Wang et al., 2018).The extracellular polysaccharide concentration increased with increasing temperature,and the extracellular protein concentration was increased and reached the maximum value at 23 °C(Fig.2).The change rates of polysaccharides and proteins released byDolichospermumin high temperatures (27–37 °C) were higher than that in low temperatures (7–15 °C).In addition, the concentrations of protein in EPSs were always lower than that of polysaccharides with the temperature rising during the growth period, indicating that theDolichospermumEPSs consisted of a major fraction of polysaccharides.This is consistent with the results reported in a lab-cultivated study ofM.aeruginosa(Qu et al., 2012a; Xu et al., 2013b).
The polysaccharide concentration of the three EPSs fractions increased with the temperature rise, while the protein concentration increased before 23 °C and then decreased (Fig.2).This might have correlation toDolichospermumtolerance to temperature that, high temperatures damaged protein structures (Wen et al.,2005).The polysaccharide concentration in S-EPS increased slowly at low temperatures (7–15 °C), and increased when the temperature was higher than 19 °C.Then, it reached the maximum at 37 °C.However, the polysaccharide concentrations in LBEPS and TB-EPS were lower than that in S-EPS and hardly influenced.The protein concentration of the three EPSs fractions increased with increasing temperature until the temperature was higher than 25 °C, indicating that the synthesis of some proteins inDolichospermumwas inhibited at the high temperature (>25 °C).Furthermore, the concentrations of protein in S-EPS and LB-EPS were higher than that of TB-EPS.Some researchers reported that inMicrocystisEPSs, the TB-EPS and S-EPS mainly consisted of polysaccharides, and LB-EPS were mainly composed of proteins (Xu et al., 2013b).In this study, polysaccharides are dispersed in S-EPS mainly, and proteins are dispersed in mainly S-EPS and LB-EPS, indicating that filamentous cyanobacteria had a different EPSs composition from unicellular cyanobacteria.
Principal component analysis (PCA) showed that the two axes explained 76.4% of the total variance of the aggregation ofDolichospermum(Fig.3).It indicated that the aggregation rates were positively correlated to the proteins in EPSs (especially S-EPS and LB-EPS proteins) and the zeta potential.However, the extracellular polysaccharides were negatively related to the aggregation.
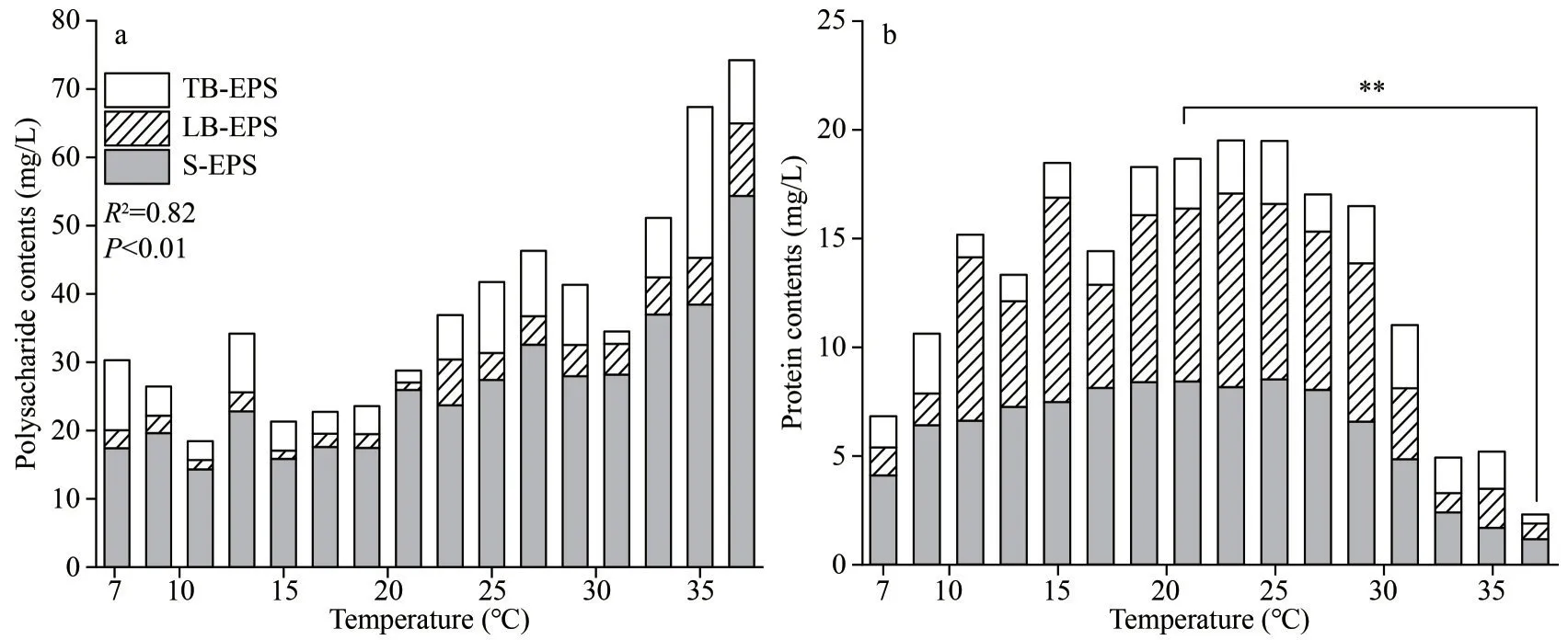
Fig.2 Variations of polysaccharides and proteins in EPSs matrix with increasing temperature
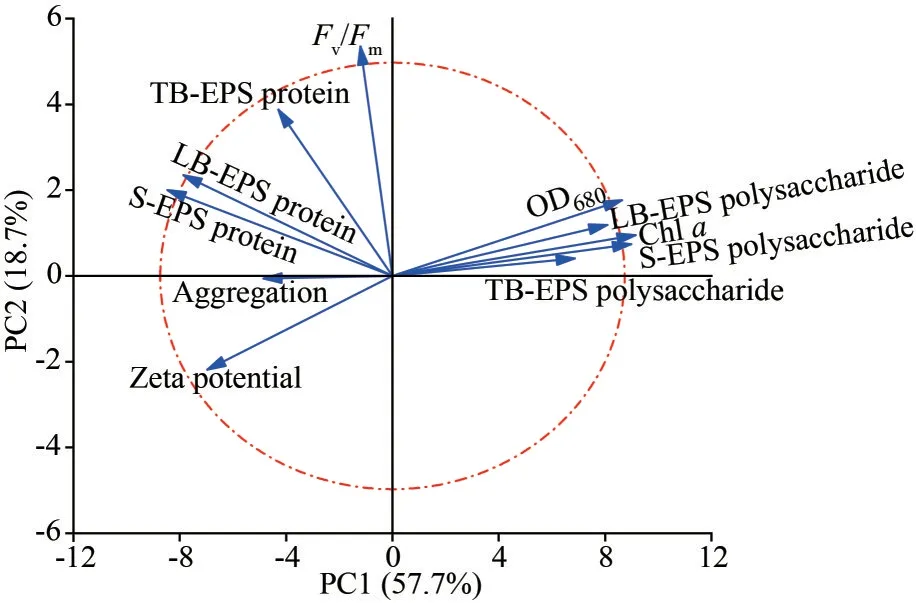
Fig.3 Principal component analysis of variables related to Dolichospermum aggregation process
3.2.2 Fluorescence EEM spectra of EPSs inDolichospermum
EEM is widely used in the characterization of organic matters.Because it could obtain intensity information of wavelength with the advantages of high sensitivity (He et al., 2017).The park A and park B were the humus like substances located at Ex/Em of 360 nm/450 nm and Ex/Em of 320 nm/450 nm, respectively (details about the data of peaks in Supplementary Fig.S1).The peak C was the protein like substances located at Ex/Em of 290 nm/350 nm.The fluorescence is related to tryptophan protein-like substances (Baker and Inverarity, 2004).Both humuslike substances (peaks A and B) and protein-like substances (peaks C) were observed in the S-EPS and LB-EPS, and only protein-like substances (peaks C)were observed in TB-EPS (Fig.4).It means that the TB-EPS ofDolichospermumwas composed of proteinlike substances.Protein is an important component of cyanobacteria cells and can be released during cell metabolism.Henderson et al.(2008) reported the presence of proteins in algae-producing organisms extracted from cyanobacteria, green algae, and diatoms.Humus-like substances may originate from the decomposition of dead cells and macromolecular organics such as proteins and polysaccharides (Qu et al., 2012a).Similar fluorescence peak positions were detected in the three EPSs fractions at different temperatures, showing that each kind of EPSs was not influenced by temperature.Nevertheless, the fluorescence intensity of EPSs fractions was different with increasing temperature, implying that the concentration of specific compounds in each kind of EPSs was affected by temperature.
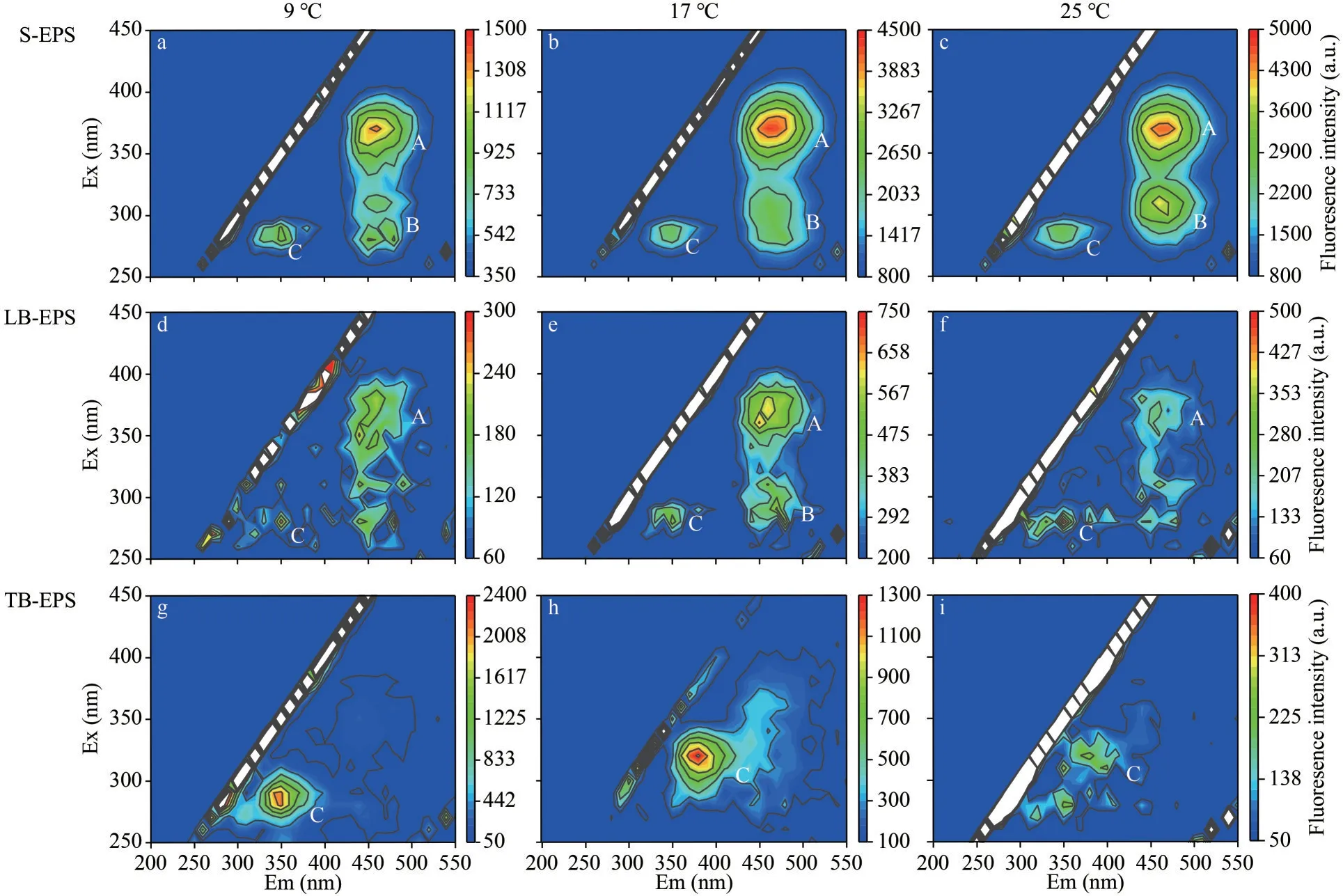
Fig.4 The EEM contours of different EPSs fractions at 9, 17, and 25 °C for Dolichospermum
3.3 Surface thermodynamics properties of the change of aggregation ability
3.3.1 Changes of surface properties ofDolichospermumduring temperature increasing
To reveal the influence of physicochemical properties of algae on the aggregation, the contact angles and zeta potential were analyzed (Table 1).The contact angle between a sample and water could characterize the hydrophobicity ofDolichospermum.Results show thatDolichospermumwas characterized as being hydrophilic (θH2O≤90°).
The zeta potential indicated the electrostatic repulsion on the surface of the algae, and the higher zeta potential value represented the less aggregation capacity.The absolute value of zeta potential increased with increasing temperature, which was consistent with the trend of the total concentration of EPSs, details described in Supplementary Fig.S2.This indicated that the EPSs secretion ofDolichospermumincreased, the negative charge on the surface of algal cells, and enhanced the repulsion between the algal cells.
3.3.2 Surface thermodynamic analysis ofDolichospermum
Surface thermodynamic analysis was applied to examine the aggregation ability ofDolichospermum(Table 2).The value of ΔGslIFEincreased from -13.76to 352.0 mJ/m2during the cultivation, indicating the decrease of surface hydrophobicity.Since the Lifshitzvan-der-Waals interaction force is always attractive,the values of ΔGslLWwere always negative, which was following the results reported by Florence (2007).However, the value of ΔGslABwas greater than that of ΔGslLWat each temperature, implying that ΔGslABwas the major contributor to ΔGslIFE.The Lewis acid-base interactions played a key role in the aggregation.
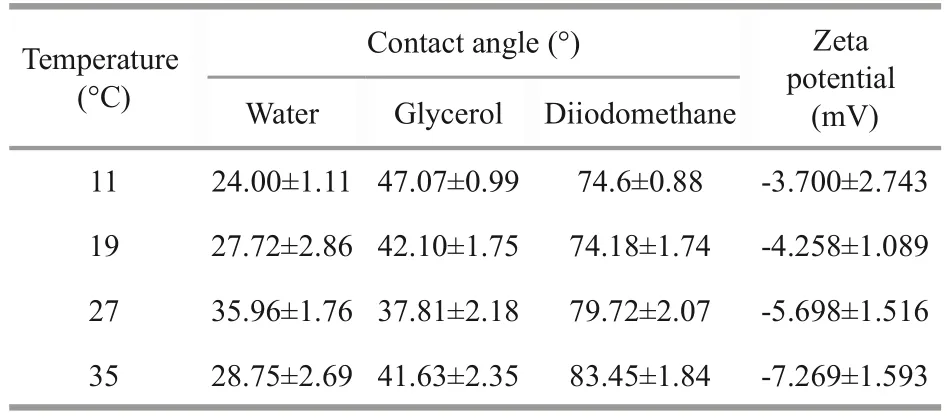
Table 1 Contact angle and zeta potential of Dolichospermum surface with different temperature
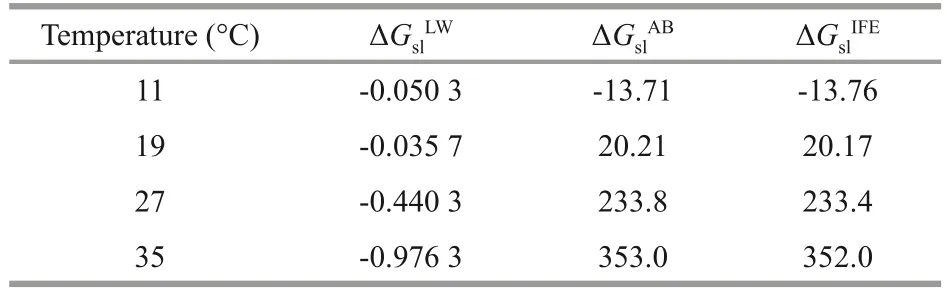
Table 2 Thermodynamic indexes of different temperature(mJ/m2)
The effective Hamaker constant (ASLS) value represented the intermolecular attraction (Funke, 1995;Liu et al., 2007).The electrostatic force (WEI) was mainly influenced by surface charge.In this study,with temperature increase, the ASLSvalue increased,and the WEIvalue gradually increased too (Fig.5).Results show that with temperature increase, the surface charge quantity ofDolichospermumincreased, the electrostatic repulsion on the surface of algae cells enhanced, the inter-particle attraction decreased, so the algae cells tended to disperse instead of aggregating, which is consistent with the results of EPSs (Fig.2) components and zeta potential.
4 DISCUSSION
4.1 The mechanism of Dolichospermum aggregation
Polysaccharides and proteins were the main components of EPSs, accounting for 70%–80% of the total EPSs (Richert et al., 2005), and most polysaccharides had hydrophilic properties, while proteins were more hydrophobicity (Liu et al., 2010).Previous studies (Liu et al., 2014; Ji et al., 2021) and this experiment showed that bound EPSs (LB-EPS and TB-EPS) had more hydrophobic components and fluorescent components than S-EPS.Therefore, it can be concluded that extracellular polysaccharides inhibited the aggregation, while protein promoted the aggregation ofDolichospermum.

Fig.5 Changes of the effective Hamaker constant (a) and the electrostatic force (b)
Furthermore, surface charge was the key factor affecting aggregation (Xing et al., 2022).If the surface negative charge between the cyanobacteria particles was large enough, the repulsion force between the particles was strong and difficult to aggregation.Tan et al.(2018) found that the increase of the ratio of protein to polysaccharide would reduce the net charge on the surface of algae cells, and then reduced the electrostatic repulsion.In addition, proteins are charge neutralizers because some proteins with positively charged groups could neutralize anionic functional groups (Liao et al., 2001).Moreover, the ratio of protein to polysaccharide was positively correlated with hydrophobicity, which is consistent with the conclusion obtained from the surface thermodynamic analysis in this study.
Above all, it could be explained that the protein of EPSs promoted the aggregation ofDolichospermumbecause it reduced the net charge on the surface of algae cells, and then reduced the electrostatic repulsion.In addition, when the polysaccharide of EPSs increased, the aggregation ofDolichospermumcould be inhibited.
4.2 The effects of temperature increasing on Dolichospermum aggregation
Temperature is an important factor affecting the growth of algae (Maheaux et al., 2016), and aromatic amino acid was thought to resist temperature stress,especially at higher temperature (Teng et al., 2019).While high temperatures (27–30 °C) did harm the formation of large colonies and degraded algae cell proteins, DNA, lipids, and membranes (Zhu et al.,2016; Babele et al., 2017), protein secretion inDolichospermumwas inhibited, which is consistent with the findings of this study.Moreover, the growth of cyanobacteria was related to tryptophan and humic substances in S-EPS, while only tryptophan substances in LB-EPS and TB-EPS (Xu et al., 2013b), the concentration of specific compounds in each type of EPSs were affected by temperature (Wei et al., 2017),which is consistent with the results of this study too.
Temperature influenced the surface characteristics ofDolichospermum, and then affected the aggregation of algae cells.The absolute value of zeta potential can represent the change of surface charge (Liu et al.,2010).The absolute value change of zeta potential showed the same trend as the aggregation rate in this study (Fig.1c).The contact angles indicated thatDolichospermumshowed hydrophilicity under the cultivation temperature.We speculated that it was becauseDolichospermumsecreted more polysaccharides than protein with the increase in temperature (Fig.2).The amount of surface charge also altered the aggregation of algae cells, which also confirmed the results of Tan et al.(2018).
In addition, hydrogen bonding, hydrophobic and electrostatic interactions between microorganisms can affect microbial aggregation (Yang et al., 2022).The change of interaction free energy indicated that algae cells tended to bond with water molecules rather than with each other and gradually showed hydrophilicity at high temperature and the Lewis acid-base played a major role in the aggregation of algae cells in process of increasing temperature.The same conclusion was obtained in the study of sludge system (Hou et al.,2015).The results of cell surface thermodynamic analysis further confirmed that the aggregation ability ofDolichospermumchanged with temperature.
Dolichospermumacclimated to a higher temperature in natural conditions, usually around 27–30 °C(Kłodawska et al., 2019).Dolichospermumincreased its metabolic activity and biomass when water temperature increased (Qian et al., 2019).Furthermore,previous research has shown that microcystins are more likely to accumulate and form blooms in the summer (Martinez et al., 2008; Liu et al., 2016), butDolichospermumblooms have occurred frequently in summer recent years (Olofsson et al., 2019; Qian et al., 2019).This was very different from the aggregation temperature determined in the laboratory.The primary reason could be the single strain culture in the laboratory, as well as the relatively simple environment.As a result, detectingDolichospermumaggregation is essential, and further researches are needed to focus on how to deal with the aggregation ofDolichospermumand the relationship with the water environment, and how to manage it.
5 CONCLUSION
The recruitment temperature in this study was 11 °C forDolichospermumgrowth, and 21 °C was the optimum temperature forDolichospermum.35 °C was the upper limit of temperature tolerance forDolichospermumgrowth.The aggregation rate decreased significantly when the cultivation temperature increased from 11 to 37 °C.The composition and concentration of EPSs affected the cell surface properties ofDolichospermumwith temperature change, and thus affected the aggregation ofDolichospermum.During the temperature increase,proteins in EPSs matrix played a major role in promoting the dispersion of algae cells and reducing the aggregation.Surface thermodynamic analysis found that the interfacial free energy and electrostatic repulsion on the surface of algae cells were enhanced,which further increased the dispersion ofDolichospermum.
6 DATA AVAILABILITY STATEMENT
All data generated and/or analyzed during this study are available from the corresponding author upon reasonable request.
7 ACKNOWLEDGMENT
We thank Xiaofeng ZHANG, Jinliu YE, and Caiyu ZOU from Nanjing Normal University for their assistance in samples measurements and field work.
 Journal of Oceanology and Limnology2023年6期
Journal of Oceanology and Limnology2023年6期
- Journal of Oceanology and Limnology的其它文章
- Trends of carbon and nutrient accumulation through time in the Andong salt marsh, Hangzhou Bay, China*
- Physical processes determining the distribution patterns of Nemopilema nomurai in the East China Sea*
- Comparison in structure and predicted function of epiphytic bacteria on Neopyropia yezoensis and Neopyropia katadae*
- Interaction between macroalgae and microplastics: Caulerpa lentillifera and Gracilaria tenuistipitata as microplastic bio-elimination vectors*
- Lake regime shift from submerged macrophyte to phytoplankton affected phosphorus speciation in sediment and eutrophic state in Caohai Lake, Guizhou, China*
- Temporal characteristics of algae-denitrifying bacteria co-occurrence patterns and denitrifier assembly in epiphytic biofilms on submerged macrophytes in Caohai Lake, SW China*
