Study on the Mechanism of Pseudostellariae Radix in Regulating Angiogenesis Based on Network Pharmacology and a Dual-screening System
Na LI Yihui CHAI Yuqi YANG Xiang PU Guo FENG Sibu MA Lailai LI


Abstract[Objectives] This study was conducted to clarify the action mechanism of Pseudostellariae Radix in regulating angiogenesis by using network pharmacology and a dual-screening system, and to provide a basis for its clinical treatment of cardiovascular diseases. [Methods] The TCMSP database was used for preliminary screening to obtain the active compounds of Pseudostellariae Radix and the protein targets of its action. GeneCards and OMIM databases were used to search for targets related to angiogenesis. Cytoscape 3.9.1 was used to construct a drug-target network and protein interaction network of Pseudostellariae Radix in angiogenesis. The GO enrichment analysis and KEGG pathway analysis of the targets of Pseudostellariae Radix in angiogenesis were carried out on Metascape platform. The effects of the screened active compounds were verified using a dual-screening system. [Results] Six active components of Pseudostellariae Radix, luteolin, acetin, beta-sitosterol, linarin, schottenol and 1-monolinolein, were screened by TCMSP database; and the six active components were predicted with 78 common target proteins related to angiogenesis, of which 19 were core targets. Pseudostellariae Radix mainly intervened in angiogenesis through domain specific binding, ubiquitin-like protein ligase binding, kinase binding and other molecular functions to regulate biological processes such as membrane microdomain, plasma membrane raft and caveola. The results of KEGG enrichment indicated that pathways in cancer, lipid and atherosclerosis, hepatitis B, apoptosis, toxoplasmosis and other key pathways might be the mechanism for the intervention of angiogenesis. The results of the dual-screening system showed that luteolin, acacetin, beta-sitosterol and linarin protected HUVECs and promoted zebrafish angiogenesis. [Conclusions] This study preliminarily demonstrated that luteolin, acacetin, beta-sitosterol and linarin could intervene in angiogenesis through multiple targets and multiple pathways, providing ideas and a scientific basis for the treatment of cardiovascular diseases.
Key wordsPseudostellariae Radix; Angiogenesis; Network pharmacology; Dual-screening system
DOI:10.19759/j.cnki.2164-4993.2023.05.022
Cardiovascular disease is the worlds leading cause of death, and more than three-quarters of cardiovascular disease deaths occur in low-income and middle-income countries[1]. Ischemic heart disease (IHD) is caused by myocardial ischemia and hypoxia due to stenosis or obstruction of vascular lumen caused by coronary atherosclerosis[2]. Meanwhile, ischemic injury will activate local and systemic nervous and hormonal systems[3], which will cause pathological changes in extracellular environment, thus promoting myocardial cell necrosis and apoptosis, ventricular remodeling and even heart failure. Traditional intervention and surgical methods have problems including postoperative vascular restenosis and occlusion[4]. Moreover, patients with severe coronary heart disease cannot undergo revascularization due to diffuse stenosis of the multi-vessel coronary artery, and commonly used drugs of Western medicine have very limited therapeutic effects[5]. Therefore, exogenous angiogenic factors or drugs that promote angiogenesis are given to promote coronary collateral circulation and the generation of new blood vessels to meet the needs of cardiac blood supply. Such method is called "therapeutic angiogenesis"[6-8]. On the one hand, angiogenesis promotes the release of a series of angiogenic factors by ischemic cells, which induce arterial generation, which further leads to the establishment of new collateral circulation, achieving self-bridging; and on the other hand, it promotes an increase in capillary density, improves perfusion, and reduces myocardial damage and necrosis[9]. Therefore, therapeutic angiogenesis is of great significance for a considerable number of patients who receive poor efficacy from traditional drugs and are not suitable for coronary artery bypass surgery and interventional therapy, or who experience postoperative restenosis[10-11].
Traditional Chinese medicine believes that the pathological changes of ischemic heart disease are mainly manifested as deficient root and excessive superficiality[12]. Pseudostellariae Radix has the effect of invigorating qi and promoting fluid production, and can be used to treat heart disease with qi deficiency and blood stasis[13]. Modern pharmacological and clinical studies have found that Pseudostellariae Radix and its formulations have cardiovascular protective effects [14-17], as well as anti-inflammatory[18-19] and antioxidant effects[20], while Inflammation and oxidation are closely related to angiogenesis[21-22]. Based on this, our research group proposed that Pseudostellariae Radix may have the activity of promoting angiogenesis, and conducted preliminary analysis using network pharmacology, and the activity was verified by a dual-screening system [human umbilical vein endothelial cells HUVECs and TG (fli1α:EGFP) zebrafish]. This study aimed to provide a scientific basis for the clinical treatment of cardiovascular diseases by clarifying the role of Pseudostellariae Radix intervening in angiogenesis.
Materials and Methods
Experimental materials
Drugs and reagents
RPMI 1640 culture medium (8121004) and fetal bovine serum (2021472), purchased from Gibco Company; trypsin digestive juice (710N0317), Cellmax Company; luteolin (A10013), acacetin (B20627), β-sitosterol (A10024), linarin (A10082) and 1-monolinolein(B74086), purchased from Shanghai Yuanye Biotechnology Co., Ltd.; schottenol (S141584), purchased from Shanghai Kewei Chemical Technology Co., Ltd.
Instruments
CO2 constant temperature incubator, Thermo Fisher Scientific; CKX53 inverted phase contrast microscope, Shanghai Mangi Optoelectronic Technology Co., Ltd.; SKY 1530 full-automatic microplate reader, Thermo Fisher Scientific; AUW120D electronic analytical balance, Shimadzu, Japan; 27310 hypoxia chamber, STEMCELL; X70 fluorescence microscope, Olympus Corporation, Japan; MF53 optical microscope, Mshot Photoelectric Technology.
Cell lines and zebrafish
HUVEC, purchased from Sciencell Company; TG (fli1 α: EGFP) zebrafish, provided by the zebrafish laboratory of Guizhou University of Traditional Chinese Medicine.
Methods
Network pharmacology
Active component screening
The chemical components and targets of Pseudostellariae Radix were collected by TCM systematic pharmacology database TCMSP (http://LSP.nwu.edu.cn/tcmsp.php). Active components were preliminarily screened according to two ADME attribute values of OB≥30% and DL≥0.18 to obtain active compounds, and targets were collected with screened active compounds.
Screening of disease targets
With "angiogenesis" as the key word, disease targets were collected using OMIM (https://www.omim.org) and Genecards (https://www.genecards.org/) databases. The target information of different databases was merged, and repeated target proteins were deleted, so as to establish a disease target database.
Establishment of cross-linking network between disease targets and drug targets
Bioinformatics & Evolutionary Genomics network platform was used to screen out potential targets of the active components of Pseudostellariae Radix intervening in angiogenesis, forming a venn diagram. The downloaded data were imported into Cytoscape(3.9.1) software, and a network topology diagram of "Pseudostellariae Radix-target-chemical component" was constructed.
Construction of protein-protein interaction network
The screened potential targets of active components from Pseudostellariae Radix intervening in angiogenesis were introduced into STRING(https://string-db.org), and "Homo sapiens" was selected in the Organism option to obtain a PPI network. And the relationship data between proteins and the PPI network running in the STRING database were downloaded.
Screening of key targets
The data of protein-protein interaction were imported into Cytoscape(3.9.1) software, and the built-in plug-in cytoNAC in Cytoscape(3.9.1) was used for calculation. Screening was performed according to three network topology characteristic attribute values of PPI networks, namely degree, closeness centrality (CC) and betweenness centrality (BC), and the data were exported.
GO enrichment analysis and KEGG pathway analysis
The potential target proteins of Pseudostellariae Radix intervening in angiogenesis were sorted out and imported into Metascape database, and GO-BP, GO-CC and GO-MF data and KEGG_pathway data were obtained by analysis. Data screening was carried out with P value ≤0.05 as the screening standard, and GO enrichment and KEGG pathway analysis were conducted according to the p value screening. KEGG pathway analysis and GO enrichment analysis mapping were carried out on the Bioinformatics online platform.
Experimental verification based on double screening system
In-vitro screening
HUVECs were inoculated into 96-well plates at a rate of 1×104/well, and after 24 h of culture, the culture medium was changed to 100 μl of M199 medium containing 2% FBS. The normal control group (NC) was incubated in a 5% CO2 incubator at 37 ℃ for 12 h; the hypoxia control (HC) was placed in a hypoxia chamber (5% CO2/95% N2), and incubated in an incubator for 12 h; and the drug groups were added with different concentrations of test drug and placed in a hypoxic chamber (5% CO2/95% N2), and incubated in an incubator for 12 h. The MTT assay method was adopted to detect endothelial cell viability.
In-vivo screening
Fertilized eggs of TG (fli1 α: EGFP) zebrafish were divided into following groups: a normal control group (NC), an anti-angiogenesis control group (AC) and drug groups. The normal control group was added to normal zebrafish culture water and cultured for 48 h. The anti-angiogenesis control group was added with Sim to result in angiogenesis obstacle. The drug groups were added with Sim, and different concentrations of test drug simultaneously. After 48 h of culture, the main blood vessels of the trunk were observed under a microscope, and analysis and quantification were conducted using Image J.
Angiogenesis protection rate (%)=(Vascular areadrug-Vascular areaAC)/(Vascular areaNC-Vascular areaAC)×100%
Statistical method
All experimental results were expressed as mean±standard deviation (x±s), and statistical analysis was conducted using the SPSS 17.0 statistical software package. If the data showed a normal distribution and the variances were homogeneous, one-way ANOVA would be used, and the least significant difference method was used for multiple comparisons between groups; and if the data did not exhibit a normal distribution, the non-parametric test method would be adopted; and P<0.05 stood for a statistical difference.
Results and Analysis
Network pharmacology research
Collection and screening of components from Pseudostellariae Radix
"Pseudostellariae Radix" was inputted into TCMSP database for component search. According to conditions of OB≥30% and DL≥0.18, after deleting components without targets in TCMSP database, a total of six effective components of "Pseudostellariae Radix" were obtained through screening (Table 1 and Fig. 1).
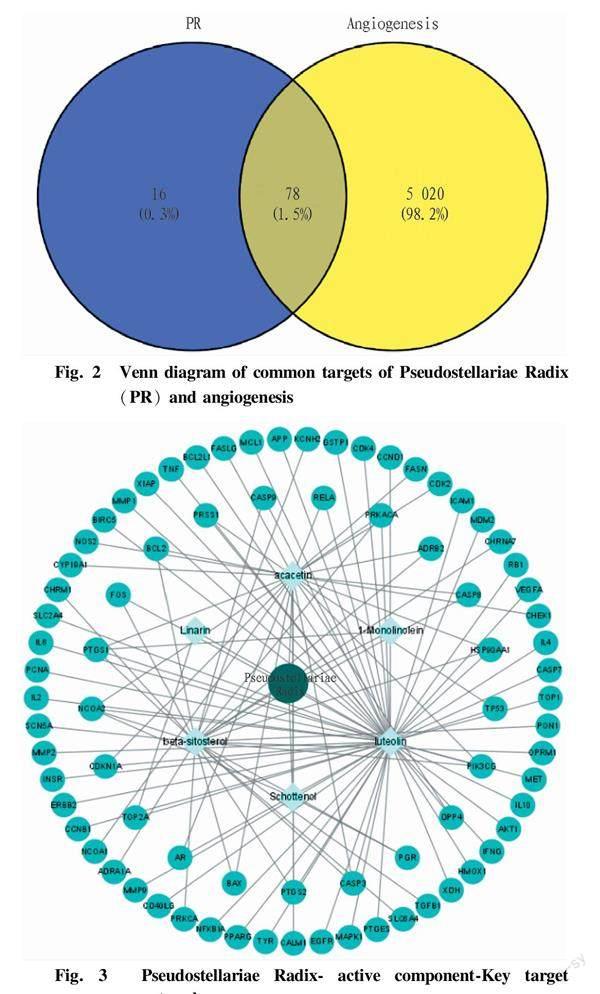
Target prediction was performed on these components, and relevant targets were imported into the protein sequence database UniProt to obtain and standardize names of target proteins. After deduplication, a total of 94 target proteins were obtained. Target proteins of angiogenesis were collected through GeneCards database, and 5 098 disease targets related to angiogenesis were screened. The Venn diagram of 5 098 disease targets and 94 drug component targets was constructed. The results showed that 78 intersected targets were common target proteins of the drug and disease (Fig. 2).
Target prediction of Pseudostellariae Radix components and construction of component-target network diagram
The network topology diagram of "active drug component-disease core targets (proteins)" of Pseudostellariae Radix was constructed by Cytoscape software. In the diagram, the peripheral circular nodes represent 78 targets (proteins), and the square nodes represent the active components of Pseudostellariae Radix, and the middle circular node represents the traditional Chinese medicine Pseudostellariae Radix. One active component can act on multiple targets, and one target can also be acted on by different active components, suggesting that Pseudostellariae Radix has the advantages of multiple components and multiple action points in intervening angiogenesis. The network includes 85 connection points and 114 relationship lines. The degree values were calculated, and the results showed that the core proteins were PTGSESR1, CASP3, RELA, TNF and JUN in descending order of degree. The active components ranked as luteolin, acacetin, beta-sitosterol, linarin, schottenol and 1-monolinolein according to the degree value from high to low (Fig. 3).
Construction of PPI network
The protein interaction spectrum was imported into Cytoscape software to screen core proteins and draw a PPI network diagram. The results showed that the network has 78 connection points and 1 046 edges, among which 19 circles in the center represent core proteins, namely RELA, PGR, BCL2L1, MAPK1, AR, MMP9, PTGSFOS, CCND1, CASP3, HSP90AA1, VEGFA, ERBBPPARG, EGFR, IL6, TNF, TP53, and AKT1 (Fig. 4).
GO functions and KEGG enrichment pathway analysis
In order to further reveal the biological information of Pseudostellariae Radixs intervention in angiogenesis, GO enrichment analysis was carried out on the 78 key targets, and the top 10 items were selected. The results showed that Pseudostellariae Radix mainly participated in regulating molecular functions such as protein domain specific binding, ubiquitin like protein ligase binding, kinase binding, ubiquitin protein ligase binding and protein kinase binding through cell components such as membrane rafts, membrane microbiome, plasma membrane raft, caveola and external side of plasma membrane, realized biological processes such as membrane microdomain, plasma membrane raft, caveola, external side of plasma membrane and side of membrane, thereby intervening in angiogenesis (Fig. 5). The top 20 pathways enriched in KEGG were visualized using a bubble diagram, and the results showed that key pathways such as pathways in cancer, lipid and astrolysis, hepatitis B, apoptosis and topolasmosis may be the mechanism of Pseudostellariae Radixs intervention in angiogenesis (Fig. 6).
Experimental verification of dual-screening system
Effects of active components from Pseudostellariae Radix on HUVEC hypoxia model
Luteolin, acacetin, beta-sitosterol and linarin in Pseudostellariae Radix increased the cell viability of hypoxic HUVECs in a dose-dependent manner. There were no statistically significant differences between schottenol and 1-monolinolein compared with the HC group (Fig. 7).
Effects of active components from Pseudostellariae Radix on Tg (Fli1α:EGFP) zebrafish anti-angiogenesis model
Luteolin, acacetin, beta-sitosterol and linarin from Pseudostellariae Radix could promote angiogenesis of zebrafish to varying degrees (Fig. 8).
Conclusions and Discussion
There is no good solution for a considerable number of patients who cannot be treated with coronary artery revascularization. Therapeutic angiogenesis may become an expected treatment model for a considerable number of patients with diffuse coronary lesions and those who have difficulties in accepting traditional revascularization treatment. Endothelial cells play a key role in the process of angiogenesis. The damage and dysfunction of endothelial cells are closely related to the occurrence and development of ischemic heart disease. Improving vascular endothelial function and promoting angiogenesis through different ways is of great significance for the prevention and treatment of ischemic heart disease[23]. Therefore, hypoxic endothelial cells were used as a model for screening active substances in this study.
Modern studies have confirmed that Pseudostellariae Radix can protect endothelial cells and promote angiogenesis[24]. In this study, six potential active substances were screened from Pseudostellariae Radix by network pharmacology. Among them, luteolin is a bioflavonoid, which exists in many medicinal plants and some commonly eaten fruits and vegetables, including green leaf spices such as parsley, sweet pepper and celery, and has many biological activity. Jia et al.[25] found that luteolin could prevent vascular inflammation caused by TNF-α; and luteolin could also increase the expression of NO in blood vessels by regulating HIF-2α-Arg-NO axis and PI3K-AKT-eNOS-NO signaling pathway, and played a role in protecting pulmonary vascular endothelial function, thus improving the symptoms of pulmonary arterial hypertension[26]. Acacetin, as a natural flavonoid, inhibits the damage of HUVECs induced by high glucose in a dose-dependent manner and improves cell survival rate[27]. ApoE-/-animal experiments showed that acacetin could also reduce ROS level and enhance the expression of reductase protein, playing an antioxidant role through phosphorylation of Nrf2 and degradation of Keap1[28]. Beta-sitosterol is one of the most common components in phytosterols, and it is also a potential herbal nutritional health product approved by the US Food and Drug Administration (FDA). Beta-sitosterol can be used to intervene atherosclerosis and other diseases. Beta-sitosterol can reduce the expression of TNF-α and COX-and alleviate the inflammatory damage of HUVECs induced by LPS[29]. Jiang et al.[30] found that human aortic endothelial cells treated with beta-sitosterol could significantly inhibit apoptosis induced by ox-LDL and improve energy metabolism and cell morphology; and linarin is a common flavonoid, which exists in many traditional Chinese medicines and is used to treat many diseases. Linarin can increase the activity of eNOS by activating PI3K/Akt/NO signal pathway, thus increasing the synthesis and release of NO and achieving the effect of relaxing blood vessels[31]. In this study, screening was conducted using endothelial cells and zebrafish, and it was found that four compounds, luteolin, acacetin, beta-sitosterol and linarin, screened by network pharmacology could effectively protect endothelial cells and promote angiogenesis in zebrafish, and were potential material basis for Pseudostellariae Radix to promote angiogenesis.
In this study, network pharmacology and a dual-screening system were used to clarify the mechanism and active substance basis of Radix Pseudostellariaes intervention in angiogenesis, in order to provide scientific basis for clinical treatment of cardiovascular diseases.
References
[1] ORGANIZATION WH. Prevention of cardiovascular disease: Pocket guidelines for assessment and management of cardiovascular risk: (WHO/ISH cardiovascular risk prediction charts for the African Region)[J]. Tetrahedron Letters, 2013, 54(22): 2817-2820.
[2] ELWOOD PC, RENAUD S, SHARP DS, et al. Ischemic heart disease and platelet aggregation. The caerphilly collaborative heart disease study[J]. Circulation, 1991, 83(1): 38-44.
[3] MITCHELL GF, LAMAS GA, PFEFFER MA. Ventricular remodeling after myocardial infarction[M] Interactive Phenomena in the Cardiac System. Springer US, 1993: 164-168.
[4] JUKEMA JW, VERSCHUREN JJ, AHMED TA, et al. Restenosis after PCI. Part 1: Patho-physiology and risk factors[J]. Nature Reviews Cardiology, 2011, 9(1):53.
[5] HUANG F, LAI W, CHAN C, et al. Comparison of bypass surgery and drug-eluting stenting in diabetic patients with left main and/or multivessel disease: A systematic review and meta-analysis of randomized and nonrandomized studies[J]. Cardiology Journal, 2014, 22(2): 123-134.
[6] ISNER JM, LOSORDO DW. Therapeutic angiogenesis for heart failure[J]. Nature Medicine, 1999, 5(5): 491.
[7] ANNEX BH, SIMONS M. Growth factor-induced therapeutic angiogenesis in the heart: protein therapy[J]. Cardiovascular Research, 2005, 65(3): 649-655.
[8] AHN A, FRISHMAN WH, GUTWEIN A, et al. Therapeutic angiogenesis: A new treatment approach for ischemic heart disease—part I[J]. Cardiology in Review, 2008, 16(4): 163.
[9] HELISCH A, WARE JA. Therapeutic angiogenesis for ischemic heart disease[J]. Advances in Experimental Medicine & Biology, 2000, 476(476): 327-350.
[10] Al SH. Therapeutic angiogenesis in cardiovascular disease[J]. Journal of Cardiothoracic Surgery, 2007, 2(1): 863.
[11] ZACHARY I, MORGAN RD. Therapeutic angiogenesis for cardiovascular disease: biological context, challenges, prospects[J]. Heart (British Cardiac Society), 2011, 97(3): 181-189.
[12] HUANG HY. Exploring the connotation of the pathogenesis of coronary heart disease with "deficient root and excessive superficiality" (continued)[J]. Journal of Beijing University of Traditional Chinese Medicine, 1995(1): 10-11. (in Chinese).
[13] XU L, GAO HC, ZHANG S, et al. Effects of Jilin Radix Ginseng, Codonopsis Radix and Radix Pseudostellariae on cardiac function in patients with heart qi deficiency[J]. Journal of Changchun University of Chinese Medicine, 1997(1): 20. (in Chinese).
[14] LIU XX, RUAN JS. Protective effect of polysaccharides from Radix Pseudostellariae on myocardial ischemia in rats[J]. Chinese Journal of Ethnomedicine and Ethnopharmacy, 2017(17): 18-20. (in Chinese).
[15] WANG Z, LIAO SG, HE Y, et al. Protective effects of fractions from Pseudostellaria heterophylla against cobalt chloride-induced hypoxic injury in H9c2 cell[J]. Journal of Ethnopharmacology, 2013, 147(2): 540-545.
[16] SUN B, WAN L, LIN XJ, et al. Study on the inhibitory effects of Pseudostellaria heterophylla polysaccharide on myocardial apoptosis of ischemia-reperfusion injury model rats[J]. China Pharmacy, 2018(1): 2175-2179. (in Chinese).
[17] YU DJ. Initially study on professor WENG Wei-liangs clinical experience of prescription and medication treating cardiovascular diseases[J]. China Journal of Traditional Chinese Medicine and Pharmacy, 2011, 026(12): 2914-2915. (in Chinese).
[18] PANG HY, HUANG LS, WU HT, et al. Mechanism study of Pseu-dostellaria heterophylla extracts on anti-inflammation induced by stress injuries[J]. Chinese Journal of Pharmaceutical Analysis, 2017(9): 133-137. (in Chinese).
[19] XU XX, HUANG YX, XIA LZ, et al. Effect of Pseudostellaria heterophylla polysaccharides on anti-oxidation capacity and pancreatic pathology of diabetic mouse [J]. Science and Technology of Food Industry, 201033(24): 392-393. (in Chinese).
[20] CHEN Z, LI S, WANG X, et al. Protective effects of Radix Pseudostellariae polysaccharides against exercise-induced oxidative stress in male rats[J]. Experimental and therapeutic medicine, 2013, 5(4): 1089-1092.
[21] KIM YW, WEST XZ, BYZOVA TV. Oxidative stress in angiogenesis and vascular disease[J]. Journal of Molecular Medicine, 2013, 91(3): 323-328.
[22] JAIPERSAD AS, LIP GYH, SILVERMAN S, et al. The role of monocytes in angiogenesis and atherosclerosis[J]. Journal of the American College of Cardiology, 2014, 63(1): 1-11.
[23] SINGHAL AK, SYMONS JD, BOUDINA S, et al. Role of endothelial cells in myocardial ischemia-reperfusion injury[J]. Vasc Dis Prev, 2010, 7(1): 1-14.
[24] LIN SB, JIANG ZS, RUAN JS, et al. Radix Pseudostellariae promotes the proliferation of human umbilical vein endothelial cells through mapk signal transduction pathway[J]. Fujian Journal of Traditional Chinese Medicine, 2017, 48(6): 16-19. (in Chinese).
[25] JIA Z, NALLASAMY P, LIU D, et al. Luteolin protects against vascular inflammation in mice and TNF-alpha-induced monocyte adhesion to endothelial cells via suppressing IΚBα/NF-κB signaling pathway[J]. J Nutr Biochem., 2015, 26(3): 293-302.
[26] JI L, SU S, XIN M, et al. Luteolin ameliorates hypoxia-induced pulmonary hypertension via regulating HIF-2α-Arg-NO axis and PI3K-AKT-eNOS-NO signaling pathway[J]. Phytomedicine. 2022(104): 154329
[27] HAN WM, CHEN XC, LI GR, et al. Acacetin protects against high glucose-induced endothelial cells injury by preserving mitochondrial function via activating Sirt1/Sirt3/AMPK signals[J]. Front Pharmacol., 2020(11): 607796.
[28] WU Y, SONG F, LI Y, et al. Acacetin exerts antioxidant potential against atherosclerosis through Nrf2 pathway in apoE-/-Mice[J]. J Cell Mol Med., 2021, 25(1): 521-534.
[29] ZHANG F, LIU Z, HE X, et al. β-Sitosterol-loaded solid lipid nanoparticles ameliorate complete Freunds adjuvant-induced arthritis in rats: involvement of NF-кB and HO-1/Nrf-2 pathway[J]. Drug Deliv., 2020, 27(1): 1329-1341.
[30] JIANG YH , LI X , NIU W, et al. β-Sitosterol regulated microRNAs in endothelial cells against an oxidized low-density lipoprotein[J]. Food Funct., 2020, 11(2): 1881-1890.
[31] YANG Y, CHEN B, LIANG KL, et al. Relaxation effect of buddleoside combined with luteolin on isolated vessels in vivo and its mechanism[J]. China Journal of Chinese Materia Medica, 2017, 42(7): 1370-1375. (in Chinese).
Editor: Yingzhi GUANGProofreader: Xinxiu ZHU
Received: July 28, 2023Accepted: September 30, 2023
Supported by Project of Science and Technology Department of Guizhou Province (ZK[2021]-546); Project of Science and Technology Department of Guizhou Province ([2019]1401); Guizhou Administration of Traditional Chinese Medicine (QZYY-2021-03); Guizhou Provincial Health Commission (gzwkj2021-464).
Na LI (1986-), female, P. R. China, devoted to research about traditional Chinese pharmacology.
*Corresponding author. Lailai LI, male, P. R. China, devoted to research about pharmacology of traditional Chinese medicine.
- 农业生物技术(英文版)的其它文章
- Simultaneous Determination of 14 β-Receptor Agonists Residues in Mutton by High Performance Liquid Chromatography-Tandem Mass Spectrometry (HPLC-MS/MS)
- Application of Mass Spectrometry (MS)-coupled Techniques in Pesticide Residue Detection
- Optimization of Extraction Process of Polyphenols from Chrysanthemum morifolium and the Development of Chrysanthemum Rice Wine
- Effects of Heat Treatment on Processing Characteristics of Pork
- Discussion on Construction and Mechanism of Practice Bases for Professional Degree Postgraduates Based on the Integration of Production and Education in Local Universities
- Research on Identification Method of Apple Diseases in Southern Xinjiang Based on Deep Learning and Its System Implementation

