Study on the Action Mechanism of Isodon suzhouensis Stems in the Treatment of Prostate Cancer Based on UPLC-Q-TOF-MS/MS Combined with Network Pharmacology and Molecular Docking
Peigui LIU Weiqing ZHANG Xianji LIU Meihui DUAN Weixian YANG Meiqi WEI


Abstract[Objectives] This study was conducted to explore the action mechanism of chemical components from stems of Isodon suzhouensis on prostate cancer based on UPLC-Q-TOF-MS/MS technique, network pharmacology, and molecular docking validation. [Methods] UPLC-Q-TOF-MS/MS was applied to search active components in stems of I. suzhouensis, and SwissTargetPrediction and PharmaMapper databases were used to predict their potential targets. The GeneCards database was adopted to screen the targets of prostate cancer, and the targets of the active components and the targets of prostate cancer were intersected to obtain common targets. A protein-protein interaction network was constructed, and also, an "active component-target-disease-pathway" network was constructed using Metascape for gene ontology function and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis. Moreover, AutoDock was adopted to perform molecular docking verification on key active components and core targets. [Results] Sixty three active components were screened from stems of I. suzhouensis, and they had 233 common targets with prostate cancer, mainly involving biological processes such as protein phosphorylation, response to hormones, active regulation of cell movement, cell response to lipids, and response to oxidative stress, as well as pathways in cancer, prostate cancer, micro ribonucleic acids in cancer, p53 signaling pathway, NF-κB signaling pathway and other signaling pathways, exerting anti-tumor effects. Molecular docking showed that the top 5 key active components had good binding ability with the top 5 core targets. [Conclusions] Active components from stems of I. suzhouensis can exert therapeutic effects on prostate cancer through multiple targets and pathways.
Key wordsStem of Isodon suzhouensis; UPLC-Q-TOF-MS/MS; Network pharmacology; Molecular docking; Prostatic cancer
DOI:10.19759/j.cnki.2164-4993.2023.05.021
Isodon suzhouensis is a perennial herbaceous plant in the Labiatae family, belonging to the genus Isodon. It is one of few medicinal and edible Chinese herbal plants in the genus Isodon[1]. I. suzhouensis is pungent in nature and bitter in taste, and attributed to the liver, lung, stomach and kidney meridians. Its functions are clearing away heat and toxic materials, inducing diuresis and reducing edema, and promoting blood circulation and removing blood stasis. I. suzhouensis is used for damp-heat jaundice, sore throat, lung abscess, epigastric pain, stranguria, edema, arthralgia, amenorrhea, mammary abscess, hemorrhoid, traumatic injury, and venomous snake bites, and it is used for treating various diseases such as malignant sores, tumors, venomous snake bites, tuberculosis, nephritis, pharyngitis, colds, and acute infectious hepatitis in the folk[2].
Prostate cancer is the most common male malignant tumor in Europe and America, and its incidence rate is the first among male malignant tumors in the United States, and its mortality rate is second only to lung cancer[3]. With the development and progress of our society and the extension of the average life span of the population, the incidence of prostate cancer is increasing significantly. At present, the early diagnosis of prostate cancer mainly depends on the level of serum prostate-specific antigen (PSA)[4]. However, as an early diagnostic marker, PSA still lacks the best specificity and sensitivity. Therefore, finding new specific molecular diagnostic markers for prostate cancer to assist or eventually replace PSA has been a hot topic in recent years. Studies on the occurrence of various cancers at the cellular and molecular levels show that RecQ helicases serve as an important family of DNA helicases, which plays a key role in the process of nucleic acid metabolism[5].
At present, the action mechanism of active components in stems of I. suzhouensis with prostate cancer is still uncertain. Therefore, based on liquid chromatography-mass spectrometry (LC-MS) combined with network pharmacology and molecular docking techniques, the active components of I. suzhouensis were analyzed, and their active targets and action mechanism for treating prostate cancer were analyzed and predicted to explore the pharmacologic material basis of I. suzhouensis. This study provides reference for the quality control of I. suzhouensis, and also some theoretical reference for exploring the treatment of prostate cancer with stems of I. suzhouensis.
Materials and Methods
Experimental materials
Medicinal materials
The medicinal materials were purchased from Bozhou medicinal materials market in Anhui Province and kept in the pharmaceutical laboratory of Peoples Hospital of Anshun City. They were identified as I. suzhouensis of Isodon in Labiatae by Yan Chen from the pharmaceutical laboratory of Peoples Hospital of Anshun City.
Samples and reagents
Sample treatment: First, 50 mg of I. suzhouensis stems was extracted by refluxing with 10 times of ethanol for 3 times, 3 h each time, and the extracts were combined. After filtration, the filtrate was concentrated under reduced pressure to recover ethanol and obtain a thick paste. Next, 10 mg of the sample was dissolved in 70% methanol water, and filtered through a 0.22 μm organic microporous filter membrane to obtain a sample solution of I. suzhouensis as the sample for LC-MS analysis.
Reagents: 95% ethanol; methanol (chromatographically pure); acetonitrile (chromatographically pure).
Instruments and conditions
Chromatographic conditions: ACE Ultracore2.5 SuperC18 (100 mm×2.1 mm); mobile phase: acetonitrile (0.1% formic acid)-0.1% formic acid water; gradient elution (0-2 min, 5% acetonitrile; 2-47 min, 95% acetonitrile; 47-50 min, 5% acetonitrile); volume flow rate: 0.3 ml/min; column temperature: 40 ℃.
Mass spectrometry conditions: HESI-II ion source; ion source voltage: 3.0 kV (+)/2.5 kV (-); capillary heating temperature: 320 ℃; sheath gas flow rate: 35 arb; auxiliary gas flow rate: 10 arb; ion source temperature: 350 ℃; mass spectrometry scanning method: Full MS ddms2; scanning range: 100-1 500 m/z; primary resolution of Full MS: 70 000, and secondary resolution: 17 500.
Data analysis: Searching was conducted with Compound discover software from Thermo Fisher Scientific.
Methods
Search and screening of active components
The UPLC-Q-TOF-MS/MS method was used to retrieve active components from stems of I. suzhouensis. Names and structural information of the compounds were obtained from PubChem database[6] (https://PubChem. NCBI. NLM. nih.gov), and the 2D structures were derived and saved as sdf structure. The compound structures were uploaded into Swiss Target Prediction database[7] (http://swisstargetprediction.ch/), and the "probability" value was set as "probability">0.01. The PharmMapper database[8-10] (http://www.lilab-ecust.cn/pharmmapper/) was used to supplement the target information of compounds that had not been collected, and the Norm Fit value was set as Norm Fit>0.9 to predict and analyze target information of compounds. All the target information was summarized, and the Uniprot (https://www.uniprot.org) database[11] was used to correct names of target genes for chemical components, so as to standardize the protein target information.
Prediction of prostate cancer targets and acquisition of drug-disease common targets
In the GeneCards database platform[19], related genes were collected using "Prostate cancer" as the keyword and setting Relevance scores>15, and duplicate values were deleted. The active component targets of I. suzhouensis stems and prostate cancer-related targets were intersected using Venny (http://www.liuxiaoyuyuan.cn/) to obtain common targets.
Construction of protein-protein interaction (PPI) network
The obtained common targets of prostate cancer and active components from I. suzhouensis stems were imported into the String online platform[20] (https://cn.string-db.org/) for analysis. The results were saved in the TSV format and imported into Cytoscape 3.7.0 software for visual analysis. The function of "Network Analyzer" was applied to perform network topology analysis on the PPI results, and a PPI network was constructed. Key targets were screened by CytoNCA, a plugin in Cytoscape 3.7.0 software.
GO and KEGG pathway analysis
The TSV format file saved in the String database was processed. node1 and node2 were merged, and duplicate values were deleted. Next, the data were imported into Metascape database[14] (http://metascape.org/gp/index.html#/main/step1) to perform analysis by selecting GO Biological Processes, GO Cellular Components, GO Molecular Functions and KEGG Pathway, respectively. And the Bioinformatics online platform (http://www.bioinformatics.com.cn/) was used for visual mapping analysis.
Construction of I. suzhouensis stem active component-target-disease-pathway network diagram
The collected common targets from active components of I. suzhouensis stems and prostate cancer, the active components, prostate cancer, the top 20 KEGG pathways and their related target data were integrated into Excel files. Next, the Excel files were imported into Cytoscape 3.7.0 software to construct an "active component-target-disease-pathway" network.
Molecular docking
The top five key active components in the "active ingredient-target-disease-pathway" network were molecularly docked with the top five core targets in the PPI network. Appropriate protein structures were screened from Uniprot database, and then, the protein structures were downloaded from PDB (https://www.rcsb.org/) database[15]. Small molecule structure files were downloaded from TCMSP (https://old.tcmsp-e.com/tcmsp.php) database[16], and Pymol software was used to delete water from protein receptors and solvent molecules from small molecules. Autoduck software was used to add all hydrogens to proteins and small molecules, respectively. Next, molecular docking was performed after setting as receptors and ligands and setting twisted bonds, and results were obtained by calculation.
Results and Analysis
Extraction of active components from stems of I. suzhouensis
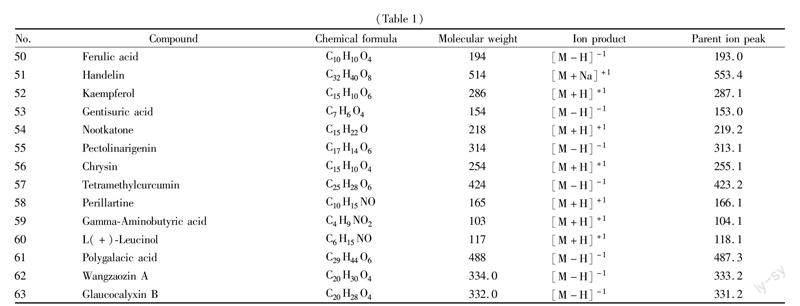
The total ion current (TLC) diagram of positive and negative ion modes was obtained from rapid analysis using UPLC-Q-TOF-MS/MS, as shown in Fig. 1. Based on the compound information generated by mass spectrometry combined with secondary fragment ions and relevant references, qualitative analysis was conducted on chemical components of I. suzhouensis stems, and a total of 63 compounds were identified from stems of I. suzhouensis. The retained components, molecular formulas, compound names, fragment ion information and other data of chemical components are shown in Table 1.
Peigui LIU et al. Study on the Action Mechanism of Isodon suzhouensis Stems in the Treatment of Prostate Cancer Based on UPLC-Q-TOF-MS/MS Combined with Network Pharmacology and Molecular Docking
Common targets from active components in stems of I. suzhouensis and prostate cancer
Sixty three chemical components were obtained by UPLC-Q-TOF-MS /MS, and 62 active components were obtained by Swiss Target Prediction and PharmMapper, while wzz-47 acquired no active targets. Totally, 684 targets were obtained after deduplication. Potential targets of prostate cancer were collected in GeneCards database, and 1 565 targets were obtained after deduplication. The targets of active components and those of prostate cancer were intersected, resulted in 233 common targets, which were the potential anti-prostate cancer targets of the active components in stems of I. suzhouensis, as shown in Fig. 2.
Construction of PPI network
The common targets were imported into the String database to obtain a PPI network using Cytoscape software, involving a total of 233 nodes and 10 914 edges, as shown in Fig. 3. Higher degree values and larger nodes indicated that the nodes were more important in the network. The top 5 targets sorted by degree values were selected as core targets: AKT1, EGFR, IL6, TNF, and MMP9.
The enrichment results of KEGG pathways showed that 198 pathways were involved, and the bubble diagram of the top 20 KEGG pathways was drawn using LogP values, as shown in Fig. 5. The results showed that active components in stems of I. suzhouensis for prevention and treatment of prostate cancer were mainly distributed in the cancer pathway, prostate cancer, micro ribonucleic acids in cancer, AGE-RAGE signal pathway in diabetes complications, toxoplasmosis, bladder cancer, axonal guidance, steroid hormone biosynthesis, transcriptional disorders in cancer, platinum resistance, etc. These pathways were closely related to the treatment of prostate cancer.
Construction of "active component-target-disease-pathway" network
Cytoscape software was employed to construct an "active component-target-disease-pathway" network, as shown in Fig. 6. The CytoNCA plugin and the "Network Analyzer" function were used for topology analysis, and the degree centrality (DC), betweenness centrality (BC) and closeness centrality (CC) were calculated. The top 5 active components were obtained as key active components: wzz-21 (morin), wzz-36 (luteolin), wzz-7 (jaceosidin), wzz-32 (genkwarin) and wzz-1 (nepetin), as shown in Table 2.
Molecular docking
Molecular docking was conducted to analyze the top 5 core targets (AKT1, EGFR, IL6, TNF and MMP9) in PPI and the top 5 key active components (morin, luteolin, jaceosidin, genkwarin and nepetin) analyzed by CytoNCA plugin in the "active component-target-disease-pathway" network. The results are shown in Table 3. The binding energy of the 5 key active components and the 5 core targets was all less than -5 kJ/mol, indicating that the stem activity of I. suzhouensis had good binding ability with the core targets, thereby exerting anti-prostate cancer effects. Pymol was employed to visualize some docking results, as shown in Fig. 7.
Conclusions and Discussion
I. suzhouensis, also known as Wangsaozi, Wenchongbuchi and Baibingbusheng, is a plant in the genus Isodon of the Labiatae family[17]. I. suzhouensis contains various effective medicinal ingredients, and has functions such as anti-tumor, antithrombotic, antibacterial, anti-inflammatory, and antioxidant. It is a rare Chinese herbal medicine that can be used for both food and medicine, but its mechanism of action is still unclear. In this study, we preliminarily explored the anti-prostate cancer mechanism and correlation of the active components from stems of I. suzhouensis, through methods such as LC-MS, network pharmacology and molecular docking.
In this study, the potential mechanism of I. suzhouensis stems in treating prostate cancer was analyzed by network pharmacology. Sixty three chemical components were quickly analyzed using UPLC-Q-TOF-MS/MS technique, and 62 active components were obtained through Swiss Target Prediction and PharmaMapper, resulting in a total of 684 targets. A total of 1 565 potential targets for prostate cancer were collected in the GeneCards database. A total of 233 common targets were obtained through intersection. Cytoscape software was employed to construct a PPI network, and the top 5 targets were selected as core targets: AKT1, EGFR, IL6, TNF and MMP9. An "active component-target-disease-pathway" network was constructed, and the top 5 active components were obtained as key active components: wzz-21 (morin), wzz-36 (luteolin), wzz-7 (jaceosidin), wzz-32 (genkwarin) and wzz-1 (nepetin). Molecular docking was performed on key active components and core targets, and their binding energy was all less than -5 kJ/mol, indicating good binding activity between the two.
The above 5 key active components all belong to flavonoids. In recent years, the anti-tumor effects of flavonoids have gradually attracted attention, such as the anti-tumor activity of quercetin, which is related to its antioxidant effect, reduction of drug resistance in tumor cells, induction of tumor cell apoptosis, and estrogen like effects[18-22]. The anti-tumor activity of silymarin is related to its antioxidant effect, inhibition of related-enzyme activity, and induction of cell cycle arrest[23-24]. AMP, as a kind of flavonoid, has a preventive effect on the growth and metastasis of prostate cancer cells. AMP has stronger inhibitory activity on androgen-sensitive LNCaP proliferation, but weaker inhibitory activity on proliferation of androgen-independent PC-3 human prostate cancer cell line, in vitro, mainly by inducing apoptosis related to downregulation of bcl-2. The inhibitory activity of AMP on the proliferation of normal prostate epithelial cells is significantly lower than that on prostate cancer cells. AMP can also inhibit the migration and invasion of PC-3 cells, which is related to the downregulation of CXCR4 expression[25].
Zhang et al.[26] used immunohistochemical methods to detect and analyze MMP9 in 51 surgical specimens of prostate cancer and 10 surgical specimens of prostate hyperplasia. The results showed that MMP9 expression was positive in 2 out of 10 cases of prostate hyperplasia, which was significantly different from that in prostate
cancer. Moreover, the expression of MMP9 was closely related to the pathological grading, Gleason score, and metastasis of prostate cancer. MMP9 expression may play an important role in the development and metastasis of prostate cancer. Multiple studies have confirmed that upregulation of MMP9 gene expression is an effective marker of tumor metastasis.
RecQ helicase is an important member of the DNA helicase family, and BLM helicase is one of the five RecQ helicases currently found in the human body. Studies on the occurrence of multiple cancers at the cellular and molecular levels have found that RecQ helicases are involved in the occurrence of leukemia, breast cancer and other cancers, and their functional abnormalities determine the multiple characteristics of related diseases in terms of cytogenetics and clinical manifestations. Multiple studies have shown that RecQ helicases are associated with the development of prostate cancer and play a certain regulatory role[27-29].
In summary, in this study, a preliminary study was conducted on the mechanism of I. suzhouensis stems in treating prostate cancer. Through network pharmacology and molecular docking, it was confirmed that stems of I. suzhouensis may be related to pathways in cancer, prostate cancer, and MicroRNAs in cancer signaling pathways, thereby exerting a therapeutic effect on prostate cancer. Next, we will investigate the relationship between helicases of the RecQ family and MMP9 expression. We aim to determine the correlation between their expression and the severity of prostate cancer lesions, as well as the correlation with MMP9 expression, by detecting the expression of helicase genes in the RecQ family at the protein and transcriptional levels. We will further determine the correlation between helicase genes of the RecQ family and prostate cancer cell metastasis, and explore the feasibility of using RecQ helicase expression as diagnostic markers based on quantitative detection for the development and progression of prostate cancer, hoping to provide a theoretical reference for exploring the treatment of prostate cancer with stems of I. suzhouensis.
References
[1] SU Y, CUI J, SHI WW, et al. Research progress in traditional Chinese medicine Isodon amethystoides[J]. Asia-Pacific Traditional Medicine, 2011, 7(6): 155-158. (in Chinese).
[2] CUI J, SHI WW, SU Y, et al. Triterpenoids constituents of Rabdosia Amethystoides (Benth) Hara[J]. Journal of Anhui University of Chinese Medicine, 2011, 30(3): 57-59. (in Chinese).
[3] ALY M, WIKLUND F, GRZNBERG H. Early detection of prostate cancer with emphasis on genetic markers[J]. Acta Oncol., 2011, 50(Suppl 1): 18-23.
[4] KLOTZ L. Active surveillance for prostate cancer: For whom[J]. J Clin Oncol., 20130(24): 3035.
[5] LARSEN NB, HICKSON ID. RecQ Helicases: Conserved guardians of genomic integrity[J]. Adv Exp Med Biol., 2013(767): 161-184.
[6] KIM S, CHEN J, CHENG T, et al. PubChem 2023 update[J]. Nucleic Acids Res., 2023, 51(D1): D1373-D1380.
[7] DAINA A, MICHIELIN O, ZOETE V. Swiss Target Prediction: updated data and new features for efficient prediction of protein targets of small molecules[J]. Nucleic Acids Res. 2019, 47(W1): W357-W364.
[8] LIU X, OUYANG S, YU B, et al. PharmMapper server: A web server for potential drug target identification using pharmacophore mapping approach[J]. Nucleic Acids Res. 2010, 38(Web Server issue):W609-W614.
[9] WANG X, PAN C, GONG J, et al. Enhancing the enrichment of pharmacophore-based target prediction for the polypharmacological profiles of drugs[J]. J Chem Inf Model., 2016, 56(6): 1175-1183.
[10] WANG X, SHEN Y, WANG S, et al. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database[J]. Nucleic Acids Res., 2017, 45(W1): W356-W360.
[11] UniProt Consortium. UniProt: the universal protein knowledgebase in 2021[J]. Nucleic Acids Res., 2021, 49(D1): D480-D489.
[12] ZHANG W, BOJORQUEZ-GOMEZ A, VELEZ DO, et al. A global transcriptional network connecting noncoding mutations to changes in tumor gene expression[J]. Nat Genet., 2018, 50(4): 613-620.
[13] SZKLARCZYK D, KIRSCH R, KOUTROULI M, et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest[J]. Nucleic Acids Res., 2023, 51(D1): D638-D646.
[14] ZHOU Y, ZHOU B, PACHE L, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets[J]. Nat Commun., 2019, 10(1): 1523.
[15] SEHNAL D, BITTRICH S, DESHPANDE M, et al. Mol* Viewer: Modern web app for 3D visualization and analysis of large biomolecular structures[J]. Nucleic Acids Res. 2021, 49(W1): W431-W437.
[16] RU J, LI P, WANG J, et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines[J]. J Cheminform., 2014(6): 13.
[17] ZHOU YP, ZHAI KF, DUAN H, et al. Research progress of Rabdosia amethystoides (Benth)[J]. Journal of Kaili University, 2018, 36(6): 47-51. (in Chinese).
[18] FENG YL, LU LP, ZHAI GY. Research progress on antitumor activity of quercetin derivatives[J]. China Journal of Chinese Materia Medica, 2020, 45(15): 3565-3574. (in Chinese).
[19] LI N, SUN C, ZHOU B, et al. Low concentration of quercetin antagonizes the cytotoxic effects of anti-neoplastic drugs in ovarian cancer[J]. PLoS One., 2014, 9(7): e100314.
[20] HE Y, ZHANG QM, ZHANG Z. Effects of quercetin on proliferation and apoptosis of PC3 cell line[J]. Journal of Southwest Medical University, 2013, 36(4): 340-342. (in Chinese).
[21] ZHU QY, WEI YF, LIU L, et al. A regulatory effect of quercetin on the expressions of Kras and Chk1 genes in human PC-3 cells[J]. Jinagsu Medical Journal, 2011, 37(23): 2744-2746. (in Chinese).
[22] YANG Y, CAO Y, ZHANG TT, et al. The pharmacologic actions progression of quercetin on estrogen related diseases[J]. Journal of International Reproductive Health/Family Planning, 2011, 30(1): 69-72. (in Chinese).
[23] WANG H, LU XM, FAN RL, et al. Mechanism of silibinin in preventing and treating prostate cancer[J]. Chinese Journal of Modern Applied Pharmacy, 2009, 26(S1): 1108-1112. (in Chinese).
[24] REN MJ, ZUO GQ. Research progress on the anti-tumor effect of silymarin[J]. International Journal of Traditional Chinese Medicine, 2005(1): 19-22. (in Chinese).
[25] NI F, GONG Y, LI L, et al. Flavonoid ampelopsin inhibits the growth and metastasis of prostate cancer in vitro and in mice[J]. PLoS One., 2017(6): e38802.
[26] ZHANG XY, HONG BF, CHEN GF, et al. Significance of MMP2 and MMP9 expression in prostate cancer[J]. National Journal of Andrology, 2005(5): 359-361, 364. (in Chinese).
[27] WU P. Effects of CRISPR/Cas9 knockout of BLM helicase gene on the proliferation of prostate cancer cells[D]. Guiyang: Guizhou University, 2018. (in Chinese).
[28] LUO PT, XU HQ, LIU ZW, et al. Construction of RNAi vectors for RNA interference of bloom helicase gene in human prostate cancer PC3 cells[J]. Chinese Journal of Cell Biology, 2015, 37(11): 1497-1502. (in Chinese).
[29] CHEN K, XU HQ, ZHAO JF, et al. Research of CRISPR/Cas9 technique to knock out bloom helicase gene in human prostate cancer PC-3 cells[J]. Chinese Journal of Cell Biology, 2018, 40(9): 1547-1553. (in Chinese).
Editor: Yingzhi GUANGProofreader: Xinxiu ZHU
Received: July 25, 2023Accepted: September 28, 2023
Supported by Science and Technology Project of Guizhou Provincial Health Commission (gzwkj2021-364); Social Development Project of Anshun City Science and Technology Bureau in 2022 (ASKS [2022] 39); Social Development Project of Anshun City Science and Technology Bureau in 2021 (ASKS [2021] 41).
Peigui LIU (1979-), male, P. R. China, associate chief physician, devoted to research about nuclear medicine and tumor markers.
*Corresponding author.
- 农业生物技术(英文版)的其它文章
- Simultaneous Determination of 14 β-Receptor Agonists Residues in Mutton by High Performance Liquid Chromatography-Tandem Mass Spectrometry (HPLC-MS/MS)
- Application of Mass Spectrometry (MS)-coupled Techniques in Pesticide Residue Detection
- Optimization of Extraction Process of Polyphenols from Chrysanthemum morifolium and the Development of Chrysanthemum Rice Wine
- Effects of Heat Treatment on Processing Characteristics of Pork
- Discussion on Construction and Mechanism of Practice Bases for Professional Degree Postgraduates Based on the Integration of Production and Education in Local Universities
- Research on Identification Method of Apple Diseases in Southern Xinjiang Based on Deep Learning and Its System Implementation

