Advances in micro-nano biosensing platforms for intracellular electrophysiology
Jiajin XUE, Min SHAO, Zhigang GAO, Ning HU,2,3
Review
Advances in micro-nano biosensing platforms for intracellular electrophysiology

1General Surgery Department, Children’s Hospital, Zhejiang University School of Medicine, Hangzhou 310052, China2Department of Chemistry, Zhejiang University, Hangzhou 310058, China3Zhejiang-Israel Joint Laboratory of Self-Assembling Functional Materials, ZJU-Hangzhou Global Scientific and Technological Innovation Center, Zhejiang University, Hangzhou 311215, China4Physical Examination Center, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou 310052, China
The establishment of a dependable electrophysiological detection platform is paramount for cardiology and neuroscience research. In the past decade, devices based on micro and nanoscale sensing and control technologies have been developed to construct electrophysiological platforms. Their unique morphological advantages and novel processing methods offer the potential for high-throughput, high-fidelity electrical signal recording. In this review, we analyze the structure, transmembrane strategies, and electrophysiological detection methods of active/passive micro and nano sensing platforms. We also provide an outlook on their vast potential for development in light of the opportunities and challenges facing micro and nano sensing technology, with the aim of pushing for higher-level electrophysiological platform construction to meet the needs of experimental research and clinical applications.
Intracellular electrophysiology; Micro-nano biosensing platforms; Cardiology and neuroscience; Intracellular action potentials
1 Introduction
The development of electrophysiological detection technology is crucial for research in cardiology and neuroscience. Cardiovascular and cerebrovascular diseases are the leading causes of morbidity and mortality worldwide (Chen et al., 2017; Timmis et al., 2018; Ma et al., 2020). Thus, developing a reliable, long-term monitoring platform for cellular electrical activity is of great significance for exploring the pathogenesis of these diseases and for timely prevention and treatment. In addition, large-scale mapping of cell-network activity must be realized as it is essential for exploring the functional mechanisms underlying the heart and brain, as well as their relationships with physiology and pathology, and understanding the functional connections of cell circuits. This requires electrophysiological recording platforms to have the ability to record in parallel on a large scale. They should be capable of simultaneously measuring and controlling the electrical activity of thousands of cells, as well as performing accurate circuit tracing.
Over the past few decades, traditional imaging techniques, such as electrocardiogram and electroencephalogram, have been primarily used for recording cellular electrical signals (Valappil et al., 2010; Hannun et al., 2019). These methods are non-invasive and suitable for analyzing large-scale cellular aggregate activity. However, the visual resolution they provide is relatively low and they do not have the ability to record individual cells. On the other hand, the development of extracellular electrodes has enabled researchers to obtain more electrophysiological information on cells. From passive microelectrode arrays to active field-effect transistors, extracellular electrodes have successfully recorded extracellular signals from cardiac cells, neural cells, and other electrically excitable cells (Thomas et al., 1972; Connolly et al., 1990; Fromherz et al., 1991; Voelker and Fromherz, 2005; Johnstone et al., 2010). Their non-invasive characteristics avoid the risk of mechanical damage to cells and allow for multi-channel, long-term monitoring of cellular electrical signals. However, the electrophysiological signals recorded by extracellular electrodes have limitations in terms of strength and quality, which make it difficult to determine detailed information about cellular electrophysiological mechanisms. In contrast, emerging intracellular recording techniques have prominent advantages in exploring intrinsic mechanisms of cellular electrophysiological changes, drawing excitation/inhibition synaptic connections, and studying regulation of potential by chemical stimuli in detail. Intracellular recording can obtain high-fidelity action potentials that contain critical information about the type, state, and density of various ion channels. Many cardiovascular and cerebrovascular diseases are closely linked to ion-channel dysfunction, and the shape of action potential is also an important indicator for evaluating cell differentiation and maturation of stem-cell-derived cardiomyocytes. Therefore, the development of intracellular recording platforms to accurately measure the duration, refractory period, and upstroke velocity of action potentials is crucial for clinical treatment and experimental research. Traditional intracellular recording methods mainly include patch-clamp technology and voltage-sensitive dye/voltage-sensitive protein-based optical imaging technology. Patch-clamp technology typically involves using an ultra-thin glass tube filled with ion solution as an electrode, tearing part of the cell membrane, and directly contacting the cytoplasm (Pantoja et al., 2004; Davie et al., 2006; Wang et al., 2015; Vardi et al., 2016). Through connection to a high-sensitivity amplifier, this technology can accurately record the amplitude and shape of electrical signals. Although patch-clamp electrodes have a high signal-to-noise ratio and high sensitivity, they cause irreversible damage to cell membranes, which is not conducive to long-term recording. Also, the complex operation process seriously affects simultaneous recording of multiple cellular electrical signals. Optical imaging technology permits large-scale parallel detection of intracellular electrical signals, but its low signal-to-noise ratio, in addition to related drug side effects or phototoxicity, limits widespread use (Matiukas et al., 2007; Lopez-Izquierdo et al., 2014). Therefore, further development in electrophysiological research calls for higher standards for intracellular recording platforms: (1) Minimal invasiveness. This requires electrodes to have relatively small volumes and enter cells gently to minimize damage to them, allowing for long-term recording. (2) High sensitivity. Reducing electrode size and maintaining a high signal-to-noise ratio and high sensitivity are important for measuring sub-threshold potentials of cells and fully mapping synaptic connections. (3) Multiplexing. Multi-cell-electrode interfaces allow for single-cell recording while enabling network-level expansion, facilitating large-scale studies with higher spatial resolution. (4) Stable cell-electrode interface. Tight sealing and stable coupling between a cell membrane and electrode are key factors for reducing signal loss and achieving long-term recording.
Micro and nano sensing technology has opened up new avenues to meet the above requirements (Spira and Hai, 2013; Zhang and Lieber, 2016; Abbott et al., 2018). The downsizing of cellular recording platforms is associated with reduced cell invasiveness and increased numbers and densities of recording sites. With the progressive evolution of nanofabrication processes, a vast range of material choices and flexible structure designs provides numerous possibilities for the development of micro-nanoelectronics. The continuous improvement of cell-membrane regulation strategies and cell-electrode interfaces has also promoted the application of micro-nano sensing and control technology in intracellular recording, providing powerful tools for neuroscience and cardiology research. This article reviews the recent research progress on micro-nano sensing platforms for intracellular recording. We summarize and compare the structures and transmembrane strategies of active/passive micro-nano sensing platforms, as well as their effects on electrophysiological detection of electrically excitable cells, and finally discuss the future development of micro-nanoelectronics.
2 Active field-effect transistors
2.1 Device structure
Active field-effect transistors often incorporate semiconductor materials such as Si and SiO2, which enable high-sensitivity intracellular recording without electrode impedance. In addition, combining metal and semiconductor materials can produce electrodes with a silicon core and a metal tip, greatly improving the functionality of micro-nano sensing and control devices when combined with complementary metal-oxide semiconductor (CMOS) technology. Lieber's group designed a series of nanowire/tube field-effect transistors (Tian et al., 2010; Duan et al., 2012; Qing et al., 2014; Zhao et al., 2019) to overcome the limitations of electrode impedance; this minimized the electrode size and record signal amplitudes to levels equivalent to patch-clamp recordings (Figs. 1a and 1b). U-shaped nanowire probes (Zhao et al., 2019) have been shown to be more sensitive for intracellular action potential recording with decreasing electrode curvature radius (Fig. 1c). The fabrication process of active field-effect transistors often uses gas-liquid-solid reactions to obtain nanowire structures and also involves manufacturing interconnects through photolithography.
2.2 Penetration strategy
Active field-effect transistors usually use chemically modified penetration strategies which utilize lipid modification to permit fusion with cell membranes (Tian et al., 2010). Lieber's group modified phospholipid bilayers on the surface of nanoscale field-effect transistors (Fig. 2). This penetration strategy has minimal impact on electrode conductivity and sensitivity, and is less mechanically invasive for cells, making it possible to record high-quality intracellular electrical signals. In addition, self-assembled monolayers of alkylthiol can be used to modify nanoelectrodes. The principle is to optimize the cell-electrode interface through fusion with the cell membrane, allowing recording of intracellular electrical signals. The chemical modification means that the electrode can penetrate the cell in a minimally invasive and gentle way, but the modification typically cannot provide precise control.
2.3 Electrophysiological detection
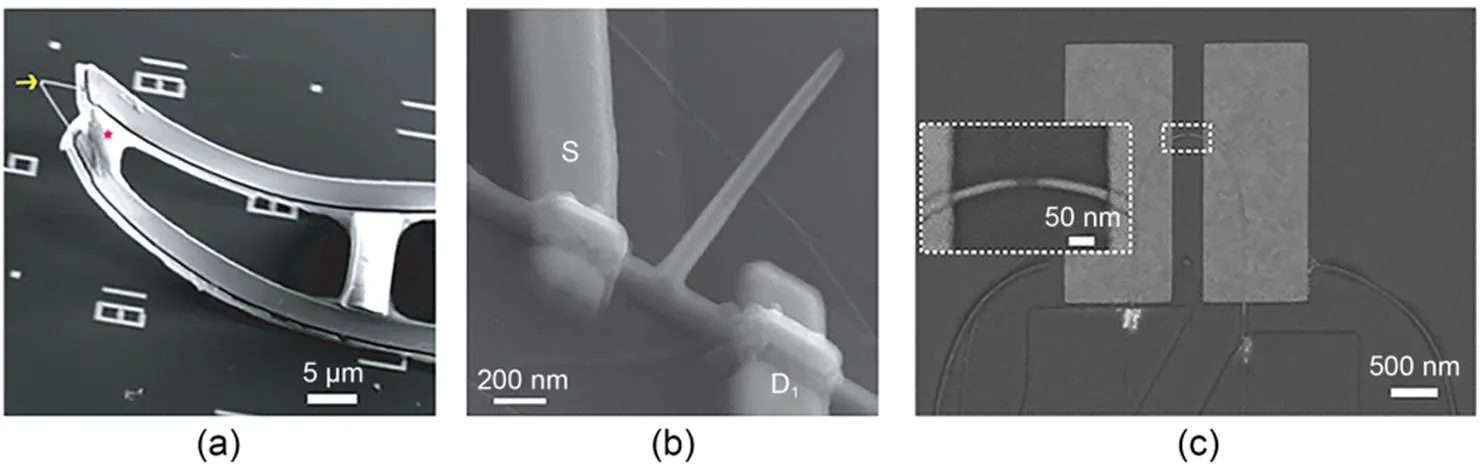
Fig. 1 Device structure of active field-effect transistors: (a) SEM image of a kinked silicon nanowire field-effect transistor (reprinted from (Tian et al., 2010), Copyright 2010, with permission from the American Association for the Advancement of Science); (b) SEM image of a branched nanotube field-effect transistor (reprinted from (Duan et al., 2012), Copyright 2012, with permission from Springer Nature); (c) SEM image of a U-shaped nanowire field-effect transistor (reprinted from (Zhao et al., 2019), Copyright 2019, with permission from Springer Nature). S and D indicate source and drain electrodes, respectively
Cardiomyocytes and neurons are two main cell types used for intracellular electrophysiological detection by micro-nano biosensing platforms. Cardiomyocytes are the main bioactive and functional units of the heart, and their unique membrane structure provides a variety of specific ion channels as the basis for action-potential generation. The action potential of cardiomyocytes can be divided into five stages: rapid depolarization (Na+inflow), early rapid repolarization (K+outflow), plateau (K+outflow, Ca2+inflow), late rapid repolarization (K+outflow), and resting state (ion pump to restore Na+, K+, and Ca2+concentrations). Neurons are the basic units of nerve organs or tissues, such as the brain, spinal cord, and retina. Excitability and conductivity are the main functions of neurons, and the electrical signals they produce are similar in principle and waveform to those produced by cardiomyocytes.
Devices constructed using micro-nano sensing and control technology (based on field-effect transistors) have achieved the closest intracellular recording of real action potentials. Bent nanowire field-effect transistors can gently contact spontaneously beating cardiac cells, and after an interval of about 40 s, gradually convert the initially recorded 3–5 mV extracellular signal into an intracellular signal with a larger amplitude (about 80 mV) (Fig. 3a) (Tian et al., 2010). Similarly, branched nanotube field-effect transistors also record characteristic action potentials in cardiac cells, with amplitudes of 75–100 mV and durations of about 200 ms; multiple independent branched nanotube field-effect transistors have been tested for simultaneous measurement of the same or different locations of cells, demonstrating their potential for extension to network-level multi-channel measurement (Fig. 3b) (Duan et al., 2012). However, the complexity of field-effect transistors hinders their large-scale manufacture, and the insufficient signal-to-noise ratio prevents accurate recording of weak and resting potentials.
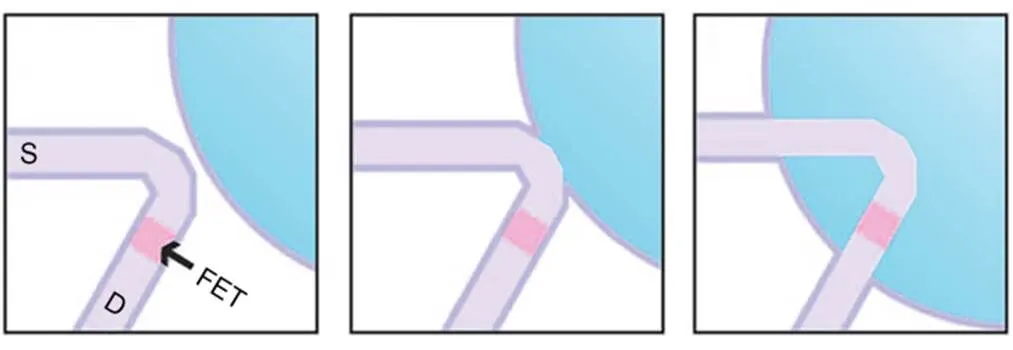
Fig. 2 Penetration strategy of active field-effect transistors (FET): kinked silicon nanowire is coated with phospholipid bilayers to fuse with cell membranes. Reprinted from (Tian et al., 2010), Copyright 2010, with permission from the American Association for the Advancement of Science
Zhao et al. (2019) designed U-shaped field-effect transistors that can continuously measure six independent neurons, obtaining potentials with consistent shape and duration, amplitudes of 60–100 mV, and a signal-to-noise ratio of 115±29. They also allow observation of sub-threshold signals similar to patch-clamp recordings of 3–5 mV, demonstrating their potential for measuring synaptic potentials. Moreover, multiple U-shaped field-effect transistor probes were employed to simultaneously measure either the same or different neurons (Fig. 3c). No significant delay or disparity in waveform was evident with regard to action potentials recorded from the same neuron by distinct electrodes. Conversely, the delay length between signals originating from distinct cells was consistent with the propagation speed of neuronal potentials. Remarkably, integrating two U-shaped field-effect transistor probes featuring different curvatures on the same substrate enables concurrent measurement of intracellular and extracellular potentials.
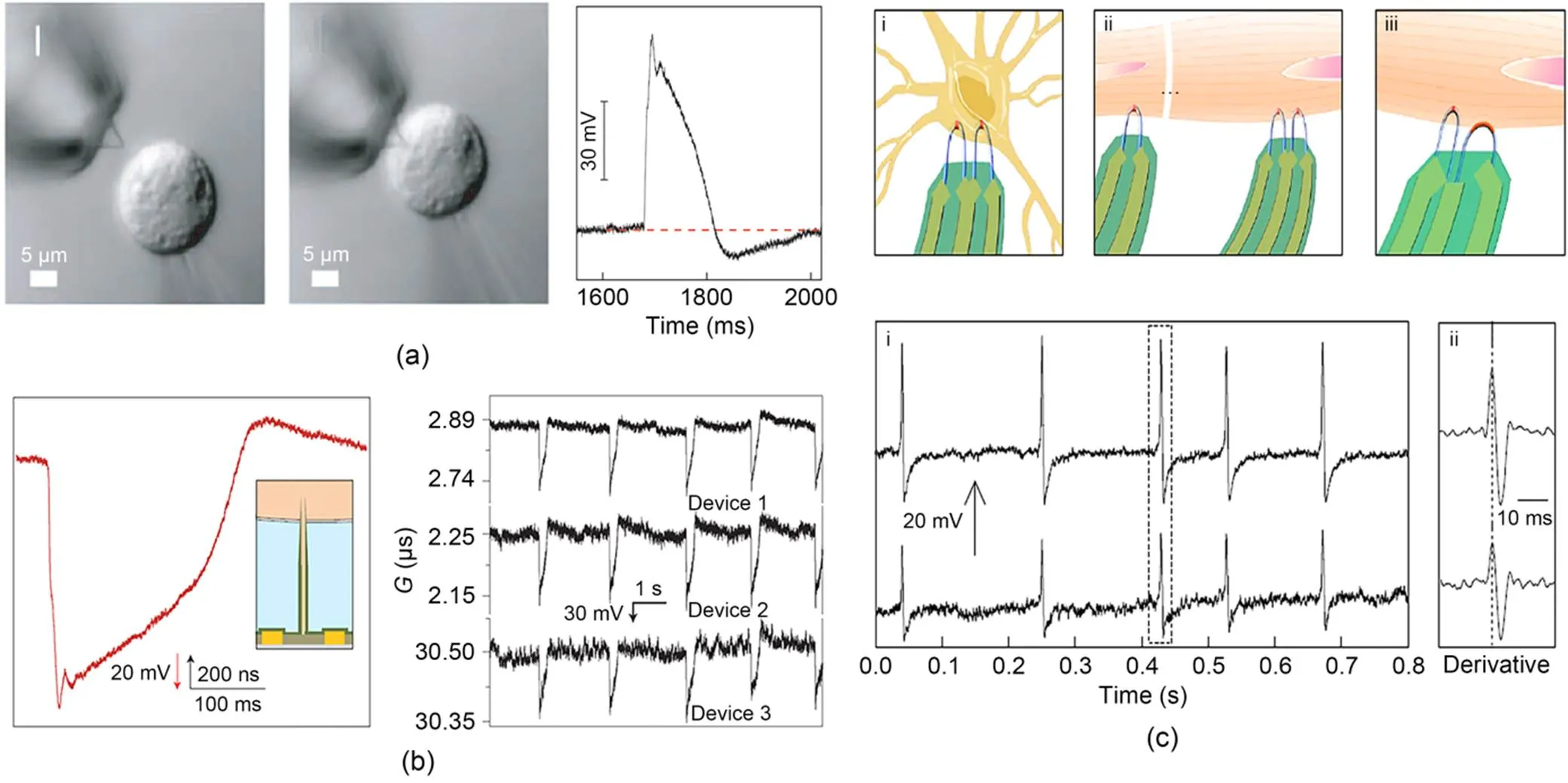
Fig. 3 Electrophysiological detection of active field-effect transistors: (a) intracellular recording from cardiomyocytes by a kinked silicon nanowire field-effect transistor (reprinted from (Tian et al., 2010), Copyright 2010, with permission from the American Association for the Advancement of Science); (b) intracellular recording by a branched nanotube field-effect transistor (reprinted from (Duan et al., 2012), Copyright 2012, with permission from Springer Nature); (c) simultaneous intracellular recording from one dorsal root ganglion (DRG) neuron by U-shaped nanowire field-effect transistors (reprinted from (Zhao et al., 2019), Copyright 2019, with permission from Springer Nature). G stands for the transconductance of the transistor
3 Passive microelectrode arrays
3.1 Device structure
Passive microelectrode arrays often use biocompatible precious metals. Gold and platinum are commonly used electrode materials that can catalyze redox reactions in solution to carry current. Charge diffuses on metal electrodes, and the magnitude of interface current is affected by electrode impedance. Meanwhile, metal oxide (iridium oxide, IrO) is also used as a highly biocompatible material for recording intracellular electrical signals (Lin et al., 2014). Compared with gold nanoelectrodes with the same surface area, IrOelectrodes have lower impedance and higher charge-storage capacity. To further reduce electrode impedance, some nanoscale materials such as platinum black and carbon nanotubes are used to modify electrodes, taking advantage of their rough characteristics and high surface-area ratio to increase the effective surface area of the electrode and improve its signal-to-noise ratio. The structural design of nanoelectrodes has a significant impact on tight coupling between cells and electrodes. Hai et al. (2010) devised a gold electrode that mimicked the morphology and dimensions of dendritic spines, effectively bolstering the adhesion and electrical connectivity between nerve cells and electrodes (Fig. 4a). However, there are drawbacks: the relatively large size of gold spines and lack of expandable multi-site measurement. With the development of micro and nanosensor control technology, vertical nanowire and nanopillar electrodes have been developed to achieve tight coupling between the electrode and cell membrane (Fig. 4b) (Robinson et al., 2012; Xie et al., 2012; Liu et al., 2017, 2022). Among these, hollow tube electrodes can extend the time for cell-membrane healing, and can even induce cell-membrane wrapping around the electrode to improve electrical signal recording time (Fig. 4c) (Lin et al., 2014). Vertical nanoelectrodes are typically constructed with lithography, deposition, and etching techniques, and lithography or deposition is then used to prepare them on CMOS integrated circuits, greatly improving the resolution of micro/nanosensors and enabling parallel recording at the network level (Abbott et al., 2017, 2020; Dipalo et al., 2018).
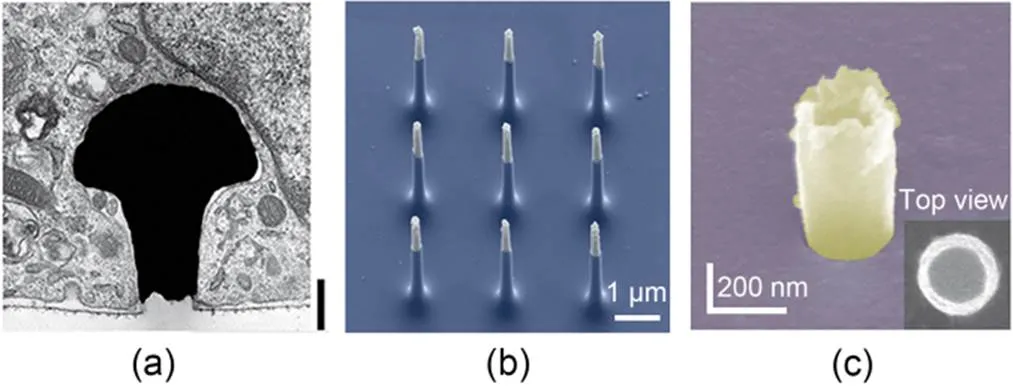
Fig. 4 Device structure of passive microelectrode arrays: (a) SEM image of a gold-spine electrode (reprinted from (Hai et al., 2010), Copyright 2010, with permission from Springer Nature); (b) SEM image of silicon nanowire electrodes (reprinted from (Robinson et al., 2012), Copyright 2012, with permission from Springer Nature); (c) SEM image of an IrOx nanotube electrode (reprinted from (Lin et al., 2014), Copyright 2014, with permission from Springer Nature)
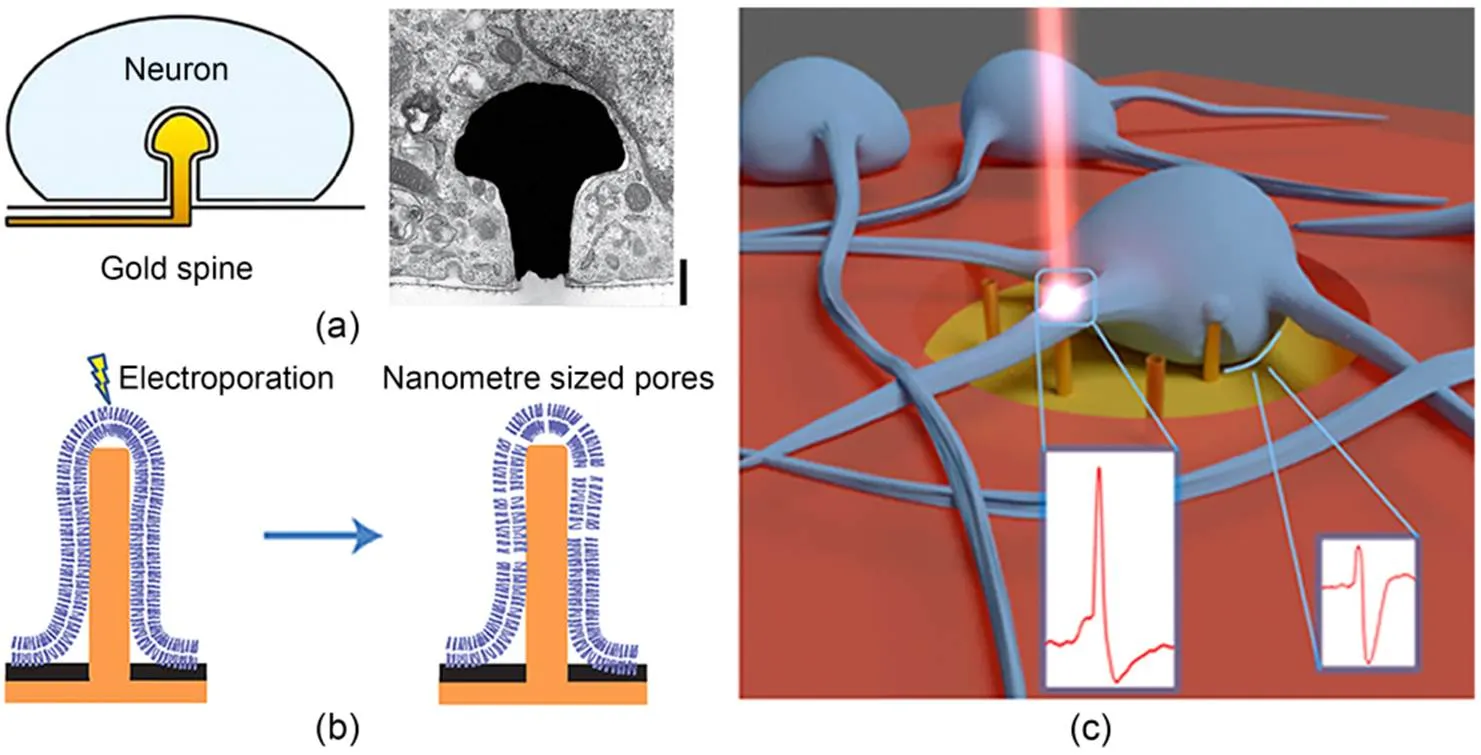
Fig. 5 Penetration strategy of passive microelectrode arrays: (a) gold-spine electrode engulfed by a neuron (reprinted from (Hai et al., 2010), Copyright 2010, with permission from Springer Nature); (b) schematic of electroporation by a nanopillar electrode (reprinted from (Xie et al., 2012), Copyright 2012, with permission from Springer Nature); (c) schematic of optoporation by 3D nanoelectrodes (Dipalo et al., 2017)
3.2 Penetration strategy
Utilizing natural cellular processes, such as phagocytosis, enables electrodes to gain access to intracellular pathways. Hai et al. (2010) designed a mushroom-shaped electrode modified by a peptide, which induces neuronal cells to actively engage in phagocytosis, thereby obtaining intracellular electrical signals with high signal-to-noise ratios and sub-threshold synaptic potentials (Fig. 5a). Meanwhile, high-aspect-ratio vertical nanowire electrodes can penetrate through the cellular membrane by virtue of their rigidity and geometrical structure. Staufer et al. (2019) reported that gold nanowire electrodes successfully penetrated cells through the mediation of adhesion forces, resulting in long-term and stable intracellular electrical signal recording. This study demonstrated that the phospholipid bilayer of the cell membrane can undergo rearrangements at the penetration site under electrode compression, forming a tight seal at the electrode interface. This method of tight coupling between the electrode and the cell achieves high signal-to-noise ratios. However, spontaneous penetration suffers from inefficiencies that limit its further development.
Electroporation is a common passive method of regulating transmembrane cell transport (Xie et al., 2012; Hu et al., 2020; Fang et al., 2022; Jahed et al., 2022; Xu et al., 2022a, 2022b). When electric pulses are applied to nanoelectrodes, it is possible to achieve cell-membrane electroporation at a lower voltage due to the small gap between the electrode and the cell, as well as the concentration of the surrounding electric field at the tip of the electrode (Fig. 5b). By applying 20 bipolar electric pulses (2.5 V, 1 s) on vertical nanowire electrodes, intracellular pathways can be successfully obtained and the quality of recorded intracellular electrical signals significantly improved compared to extracellular recordings. However, within approximately 10 min after electroporation, the recorded intracellular electrical signals gradually decay and become similar to extracellular signals. This is due to activation of the cell-membrane healing mechanism after electroporation, which seals the cell membrane and ejects the nanoelectrode from the cell. Therefore, although electroporation, as a commonly used penetration method, has the advantages of high throughput and ease of operation, it may interfere with cell signals, and its transient nature within the cell is not suitable for long-term stable intracellular physiological monitoring.
Another strategy for passive cell-membrane regulation is optoporation, which enables precise control of different regions of the cell. Dipalo et al. (2017, 2018, 2021) integrated vertical gold nanoelectrodes with plasma optics, culminating in the realization of high signal-to-noise ratios, long-term stability, and consistent intracellular recording (Fig. 5c). The nanoelectrodes selectively created nanopores on the cellular membrane with short laser pulses that solely occurred at the electrode tip, without disrupting the seal between the cell membrane and the nanoelectrode. This circumvented interference with spontaneous electrical activity. Unlike electroporation, optoporation serves as a viable means for simultaneous intracellular and extracellular electrical signal recording, devoid of signal attenuation and capable of continuous recording. Moreover, optoporation allows for individual control of each nanoelectrode, achieving precise selection of cell electrical signal recording regions; it reduces the likelihood of electrical interference but suffers from low-throughput regulation.
3.3 Electrophysiological detection
Cui's group utilized nanopillar electrodes in combination with electroporation to record action potentials of 11.8 mV from HL-1 cells (Xie et al., 2012). The electrodes achieved high-resolution detailed information that could be used to distinguish pacemaker and non-pacemaker cells with different-shaped action potentials, as well as to validate the effects of nifedipine and quinidine on action-potential shortening and prolongation, respectively (Xie et al., 2012). However, the recorded potentials decayed gradually within 10 min, eventually becoming extracellular potentials. This means that they would not be suitable for long-term recording applications (Fig. 6a). This constraint was minimized by iridium oxide nanotube electrodes, which were able to record intracellular potentials of about 15 mV and maintain them without attenuation for a certain period of time, obtaining an intracellular pathway of almost 1 h (Fig. 6b) (Lin et al., 2014). Similarly, nanotemplate electrodes combined with electroporation were able to record maximum intracellular action potentials of 6.97 mV for up to 600 s (Xu et al., 2022b). A nanovolcano electrode combined with chemically modified transmembrane strategies achieved intracellular potentials of up to 20 mV while allowing stable recording for up to 1 h (Desbiolles et al., 2019). The nanocrown electrode can record intracellular action potentials with an amplitude of about 63 mV, which decreases to about 30 mV at 1 h (Jahed et al., 2022). Furthermore, scalability of nanoelectrodes is greatly improved by combining them with CMOS circuits. Employing vertical nanowire electrodes, Abbott et al. (2017) assembled 1024 "pixels" to concurrently capture the membrane potential changes of hundreds of interconnected cardiac muscle cells. This electrode has distinct advantages for investigating the propagation of membrane potential across networks and discerning drug effects, substantiated by the lengthening of the action-potential duration by ATX-II at both the individual cell and network levels.
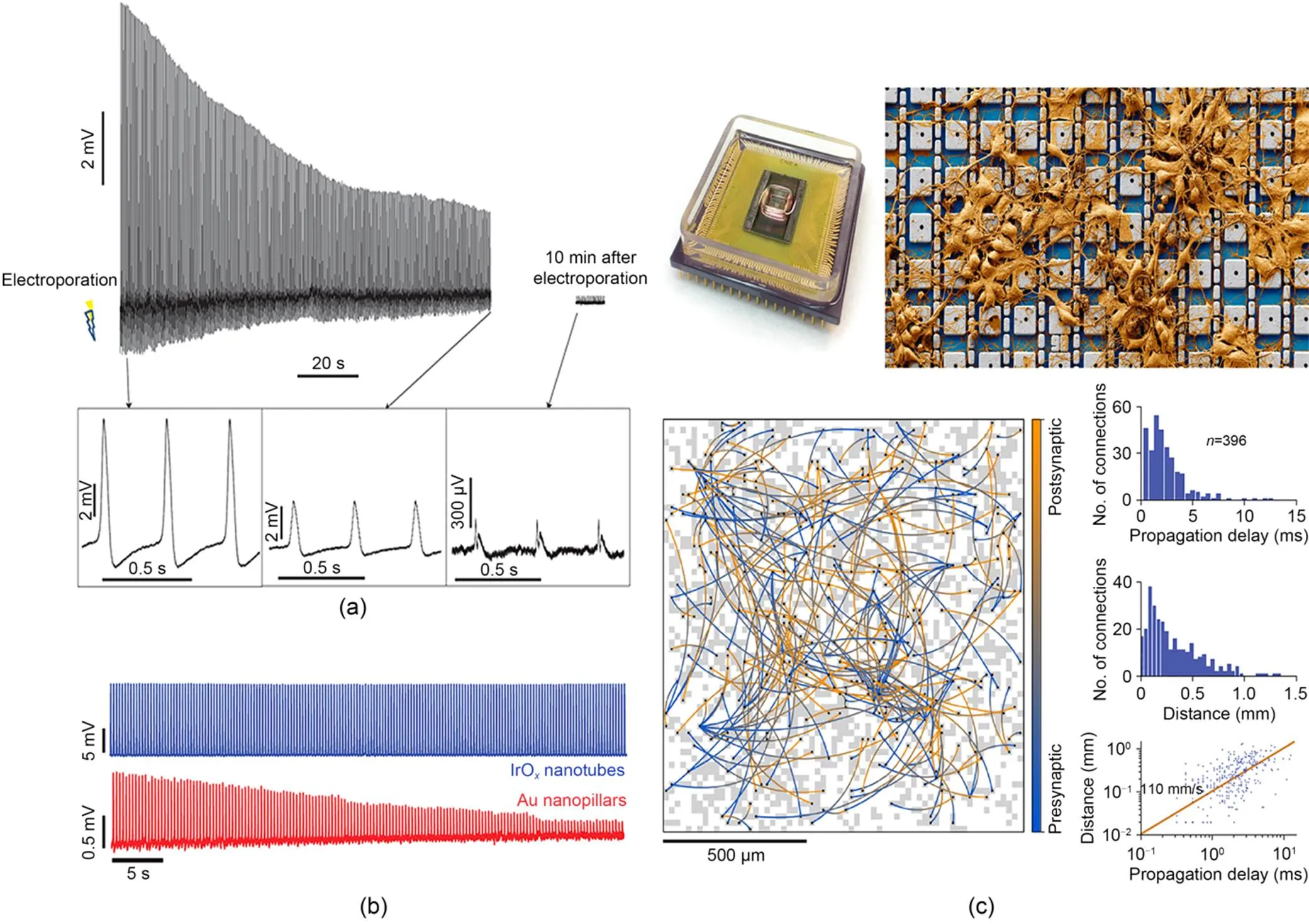
Fig. 6 Electrophysiological detection of passive microelectrode arrays: (a) intracellular measurement of action potentials with a nanopillar electrode (reprinted from (Xie et al., 2012), Copyright 2012, with permission from Springer Nature); (b) prolonged intracellular recording of HL-1 action potentials by IrOx nanotube electrodes (reprinted from (Lin et al., 2014), Copyright 2014, with permission from Springer Nature); (c) connections between presynaptic and postsynaptic neurons mapped across a neuronal network, using a nanoelectrode array (reprinted from (Abbott et al., 2020), Copyright 2020, with permission from Springer Nature)
For neurons, the maximum signal amplitude recorded by vertical nanoelectrodes is usually around 20 mV. Hai et al. (2010) deployed mushroom-shaped electrodes to detect the excitatory postsynaptic potentials (EPSPs) of three neurons. By administering depolarizing and hyperpolarizing pulses to adjacent cells, a uniform potential was recorded in the middle cell. Vertical nanowire electrodes have also been shown to have high signal-to-noise ratios, which helps them to achieve synaptic potential recording, and Park's group found that averaging multiple waveforms under the same conditions increased the signal-to-noise ratio from around 100 to >1000 (Robinson et al., 2012). Furthermore, multiple vertical nanotube/nanowire electrodes have been employed to concurrently capture intracellular potentials of neural cells, showing their aptitude for multiplexing. In particular, Abbott et al. (2020) integrated 4096 nanoelectrodes (about 44% of which were coupled to neural cells) with an average amplitude of about 200 μV and individual amplitudes up to 10 mV. Their electrodes succeeded in detecting the transmission of synaptic potentials and portraying the process of action-potential generation, in addition to accurately measuring the amplitude of synaptic potentials and mapping 304 synaptic connections within a span of 19 min (Fig. 6c).
4 Summary and perspective
The development of micro-nano sensing technology is of great significance for improving the recording of intracellular electrical signals. Firstly, in the design of micro-nano sensing and control devices, utilizing semiconductor and metal materials in conjunction can improve electrode recording efficiency while offering compatibility with CMOS technology; the electrode structure at the nanoscale successfully reduces the extent of injury inflicted during intracellular recording. Secondly, in terms of intracellular transmembrane regulation, various passive transmembrane methods (primarily electroporation) significantly improve the penetration efficiency of electrodes, allowing for stable recording of intracellular action potentials. Active field-effect transistor devices are not affected by interface impedance and can record highly sensitive, full-amplitude intracellular potential. They are usually combined with chemical modification to enter the cell in a gentle way, but their detection efficiency, resolution, and stability need to be further optimized. Passive microelectrode array devices have a high signal-to-noise ratio, and are usually combined with electroporation or optoporation to obtain intracellular access, with high efficiency, stability, and controllability. Optimizing the electrode-cell interface can improve the recorded signal amplitude, and the interface can be integrated with high-spatial-resolution, addressable, high-throughput CMOS circuits. Micro and nano electronics are widely used in drug screening, disease modeling, and synapse mapping in cardiac and neural cells due to their minimally invasive, accurate, sensitive, stable, and multiplexing characteristics; this will provide new ideas for mechanism research and disease-treatment development for the heart and brain.
Looking forward, many challenges and development opportunities remain for the application of micro-nano sensing and control technology in intracellular recording. Firstly, it is imperative to optimize the structural design of devices, enhance their efficacy, and streamline production methods. No device has yet simultaneously achieved high-fidelity, high-sensitivity, and large-scale intracellular recording. Therefore, developing electrodes that are scalable while maintaining high accuracy and high signal-to-noise ratio recording is of great significance for experimental research and clinical applications. At the same time, reducing manufacturing costs would also lay a foundation for commercialization of micro-nano sensing and control devices. Optimizing the electrode-cell interface is necessary to improve device performance, and developing nanostructures that are tightly coupled to the cell (using high-performance materials and low-cost processes) as well as combining them with CMOS processes, can enable accurate, high-resolution intracellular recording. In addition, it is essential to optimize cell-membrane penetration strategies and develop signal recording systems. Efficient and large-scale intracellular recording can be achieved by adjusting electroporation/optoporation strategies and integrating a perforation recording system. Secondly, multi-functional detection capabilities are also a new trend in the development of nanoelectrodes. Integrating multiple functions in addition to intracellular recording is conducive to achieving accurate control and synchronous monitoring of cells. Future nanodevices are anticipated to encompass electrical/optical stimulation and biological/chemical sensing, as well as intracellular delivery/extraction capabilities. The amalgamation of these functionalities should significantly contribute to the realm of precise regulation and synchronous monitoring of cellular behavior. Notably, electrical stimulation emerges as an efficacious strategy for orchestrating cell activity and expression, with its fusion to nanoelectronics harboring tremendous potential for diverse biomedical milieus. Further bridging the divide, microfluidic technology holds immense promise as a conduit for marrying nanoelectronics, thereby engendering multifunctional transmission modalities and amplifying signal-monitoring prowess. Finally, integrating nanoelectrodes into implantable devices for in vivo-intracellular connections is a promising application. Nanoelectrodes offer several benefits, including minimal invasiveness to biological systems, prevention of inflammatory responses in tissues, and preservation of functional integrity in prolonged connections. Based on a stable micro-nano electronic-biological interface, it is possible to study neural circuit dynamics on a large scale in vivo, and even remotely monitor physiological processes and repair nerve damage. As micro-nano sensing and control technology advances, its broad adoption promises to lead to substantial breakthroughs in the fields of neuroscience and cardiology, as well as in related domains like pathology and pharmacology, both in terms of research and clinical treatment.
Acknowledgments
The work is supported by the National Natural Science Foundation of China (Nos. 62171483 and 82061148011), the Zhejiang Provincial Natural Science Foundation of China (No. LZ23F010004), the "Pioneer" and "Leading Goose" R&D Program of Zhejiang Province (No. 2023C03029), and the Traditional Chinese Medicine Project of Zhejiang Province (No. 2021ZA084), China.
Author contributions
Jiajin XUE and Min SHAO prepared the manuscript. Ning HU and Zhigang GAO conceptualized the study, supervised the research, managed the project, and acquired funding. All authors contributed to the discussions and writing of the final manuscript.
Conflict of interest
Jiajin XUE, Min SHAO, Zhigang GAO, and Ning HU declare that they have no conflict of interest.
Abbott J, Ye TY, Qin L, et al., 2017. CMOS nanoelectrode array for all-electrical intracellular electrophysiological imaging., 12(5):460-466. https://doi.org/10.1038/nnano.2017.3
Abbott J, Ye TY, Ham D, et al., 2018. Optimizing nanoelectrode arrays for scalable intracellular electrophysiology., 51(3):600-608. https://doi.org/10.1021/acs.accounts.7b00519
Abbott J, Ye TY, Krenek K, et al., 2020. A nanoelectrode array for obtaining intracellular recordings from thousands of connected neurons., 4(2):232-241. https://doi.org/10.1038/s41551-019-0455-7
Chen WW, Gao RL, Liu LS, et al., 2017. China cardiovascular diseases report 2015: a summary., 14(1):1-10. https://doi.org/10.11909/j.issn.1671-5411.2017.01.012
Connolly P, Clark P, Curtis ASG, et al., 1990. An extracellular microelectrode array for monitoring electrogenic cells in culture., 5(3):223-234. https://doi.org/10.1016/0956-5663(90)80011-2
Davie JT, Kole MHP, Letzkus JJ, et al., 2006. Dendritic patch-clamp recording., 1(3):1235-1247. https://doi.org/10.1038/nprot.2006.164
Desbiolles BXE, de Coulon E, Bertsch A, et al., 2019. Intracellular recording of cardiomyocyte action potentials with nanopatterned volcano-shaped microelectrode arrays., 19(9):6173-6181. https://doi.org/10.1021/acs.nanolett.9b02209
Dipalo M, Amin H, Lovato L, et al., 2017. Intracellular and extracellular recording of spontaneous action potentials in mammalian neurons and cardiac cells with 3D plasmonic nanoelectrodes., 17(6):3932-3939. https://doi.org/10.1021/acs.nanolett.7b01523
Dipalo M, Melle G, Lovato L, et al., 2018. Plasmonic meta-electrodes allow intracellular recordings at network level on high-density CMOS-multi-electrode arrays., 13(10):965-971. https://doi.org/10.1038/s41565-018-0222-z
Dipalo M, Rastogi SK, Matino L, et al., 2021. Intracellular action potential recordings from cardiomyocytes by ultrafast pulsed laser irradiation of fuzzy graphene microelectrodes., 7(15):eabd5175. https://doi.org/10.1126/sciadv.abd5175
Duan XJ, Gao RX, Xie P, et al., 2012. Intracellular recordings of action potentials by an extracellular nanoscale field-effect transistor., 7(3):174-179. https://doi.org/10.1038/nnano.2011.223
Fang JR, Xu DX, Wang H, et al., 2022. Scalable and robust hollow nanopillar electrode for enhanced intracellular action potential recording., 23(1):243-251. https://doi.org/10.1021/acs.nanolett.2c04222
Fromherz P, Offenhäusser A, Vetter T, et al., 1991. A neuron-silicon junction: a Retzius cell of the leech on an insulated-gate field-effect transistor., 252(5010):1290-1293. https://doi.org/10.1126/science.1925540
Hai A, Shappir J, Spira ME, 2010. In-cell recordings by extracellular microelectrodes., 7(3):200-202. https://doi.org/10.1038/nmeth.1420
Hannun AY, Rajpurkar P, Haghpanahi M, et al., 2019. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network., 25(1):65-69. https://doi.org/10.1038/s41591-018-0268-3
Hu N, Xu DX, Fang JR, et al., 2020. Intracellular recording of cardiomyocyte action potentials by nanobranched microelectrode array., 169:112588. https://doi.org/10.1016/j.bios.2020.112588
Jahed Z, Yang Y, Tsai CT, et al., 2022. Nanocrown electrodes for parallel and robust intracellular recording of cardiomyocytes., 13(1):2253. https://doi.org/10.1038/s41467-022-29726-2
Johnstone AFM, Gross GW, Weiss DG, et al., 2010. Microelectrode arrays: a physiologically based neurotoxicity testing platform for the 21st century., 31(4):331-350. https://doi.org/10.1016/j.neuro.2010.04.001
Lin ZC, Xie C, Osakada Y, et al., 2014. Iridium oxide nanotube electrodes for sensitive and prolonged intracellular measurement of action potentials., 5:3206. https://doi.org/10.1038/ncomms4206
Liu R, Chen RJ, Elthakeb AT, et al., 2017. High density individually addressable nanowire arrays record intracellular activity from primary rodent and human stem cell derived neurons., 17(5):2757-2764. https://doi.org/10.1021/acs.nanolett.6b04752
Liu R, Lee J, Tchoe Y, et al., 2022. Ultra-sharp nanowire arrays natively permeate, record, and stimulate intracellular activity in neuronal and cardiac networks., 32(8):2108378. https://doi.org/10.1002/adfm.202108378
Lopez-Izquierdo A, Warren M, Riedel M, et al., 2014. A near-infrared fluorescent voltage-sensitive dye allows for moderate-throughput electrophysiological analyses of human induced pluripotent stem cell-derived cardiomyocytes., 307(9):H1370-H1377. https://doi.org/10.1152/ajpheart.00344.2014
Ma LY, Chen WW, Gao RL, et al., 2020. China cardiovascular diseases report 2018: an updated summary., 17(1):1. https://doi.org/10.11909/j.issn.1671-5411.2020.01.001
Matiukas A, Mitrea BG, Qin MC, et al., 2007. Near-infrared voltage-sensitive fluorescent dyes optimized for optical mapping in blood-perfused myocardium., 4(11):1441-1451. https://doi.org/10.1016/j.hrthm.2007.07.012
Pantoja R, Nagarah JM, Starace DM, et al., 2004. Silicon chip-based patch-clamp electrodes integrated with PDMS microfluidics., 20(3):509-517. https://doi.org/10.1016/j.bios.2004.02.020
Qing Q, Jiang Z, Xu L, et al., 2014. Free-standing kinked nanowire transistor probes for targeted intracellular recording in three dimensions., 9(2):142-147. https://doi.org/10.1038/nnano.2013.273
Robinson JT, Jorgolli M, Shalek AK, et al., 2012. Vertical nanowire electrode arrays as a scalable platform for intracellular interfacing to neuronal circuits., 7(3):180-184. https://doi.org/10.1038/nnano.2011.249
Spira ME, Hai A, 2013. Multi-electrode array technologies for neuroscience and cardiology., 8(2):83-94. https://doi.org/10.1038/nnano.2012.265
Staufer O, Weber S, Bengtson CP, et al., 2019. Adhesion stabilized enintracellular electrical recordings from multicellular assemblies., 19(5):3244-3255. https://doi.org/10.1021/acs.nanolett.9b00784
Thomas C, Springer P, Loeb G, et al., 1972. A miniature microelectrode array to monitor the bioelectric activity of cultured cells., 74(1):61-66. https://doi.org/10.1016/0014-4827(72)90481-8
Tian BZ, Cohen-Karni T, Qing Q, et al., 2010. Three-dimensional, flexible nanoscale field-effect transistors as localized bioprobes., 329(5993):830-834. https://doi.org/10.1126/science.1192033
Timmis A, Townsend N, Gale C, et al., 2018. European society of cardiology: cardiovascular disease statistics 2017., 39(7):508-579. https://doi.org/10.1093/eurheartj/ehx628
Valappil RA, Black JE, Broderick MJ, et al., 2010. Exploring the electrocardiogram as a potential tool to screen for premotor Parkinson’s disease., 25(14):2296-2303. https://doi.org/10.1002/mds.23348
Vardi R, Goldental A, Sardi S, et al., 2016. Simultaneous multi-patch-clamp and extracellular-array recordings: single neuron reflects network activity., 6:36228. https://doi.org/10.1038/srep36228
Voelker M, Fromherz P, 2005. Signal transmission from individual mammalian nerve cell to field-effect transistor., 1(2):206-210. https://doi.org/10.1002/smll.200400077
Wang GF, Wyskiel DR, Yang WG, et al., 2015. An optogenetics- and imaging-assisted simultaneous multiple patch-clamp recording system for decoding complex neural circuits., 10(3):397-412. https://doi.org/10.1038/nprot.2015.019
Xie C, Lin ZL, Hanson L, et al., 2012. Intracellular recording of action potentials by nanopillar electroporation., 7(3):185-190. https://doi.org/10.1038/nnano.2012.8
Xu DX, Fang JR, Zhang MY, et al., 2022a. Porous polyethylene terephthalate nanotemplate electrodes for sensitive intracellular recording of action potentials., 22(6):2479-2489. https://doi.org/10.1021/acs.nanolett.2c00258
Xu DX, Fang JR, Wang H, et al., 2022b. Scalable nanotrap matrix enhanced electroporation for intracellular recording of action potential., 22(18):7467-7476. https://doi.org/10.1021/acs.nanolett.2c02398
Zhang AQ, Lieber CM, 2016. Nano-bioelectronics., 116(1):215-257. https://doi.org/10.1021/acs.chemrev.5b00608
Zhao YL, You SS, Zhang AQ, et al., 2019. Scalable ultrasmall three-dimensional nanowire transistor probes for intracellular recording., 14(8):783-790. https://doi.org/10.1038/s41565-019-0478-y
题目:面向细胞内电生理的微纳生物传感平台研究进展
作者:薛佳金1,邵敏4,高志刚1,胡宁1,2,3
机构:1浙江大学医学院附属儿童医院,普外科,中国杭州,310052;2浙江大学,化学系,中国杭州,310058;3浙江大学杭州国际科创中心,浙江-以色列自组装功能材料联合实验室,中国杭州,311215;4浙江大学医学院第二附属医院,体检中心,中国杭州,310052
摘要:建立可靠的电生理检测平台对于心脏学和神经科学的研究至关重要。在过去的十年中,基于微纳传感和控制技术的器件已经被开发用于构建电生理平台,其独特的形态优势和新颖的处理方法为实现高通量、高保真的电信号记录提供了潜力。在本文中,我们分析了有源/无源微纳传感平台的结构、跨膜策略和电生理检测。结合微纳米传感技术面临的机遇和挑战,展望了微纳米传感技术的巨大发展潜力,以推动更高水平的电生理平台建设,满足实验研究和临床应用的需要。
关键词:细胞内电生理;微纳生物传感平台;心脏学和神经科学;细胞内动作电位
https://doi.org/10.1631/jzus.A2300267
https://doi.org/10.1631/jzus.A2300267
Ning HU, huning@zju.edu.cn
© Zhejiang University Press 2023
May 18, 2023;
July 16, 2023;
Aug. 22, 2023;
Sept. 11, 2023
 Journal of Zhejiang University-Science A(Applied Physics & Engineering)2023年11期
Journal of Zhejiang University-Science A(Applied Physics & Engineering)2023年11期
- Journal of Zhejiang University-Science A(Applied Physics & Engineering)的其它文章
- Experimental and theoretical analysis of a hybrid vibration energy harvester with integrated piezoelectric and electromagnetic interaction
- Biomaterial types, properties, medical applications, and other factors: a recent review
- Correlation between travel experiences and post-COVID outbound tourism intention: a case study from China
- Experimental investigation on cenosphere-aluminum syntactic foam-filled tubes under axial impact loading
- Short-term tunnel-settlement prediction based on Bayesian wavelet: a probability analysis method
- Effect of carbon dioxide concentration on the combustion characteristics of boron agglomerates in oxygen-containing atmospheres
