Genetic abnormalities assist in pathological diagnosis and EBVpositive cell density impact survival in Chinese angioimmunoblastic T-cell lymphoma patients
Yunfei Shi ,Haojie Wang ,Yanfei Liu ,Mengping Long ,Ning Ding ,Lan Mi ,Yumei Lai,Lixin Zhou,Xinting Diao,Xianghong Li,Weiping Liu,Jun Zhu
1Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing),Department of Pathology,Peking University Cancer Hospital &Institute,Beijing 100142,China;2 Institute of Genetics and Developmental Biology,Chinese Academy of Sciences,Beijing 100101,China;3Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing),Department of Lymphoma,Peking University Cancer Hospital &Institute,Beijing 100142,China
Abstract Objective: To explore the application of genetic abnormalities in the diagnosis of angioimmunoblastic T-cell lymphoma (AITL) and the reliable pathological prognostic factors.Methods: This study included 53 AITL cases,which were reviewed for morphological patterns,immunophenotypes,presence of Hodgkin and Reed-Sternberg (HRS)-like cells,and co-occurrence of B cell proliferation.The Epstein-Barr virus (EBV)-positive cells in tissues were counted,and cases were classified into“EBV encoded RNA (EBER) high-density” group if >50/HPF.Targeted exome sequencing was performed.Results: Mutation data can assist AITL diagnosis: 1) with considerable HRS-like cells (20 cases): RHOA mutated in 14 cases (IDH2 co-mutated in 3 cases,4 cases with rare RHOA mutation),TET2 was mutated in 5 cases (1 case comutated with DNMT3A),and DNMT3A mutated in 1 case;2) accompanied with B cell lymphoma (7 cases): RHOA mutated in 4 cases (1 case had IDH2 mutation),TET2 mutated in 2 cases and DNMT3A mutated in 1 case;3) mimic peripheral T cell lymphoma,not otherwise specified (5 cases): RHOA mutated in 2 cases (IDH2 co-mutated in 1 case),TET2 mutated in 3 cases,and DNMT3A mutated in 1 case;4) pattern 1 (1 case),RHOA and TET2 co-mutated.Besides RHOAG17V (30/35),rare variant included RHOAK18N,RHOAR68H,RHOAC83Y,RHOAD120G and RHOAG17del,IDH2R172 co-mutated with IDH2M397V in one case.There were recurrent mutations of FAT3,PCLO and PIEZO1 and genes of epigenetic remodeling,T-cell activation,APC and PI3K/AKT pathway.EBER high-density independently indicated adverse overall survival and progression-free survival (P=0.046 and P=0.008,Kaplan-Meier/log-rank).Conclusions: Over half AITL cases might be confused in diagnosis for certain conditions without mutation data.Targeted exome sequencing with a comprehensive panel is crucial to detect both hot-spot and rare mutation variants for RHOA and IDH2 and other recurrent mutated genes in addition to TET2 and DNMT3A.EBER highdensity independently indicated adverse survival.
Keywords: T cell;lymphoma;mutation;pathology;prognosis
Introduction
Angioimmunoblastic T-cell lymphoma (AITL) is a subtype of mature peripheral T-cell lymphoma (PTCL)derived from follicular T helper cells that represents nearly one-third of newly diagnosed PTCL cases (1-3).Most patients are diagnosed with AITL accompanied by aggressive clinical course,B-symptoms (weight loss,fever,and generalized lymphadenopathy) and often autoimmune disorders (2,4).
The characteristic histopathology of AITL is partial/complete effacement of the nodal architecture by medium-sized T cells,with clear/pale cytoplasm,prominent proliferation of high endothelial venules(HEVs) and an irregular,expanded follicular dendritic cell(FDC) meshwork (1,5).Variable numbers of Epstein-Barr virus (EBV)-positive cells are observed,mostly comprising bystander immunoblastic B cells and plasma cells;some cases may progress into secondary B-cell lymphoma,most of which are EBV-positive diffuse large B-cell lymphoma(DLBCL,called “large B transformation”) (5).Hodgkin and Reed-Sternberg (HRS)-like cells that mimic classic Hodgkin lymphoma are often observed (5,6).The B-cell proliferation including HRS-like cell proliferation can raise great challenges in routine pathological diagnostic practices (5).
High-throughput genomic sequencing has recently shed new light on the mutational profile of AITL,revealing recurrent somatic mutations in epigenetic-associated genes,includingTET2andDNMT3A,and hot-spot mutations involvingRHOAG17VandIDH2R172(7-9).RHOAG17Vhas been identified in up to 70% of AITLs and plays a vital role in the oncogenesis of ALTL (10,11).IDH2R172mutation was identified in approximately onethird of AITL cases and can lead to the inhibition of DNA hydroxylases including TET2.The overall incidence ofRHOAG17Vand/orIDH2R172mutation in AITL is approximately 70%;these mutations are specific to AITL and may be helpful in the diagnosis of AITL (12).
The aim of this study was to examine the genetic mutation landscape and prevalence in AITL cases in China and the impact on routine diagnosis processes and to evaluate the pathological factors of AITL on survival.
Materials and methods
Patients and samples
This study included 53 cases of AITL diagnosed in Peking University Cancer Hospital between December 2008 and December 2021.Formalin-fixed paraffin embedded (FFPE)tumor tissue was collected for sequencing.Treatment strategies and response was evaluated using the Lugano criteria.Follow-up data were obtained from medical records and confirmed by phone calls,if necessary.All study procedures were approved by the Ethical Committee of Peking University Cancer Hospital (No.2021KT06),and written consent was obtained from all participants in this study.
Histology and immunohistochemistry (IHC)
Hematoxylin and eosin (H&E)-stained slides for each case were collected and analyzed to determine the morphologic patterns.Pattern 1 was inconspicuous neoplastic T cells partially involving the lymph node,accompanied by hyperplastic follicles mimicking reactive follicular hyperplasia;pattern 2 was characterized by prominent perifollicular neoplastic T-cell infiltrates,with a few residual atretic follicles;in pattern 3,the nodal architecture was totally effaced by tumoral T-cells without follicular structures.
IHC was performed on FFPE sections on an automated Ventana Benchmark immunostainer (Ventana,Tucson,Arizona,USA) using UltraView detection kits (Ventana).The antibodies used for IHC and the evaluation criteria are listed inSupplementary Table S1.The T follicular helper(TFH) cell phenotype was defined as cases in which the tumor cells expressed at least two TFH markers (5).
In situ hybridization (ISH)
EBV status was determined by ISH to detect EBV-encoded RNA 1 and 2 (EBER1/2s) using peroxidase-labelled probes ISH-7001UM (Beijing Zhongshan Golden Bridge Biotechnology,Beijing,China).Tissues from an EBVpositive nasopharyngeal carcinoma were used as a positive control,and positive cells (PCs) were defined as the presence of moderate or higher intensity staining (brown/dark brown) in the nucleus.The number of PCs were recorded in high-power fields (HPFs).The EBER-positive cells were distributed in two patterns: an evenly “scattered”pattern and a focally “hot-spot” pattern.For samples with a“scattered” pattern,10 HPFs were counted,and the“absolute” average number of PCs/HPF was determined.For samples with a “hot-spot” pattern,the numbers of PCs/HPF were calculated as described and the proportion of “hot-spot” areas to the total tumor area were also calculated;the two values were multiplied to obtain the“relative” mean numbers of PCs/HPF. Finally,we determined the “absolute/relative” mean counts of PCs of EBER,and the cases were divided into the “EBER highdensity” group (>50/HPF) and the “EBER low-density”group (≤50/HPF).
All the H&E,IHC and ISH slides were reviewed by at least two pathologists independently;whether the morphological diagnosis consensus for AITL was reached or not was also recorded.
T-cell and B-cell clonality analysis
Polymerase chain reaction (PCR) amplifications of T-cell receptor genes and immunoglobulin gene rearrangements were performed for detecting monoclonality when necessary following the BIOMED-2 protocol as described previously (13).DNA was extracted from FFPE tissue sections using the QIAamp DNA FFPE Tissue Kit(QIAGEN,Hamburg,Germany) following the manufacturer’s protocol.PCR for T-cell receptor (TCR)and immunoglobulin (IG) gene rearrangements was performed using commercial BIOMED-2 multiplex PCR kits (Righton Gene,Shanghai,China).After separation by capillary electrophoresis,PCR products were subjected to GeneScan analysis on an ABI 9700Genetic Analyzer(Applied Biosystems,CA,USA) and analyzed using the GeneMapper software (Version 4.0;Applied Biosystems,CA,USA).
Next-generation sequencing and sequencing data analysis
AITL patients (n=53) with qualified DNA were submitted for targeted exome sequencing (TES) with a customed panel (41 genes,includedTET2,DNMTA3,RHOAandIDH2).Thirty-seven cases undergone a larger panel across 659 genes (all 41 genes were included in the large panel) as reported previously.Genomic DNA (gDNA) extraction from FFPE tissues,library preparation and target gene enrichment were performed as reported previously and following the manufacturer’s protocol (14).Briefly,the gDNA libraries were subjected to high-throughput sequencing with 150-bp pair-end reads on the NovaSeq 60,000 Sequencing System (Illumina,San Diego,CA,USA).The average sequencing depth of tissues was approximately 500×.Sequence reads were aligned using BWA (Version 0.5.9;Broad Institute,CA,USA).Single nucleotide variants (SNVs) were called using MuTect(Version 1.1.4;Broad Institute,CA,USA).Small insertions and deletions (Indels) were determined by GATK (Version 4.0.4;Broad Institute,CA,USA).All final candidate variants were manually reviewed using the IGV browser(Broad Institute,CA,USA).
Statistical analysis
Overall survival (OS) was defined as the time from diagnosis to the last date of follow-up or death,while progression-free survival (PFS) was determined as the interval between diagnosis and the date of relapse,progression,death or last follow-up.Statistical analyses for the differences in OS and PFS rates were performed with IBM SPSS Statistics (Version 22.0;IBM Corp.,New York,USA).P<0.05 was considered as statistically significant.Univariable survival analysis was assessed by Kaplan-Meier and log-rank methods;Cox proportional-hazard model was used for the evaluation of simultaneous influence of all covariates on survival.
March 12, 2007 issue - A few years ago, just as her father was about to disappear into the fog of dementia(), journalist Lucinda Franks stumbled upon a small box in a corner of his dilapidated() apartment. The contents shocked her. Beneath some mysterious maps and crumpled1 foreign bank notes, she found a military cap embellished2 with the raised metal insignia(,) of an eagle, a skull3 and crossbones—and a swastika. Franks knew little about her father s military service during World War II, and had always sensed that he was hiding something. Now questions consumed her. Was my sphinx-like father presenting one character and living another? she writes in her new memoir4, My Father s Secret War. Whose side was he really on? When she pressed for an explanation, her father refused to talk, citing a decades-old pledge of secrecy5.
Results
Clinical features
Among the 53 cases included in this study,approximately 58.5% (31/53) of cases were males and the mean age was 59(range: 36-84) years.Comprehensive clinical characteristics and treatment data were available for 42 of the 53 cases: 40(95.2%) patients presented with advanced stage (stage III-IV) at diagnosis,23 (54.8%) patients presented with B symptoms,15 (35.7%) patients had skin rash,22 (52.4%)had high IPI scores (score 3-5) and 21 (50.0%) had high Prognostic Index for T-cell lymphoma (PIT) scores (score 2-3).In the study group,5 (11.9%) patients had an Eastern Cooperative Oncology Group (ECOG) performance status score of >1;31 (73.8%) had an involvement of more than five nodal areas;16 (38.1%) had more than one extra nodal involvement;6 (14.3%) had bone marrow involvement,29(69.0%) had elevated serum lactate dehydrogenase (LDH)levels and 24 (57.1%) had elevated beta-2 macroglobulin(β2-MG).Among the 53 patients,41 patients received 2-8 cycles chemotherapy;the overall response rate (ORR) was 69.0% and the complete response (CR) rate was 50.0%.Eight patients received autologous hematopoietic stem cell transplantation (AHSCT).Further details are shown inSupplementary Table S2.
Pathological features and clonality analysis results
Regarding morphological pattern,1.9% (1/53) of cases were classified as pattern 1,62.3% (33/53) as pattern 2 and 35.8% (19/53) as pattern 3 (Figure 1A-C).While the amounts of neoplastic T cells varied in each case,most cells were small to medium sizes with a pale/clear cytoplasm with mild nuclear atypia.Regarding IHC results,98.1%(52/53) of cases showed dual CD3+/CD4+;49 cases were found to show the TFH phenotype,while TFH biomarkers for the other 4 cases were not sufficient: the positive rate for CD10,BCL6,PD-1 and CXCL13 was 76.9% (40/52),27.7% (13/47),94.6% (35/37) and 93.9% (46/49),respectively (Supplementary Figure S1A-D).Regarding the extra-GC proliferation of the FDC meshwork (which occupied 0-60.0% of tumor areas,mean 25.3%),and among 73.6% (39/53) of cases the meshwork proliferated markedly (occupied ≥20.0% tumor area).Approximately 13.2% (7/53) of the patients also had B-cell lymphoma (five were DLBCL,shown inSupplementary Figure S2A-C).Approximately 37.7% (20/53) of cases had scattered HRSlike cells highlighted by CD30 staining,resembling classic Hodgkin lymphoma (CHL),shown inSupplementary Figure S2D,E.Regarding the EBV-positive cell counts(range,0-800 PC/HPF,average 92.5),34.0% (18/53) of cases were classified in the “EBER high-density” group(>50/HPF),and 66.0% (35/53) of cases belonged to the“EBER low-density” group (five were completely negative;Figure 1D,E).
T-cell and/or B-cell clonality analysis was performed on 43.4% (23/53) and 30.2% (16/53) of AITL cases.TCR and IG rearrangements were observed in 78.3% (18/23) and 43.8% (7/16),respectively.For the 16 cases with simultaneous TCR/IG detection,43.8% (7/16) were TCR+/IG-,25.0% (4/16) were TCR+/IG+,12.5% (2/16)were TCR-/IG-and 18.8% (3/16) were TCR-/IG+.

Figure 1 Representative images of morphological patterns and EBER-positive cells in patients with AITL.(A) In cases with pattern 1,neoplastic T cells are inconspicuous,with follicles mimicking reactive hyperplasia with ill-defined mantle zones (H&E,100×);(B) In cases with pattern 2,prominent perifollicular neoplastic T-cell infiltrates are seen,with a few residual atretic follicles (H&E,100×);(C) In cases with pattern 3,significant infiltration of the paracortical area and disappearance of follicles is observed (H&E,100×);(D) Cases were classified into two groups on the basis of EBER-positive cell count in tumor tissues (EBER,400×): “EBER high-density” (>50/HPF) or“EBER low-density” group (≤50/HPF).Noting there was unspecific staining on the cytoplasm of plasma cells in the low density group.EBER,Epstein-Barr virus encoded RNA;AITL,angioimmunoblastic T-cell lymphoma;H&E,hematoxylin and eosin.
Results of TES
Positive findings for the TES with the 41 gene panel were found in 52 cases;the mutational profile is shown inFigure 2and listed inSupplementary Table S3.RHOAmutation was identified in 67.3% (35/52) of cases,with a variant allelic frequency (VAF) range of 2.4%-31.4%(median 9.6%). The hot-spot missense mutation ofRHOAG17Vwas found in 30 cases,and we also found a rare in-frame deletion asRHOAG17del(VAF 10.1%);four cases showed the rare missense mutation variantsRHOAK18N,RHOAR68H,RHOAC83YandRHOAD120G(VAF was 3.9%,23.1%,5.1% and 5.7%,respectively).IDH2R172was seen in 25.0% (13/52) cases,and the VAF was 4.1%-25.2% (mean 10.0%).IDH2R172was co-mutated atIDH2M397V(VAF was 48.1%) in one case.IDH2mutations were confined to cases with mutatedRHOA.

Figure 2 Gene mutation profile in AITL patients by TES (n=53,with a panel of 41 genes).Each column represents an individual patient(52 cases were shown and 1 cases without any mutation).The color indicates the type of mutation;the mutation frequency and number in each gene are listed on the left and right,respectively,and the number of mutations in each case is indicated by bar graphs on the top of the plot.AITL,angioimmunoblastic T-cell lymphoma;TES,targeted exome sequencing.
The mutation frequency ofTET2andDNMT3Awas 82.7% (43/52) and 25.0% (13/52),respectively.The results identified 95 mutations inTET2(including 30 frameshift mutations,24 missense mutations,20 nonsense mutations,12 stop-gain mutations,5 splice-site mutations,1 mutation in coding sequences and 3 nonsynonymous single nucleotide variant mutations);the VAF was 1.1%-99.6%(median 20.1%).Although there were eight mutations inTET2I1762V,no hot-spot mutation was seen.There were 13 mutations forDNMT3A(including 9 missense mutations,1 nonsense mutation,1 stop gain mutation,and 2 splice site mutations),and the VAF was 3.7%-42.0% (median 31.0%),also no hot-spot mutations were seen (Figure 3,Supplementary Table S3).The results of the 37 cases examined with the larger TES panel (659 genes) are summarized inFigure 4andSupplementary Table S4.In addition toTET2(81.1%,30/37),RHOA(59.5%,22/37),DNMT3A(24.3%,9/37),andIDH2(24.3%,9/37),other frequently mutated genes (occurred in >20% cases) wereFAT3,PCLOandPIEZO1,with occurrence rates of 24.3%(9/37),21.6% (8/37) and 21.6% (8/37),respectively.Overall,there were 12 missense mutations forFAT3(VAF 4.3%-51.4%,median 48.0%),10 missense mutations and 1 deletion mutation in coding sequences forPCLO(VAF 1.0%-49.0%,median 45.1%) and 10 missense mutations forPIEZO1(VAF 17.6%-59.0%,median 46.2%);and no hot-spot mutations were seen (Supplementary Figure S3A-C,Supplementary Table S4).Most involved signal pathways included epigenetic remolding pathway (TET2,DNMT3A,IDH2,ARID1A,ARID1B,MLL3),T-cell activation pathway (RHOA,NOTCH1,NOCTH2),APC(Wnt) pathway (CH3,CH8,APC) and PI3K/AKT pathway(RELN,PLCG1),as seen inFigure 4.
Association between genetic mutations and pathologic features

Figure 3 Mutation sites in RHOA (A),IDH2 (B),TET2 (C) and DNMT3A (D) in AITL detected by TES.Schematic diagrams of the location of coding mutations identified in genes (generated using Mutation Mapper);different colors indicate different types of mutations.

Figure 4 Gene mutation profile in partial AITL patients by large panel of TES (n=37,with a panel of 659 genes).Each column represents an individual patient.The color indicates the type of mutation;the mutation frequency and number in each gene are listed on the left and right,respectively,and the number of mutations in each case is indicated by bar graphs on the top of the plot.AITL,angioimmunoblastic T-cell lymphoma;TES,targeted exome sequencing.
The mutation status and their pathologic features for all AITL cases are integrated,analyzed and correlated inFigure 5.Using the mutation status ofIDH2,RHOA,TET2andDNMT3A,the cases were divided into four groups: 1)IDH2andRHOAco-mutated group (13 cases): 11 cases were pattern 2 and 2 cases were pattern 3;12 evaluable cases showed the TFH phenotype,3 cases were classified in the “EBER high-density” group,10 cases showed significant extra-GC FDC meshwork proliferation,1 case had a previous follicular lymphoma (diagnosed and treated 7 years earlier);3 cases showed substantial HRS-like cells;5 cases were analyzed for TCR/IG simultaneously: 3 cases of TCR+/IG-,1 case of TCR+/IG+,1 case of TCR-/IG+and 1 case of TCR+(no IG performed).In this group,seven cases were diagnosed as AITL without next-generation sequencing (NGS),and NGS make sense in the other six cases;2)RHOAmutated group (IDH2as wild type,22 cases): 1 case was pattern 1,12 cases were pattern 2 and 9 cases were pattern 3;20 cases showed the TFH phenotype,9 cases were classified in the “EBER high-density” group,17 cases showed significant extra-GC FDC meshwork proliferation,3 cases showed large B transformation,11 cases showed many HRS-like cells;6 cases were analyzed for TCR/IG simultaneously: 2 cases of TCR+/IG-,1 case of TCR+/IG+,and 2 cases of TCR-/IG+,1 case of TCR—/IG— and 5 cases of TCR+(no IG results).In six cases,AITL were diagnosed without NGS,and NGS make sense in the cases left (n=16);3)TET2and/orDNMT3Amutated group (withoutRHOAorIDH2mutation,12 cases): 5 cases were pattern 2 and 7 cases were pattern 3;all cases were found with the typical TFH phenotype;3 cases were classified in the “EBER high-density” group and 9 cases were in the “EBER low-density” group (1 case negative);6 cases were found with significant FDC proliferation,2 cases had large B transformation and the other case relapsed as CHL 7 years later;6 cases were found with considerable HRS-like cells;3 cases were analyzed for TCR/IG simultaneously: 1 case was TCR+/IG-and 2 cases were TCR+/IG+.Only 2 cases were diagnosed for AITL without NGS,and NGS support diagnosis in 6 of the other 10 cases;and 4) Non-mutated group (6 cases): 5 cases were pattern 2 and 1 case was pattern 3;5 evaluable cases showed the typical TFH phenotype,3 cases belonged to the “EBER high-density”group,all cases showed significant FDC proliferation,all were without large B transformation or with minimum HRS-like cells;2 cases were analyzed for TCR/IG simultaneously: 1 case was TCR+/IG-,1 case was TCR-/IG-;1 case was TCR+(no IG).All five cases were diagnosed as AITL by morphology and IHC,NGS reinforced the diagnosis in one case with a rare translocation asSYK::ITK.
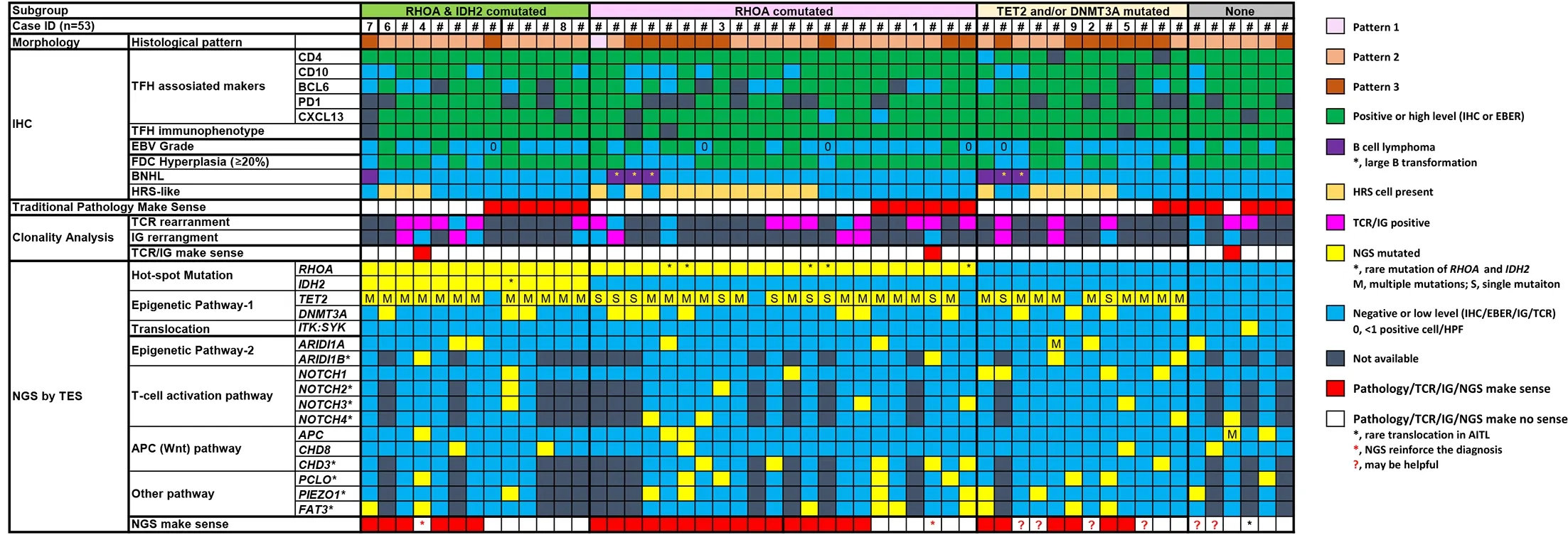
Figure 5 Overview of clinical,pathological,and molecular genetic findings in AITL cases (n=53).Each column of the heat map represents one AITL case and each line indicates the specific analysis.AITL,angioimmunoblastic T-cell lymphoma;HRS,Hodgkin and Reed-Sternberg;NGS,next-generation sequencing;IHC,immunohistochemistry;EBER,Epstein-Barr virus-encoded RNA;TFH,follicular T helper cell;TCR,T-cell receptor;IG,immunoglobulin.
Association between EBV-positive cell density,genetic mutations and survival
For the 42 patients with clinical and survival data,the OS ranged from 2.0 to 84.3 months.Twenty-one patients(50.0%) died.The median OS was 29.9 months,and the expected OS was 40.0% in 5 years;PFS ranged from 1.5 to 84.3 months,and 36 cases (85.7%) had PFS events.The median PFS time was 9.0 months,and the expected PFS rate was 29.0% at 2 years and 7.0% at 5 years.We analyzed the impacts of clinical parameters,EBV-positive cell density and genetic mutations on survival.Univariate and multivariate analyses showed that “EBER high-density”was an independent adverse survival factor for OS(P=0.046,Figure 6A,univariate Kaplan-Meier estimations,log-rank test). “EBER high-density” was also an independent adverse survival factor for PFS (P=0.008,Figure 6B,univariate Kaplan-Meier estimations,log-rank test).All the prognostic parameters involved in Cox regression multivariate survival analyses and the results are listed inTable 1.
Discussion
Regarding the morphological patterns,most of the cases in this study were pattern 2 or pattern 3,which was typical for AITL (1,5). The TFH biomarker panel including CXCL13,CD10,BCL6 and PD-1 was sufficient for the evaluation of the TFH phenotype of the CD3+/CD4+(usually) lymphoma cells (1,5,15).Other morphological features such as significant extra-GC FDC meshwork and variable EBV-positive cells are also important features(1,5).Morphologically,only 21 out of 53 cases reached consensus for AITL when reviewed.The main diagnosis challenges are as follows: 1) morphological pattern 1,which is difficult to distinguish from localized perifollicular T cell hyperplasia;2) AITL can be accompanied by B cell proliferation,which can progress into B-cell lymphoma,and it is easy for AITL to be misdiagnosed as B-cell lymphoma;and 3) the significantly enlarged immunoblastic B cells in AITL can mimic HRS cells,as the so-called“HRS-like” cells,leading to misdiagnosis as CHL (5,16).

Figure 6 EBER density in tumor tissues was associated with clinical outcomes in AITL.Patients with “EBER high-density” group(>50/HPF) exhibited a significantly shortened OS (P=0.046) (A) and shortened PFS (P=0.008) (B).Univariate Kaplan-Meier estimations with the log-rank test was performed.EBER,Epstein-Barr virus encoded RNA;AITL,angioimmunoblastic T-cell lymphoma;OS,overall survival;PFS,progression-free survival.
While TCR/IG clonality analysis as TCR+/IG-can strongly support PTCL including AITL(1,17,18),approximately 25%-30% of AITL cases were TCR+/IG+and 11%-25% were TCR-/IG± (6,19,20).The simultaneous TCR/IG clonality analysis performed in 15 morphologically unclear cases and was only helpful in 2 cases;this might be caused by the robust B cell infiltrates or minority of tumoral T-cells in AITL.From traditional morphological and clonality analysis,more than half of AITL cases were challenging in diagnosis in our study;the differential diagnoses mainly included CHL,B-cell lymphoma (especially EBV+DLBCL),peripheral T cell lymphoma,not otherwise specified (PTCL-NOS) and reactive hyperplasia.
TES has been widely used for tumor gene mutation detection,including in lymphoma,molecular typing and differential diagnosis,prognosis and progress/transformation evaluation,treatment-target screening and minimal residual disease monitoring.In terms of assisting the diagnosis of B-cell lymphoma,BRAFV600EandMYD88L265Pmutations are important in the diagnosis of hairy cell leukemia and lymphoplasmacytic lymphoma,respectively (1,12).AITL is also considered as a unique molecular subtype at the genetic level (7) and is characterized by frequent mutations ofTET2(47%-92%),DNMT3A(20%-38%),IDH2R172(20%-45%) andRHOAG17V(50%-70%) (7,21-25).Mutations involvingRHOAG17VorIDH2R172were even considered as desirable diagnostic criteria the in the coming 5th edition of WHO lymphoma classification (1).TES showed that the mutation rates ofRHOA,IDH2,TET2andDNMT3Ain our study were 67.3%,25.0%,82.7% and 25.0%,respectively,and all cases ofIDH2mutation occurred in patients withRHOAmutation;these results are consistent with previous reports.The mutations of these genes in AITL were studied in another center in China (26): the mutation rates were 30.6% forRHOA,11.3% forIDH2,14.5% forTET2and 11.3% forDNMT3A;these rates were much lower compared with our results,as all sequencing was done by Sanger sequencing;the specific mutation sites and variants were not clear.
The majority of mutations inIDH2andRHOAwere hotspot mutations,such asIDH2R172andRHOAG17V;TET2mutation was more inclined to occur asTET2I1762V(8/95).Most mutation sites ofTET2andDNMT3Awere distributed randomly,which is consistent with the literature (7,27).Five cases were found with rareRHOAmutations(RHOAK18N,RHOAR68H,RHOAC83YandRHOAD120Gand a rare in-frame deletion as ofRHOAG17del(VAF10.1%);none of these events occurred in cases withIDH2R172mutation.TheRHOAK18Nmutation was previously reported (28),and there were also otherRHOAmutation variants (29,30) in AITL.TheseRHOAmutation variants might be related to the pathogenesis and heterogeneity of clinical and pathological manifestations.With respect to the existence ofRHOAmutation variants,previously researchers tried to develop quantitative PCR or Sanger sequencing focused on hotspot mutationRHOAG17V(21,22,31,32) alone to assist diagnosis,it is important as shown by our research that it might be insufficient to support pathological diagnosis.In one case,IDH2R172T(VAF 6.0%) was found to be comutated at both andIDH2M397V(VAF 48.1%) and might be an incident finding.These findings strongly suggest the importance of using NGS (TES,specifically) rather than other PCR-based platforms in the molecular pathological diagnosis of AITL,as more rare mutations ofRHOA,IDH2or other genes might be identified to assist diagnosis.
The median VAFs ofRHOAandIDH2(9.6% and 10.0%,respectively) were much lower than those ofTET2andDNMT3A(20.1% and 30.0%),suggesting thatRHOAandIDH2mutations were tumor cell-specific mutations,butTET2andDNMT3Amutation already existed and derived from the hematopoietic stem cells (7,27).As mutation ofTET2and/orDNMT3Aseemed less specific thanRHOAandIDH2for AITL (33-35),if gene mutations were also found inFAT3,PCLOandPIEZO1(occurrence >20%) or were associated with signaling pathways including the epigenetic remodeling pathway,T-cell activation pathway or APC(Wnt) pathway,the evidence together withTET2and/orDNMT3Amutation would support the diagnosis of AITL(14,28,34,36).What’s more,mutations ofFAT3,PCLOandPIEZO1were not previously identified as related to AITL.This maybe because we used a more comprehensive panel(659 genes) for TES.However,more AITL cases and detection by TES with relative comprehensive gene panel like ours or even with whole exome sequencing are required to obtain more data and more convincing conclusions.
After integrated analysis with pathological features and TES results,we found that TES may be used for differential diagnosis in the following situations: 1) cases accompanied by HRS-like cells,which need to be differentiated from CHL (20 cases): cases withRHOAmutation (14 cases total: 3 cases co-mutated withIDH2,4 cases with rareRHOAmutation),5 cases withTET2mutation (1 case co-mutated withDNMT3A) and 1 case withDNMT3Amutation;2) cases accompanied by B cellderived lymphoma (7 cases):RHOAmutation was found in 4 cases (1 case had extraIDH2mutation),TET2mutation was found in 3 cases andDNMT3Amutation was found in 1 case;3) differentiating from PTCL-NOS: 5 cases with minimal CD21-positive FDC meshwork proliferation and low EBV-positive cell density resembled PTCL-NOS.RHOAwas mutated in 2 cases (in 1 caseIDH2was also mutated),TET2was mutated in other 2 cases,andDNMT3Awas mutated in 1 case;and 4) histological pattern 1,RHOAandTET2were co-mutated in this case.
There is a lack of reliable prognostic indicators for AITL (4),even in our single center,and there may be differences regarding different periods,scales and series of the studies;there are also no reliable pathological prognostic indicators (2,14,20,36-38).We found that a high count of EBV-positive cells (>50/HPF) was linked with a significantly poorer prognosis and was an independent prognostic indicator for OS and PFS.We used a cut-off value of >10/HPF in our previous study(n=61) (39),but it did not work in this series (30 overlapped cases).A cut off value of >50/HPF would work in both series and is easier to use in practice.Shen and Liang,et al.(40,41) found that patients with high circulating EBV DNA copy number had poor prognosis in PTCL and AITL.Our study indicated that the EBV high-density in tumor tissue might manifest or be paralleled with high circulating EBV DNA load;more studies must be done to examine this hypothesis.Although there are reports suggesting that EBER-negative individuals have a worse prognosis,the previous studies calculated the percentage of EBV-positive cells in all cells,which is quite different from our evaluation method focused on quantity;additionally,the reports only involved younger (≤60 years old) AITL patients (42).The clinical impacts of the frequent mutations in AITL remain controversial (14,43).A series from the USA (21) and another of two centers from Japan (29,44) showed thatRHOAG17Vdid not impact the survival of AITL patients;however,another study from Taiwan,China found thatRHOAG17Vmutation indicated poorer PFS (n=31) (45).In studies from Stanford (24) and New Delhi (n=33 and n=37),RHOAG17V-mutated AITL cases had a better survival(46).We previously found thatRHOAmutations may be linked with better survival (14,39),but the number of cases was smaller (n=30),as the follow-up time prolonged,the prognostic value disappeared.While previous research across PTCL from our center (14) foundTET2mutation as an adverse prognostic factor,the analysis involved other types of PTCL cases,which were usually negative forTET2mutation.
In summary,AITL is a unique lymphoma identity (47).While morphology and immune phenotype are the general methods to diagnose AITL,more than half of AITL cases might still be confused in routine diagnosis mainly for the occurrence of HRS-like cells or B cell lymphoma,the reduction of extra-GC FDC meshwork or the inconspicuous tumoral T cells in pattern 1.TCR/IGH clonality analysis would be much less helpful in this circumstance than in other types of PTCL.RHOAandIDH2are also genes associated with hot-spot mutations in Chinese AITL patients and can effectively supported the diagnosis;however,there were some cases with rare variants ofRHOAorIDH2mutation.For cases with onlyTET2and/orDNMT3Amutation,extra mutation ofFAT3,PCLOandPIEZO1(all occurrence >20%) or genes associated with specific signaling pathways might also support the diagnosis.
Conclusions
It is crucial to use TES with a relative comprehensive panel to assist the diagnosis of AITL cases.Finally,it is important to realize that the increased density of EBV-positive cells in AITL tumor tissues is an independent adverse prognostic factor.
Acknowledgements
This study was supported by “Hygiene and Health Development Scientific Research Fostering Plan of Haidian District Beijing” (No.HP2021-31-50302).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
 Chinese Journal of Cancer Research2023年5期
Chinese Journal of Cancer Research2023年5期
- Chinese Journal of Cancer Research的其它文章
- OpenNAU: An open-source platform for normalizing,analyzing,and visualizing cancer untargeted metabolomics data
- Baseline radiologic features as predictors of efficacy in patients with pancreatic neuroendocrine tumors with liver metastases receiving surufatinib
- A novel multimodal prediction model based on DNA methylation biomarkers and low-dose computed tomography images for identifying early-stage lung cancer
- Genetic susceptibility loci of lung cancer are associated with malignant risk of pulmonary nodules and improve malignancy diagnosis based on CEA levels
- Update of latest data for combined therapy for esophageal cancer using radiotherapy and immunotherapy: A focus on efficacy,safety,and biomarkers
- Inspired by novel radiopharmaceuticals: Rush hour of nuclear medicine
