A novel mutation in ACS11 leads to androecy in cucumber
WANG Jie, Ll Shuai, CHEN Chen, ZHANG Qi-qi, ZHANG Hui-min, CUl Qing-zhi,CAl Guang-hua, ZHANG Xiao-peng, CHAl Sen, WAN Li, YANG Xue-yong, ZHANG Zhong-hua,HUANG San-wen,5, CHEN Hui-ming, SUN Jin-jing#
1 College of Horticulture, Qingdao Agricultural University, Qingdao 266109, P.R.China
2 State Key Laboratory of Vegetable Biobreeding, Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences,Beijing 100081, P.R.China
3 Hunan Vegetable Research Institute, Hunan Academy of Agricultural Sciences, Changsha 410125, P.R.China
4 Genome Analysis Laboratory of the Ministry of Agriculture and Rural Affairs, Agricultural Genomics Institute at Shenzhen,Chinese Academy of Agricultural Sciences, Shenzhen 518124, P.R.China
5 Chinese Academy of Tropical Agriculture Sciences, Haikou 571101, P.R.China
6 College of Horticulture, Hunan Agricultural University, Changsha 410128, P.R.China
Abstract Sex determination in plants gives rise to unisexual flowers.A better understanding of the regulatory mechanism underlying the production of unisexual flowers will help to clarify the process of sex determination in plants and allow researchers and farmers to harness heterosis.Androecious cucumber (Cucumis sativus L.) plants can be used as the male parent when planted alongside a gynoecious line to produce heterozygous seeds, thus reducing the cost of seed production.The isolation and characterization of additional androecious genotypes in varied backgrounds will increase the pool of available germplasm for breeding.Here, we discovered an androecious mutant in a previously generated ethyl methanesulfonate (EMS)-mutagenized library of the cucumber inbred line ‘406’.Genetic analysis, whole-genome resequencing, and molecular marker-assisted verification demonstrated that a nonsynonymous mutation in the ethylene biosynthetic gene 1-AMINOCYCLOPROPANE-1-CARBOXYLATE SYNTHASE 11 (ACS11) conferred androecy.The mutation caused an amino acid change from serine (Ser) to phenylalanine (Phe) at position 301 (S301F).In vitro enzyme activity assays revealed that this S301F mutation leads to a complete loss of enzymatic activity.This study provides a new germplasm for use in cucumber breeding as the androecious male parent, and it offers new insights into the catalytic mechanism of ACS enzymes.
Keywords: cucumber, androecy, ethylene, marker-assisted breeding
1.lntroduction
Unisexual flowers can effectively promote plant outcrossing and increase genetic diversity.Studying the underlying mechanism of unisexual flowers is of great scientific significance and practical interest (Tanurdzic and Banks 2004).Edible seeds and fruits are usually produced from either female or bisexual flowers.Therefore, knowing how unisexual flowers develop also has important production and application value.
Cucumber (CucumissativusL.) has a variety of flower sex types, and it has been used as a model system for analyzing unisexual flower development (Tanurdzic and Banks 2004; Ma and Pannell 2016).Male and female flowers can be distinguished by their arrested carpels or arrested stamen primordia, respectively; moreover,hermaphroditic flowers are formed when neither the pistil nor the stamen is arrested (Baiet al.2004).Based on the flower sex types present on a single plant, cucumbers can be categorized as either monoecious (with separate male and female flowers), androecious (with only male flowers),gynoecious (with only female flowers), hermaphroditic (with only hermaphroditic flowers), andromonoecious (with male and hermaphroditic flowers), gynomonoecious (with female and hermaphroditic flowers), or trimonoecious (with male,female, and hermaphroditic flowers) (Kubicki 1969a, b, c).
Five genes essential for the regulation of unisexual flower development have been identified in cucumber and nameda(androecious),F(Female),M(Monoecious),g(gynoecious), andACO2(1-aminocyclopropane-1-carboxylicoxidase2).TheAgene encodes ACS11(1-aminocyclopropane-1-carboxylate synthase 11) and is specifically expressed in the carpel of the flower bud that will become a female flower.ACS11 and ACO2 participate in ethylene biosynthesis and repress the expression ofG, which encodes a transcription factor from the WIP family, CsWIP1, andGrepressesMexpression.Mencodes CsACS2, which promotes carpel development and inhibits stamen development.Therefore, in the flower buds in whichACS11is expressed,CsWIP1expression is repressed andCsACS2expression is activated, resulting in arrested stamens and fully developed carpels, thus producing female flowers.Conversely, flower buds in whichACS11is not expressed develop into male flowers(Saitoet al.2007; Boualemet al.2008, 2015, 2009;Martinet al.2009; Chenet al.2016; Huet al.2017).TheFgene encodes ACS1G, another ACS enzyme whose encoding gene originated from the tandem duplication of a 30.2-kb genomic region, acquiring a novel expression pattern with expression in all early-stage flower buds; and ACS1G cooperates with ACO2 for ethylene production and the development of all flower buds into female flowers(Zhanget al.2015, 2021).
In cucumber breeding, heterosis can significantly improve the yield, quality, and disease resistance of hybrid plants, but it requires a significant amount of time and effort to artificially pollinate the cucumber plants in a net chamber or greenhouse to obtain hybrid seeds.The cost of seed production would be reduced substantially if androecious lines could be grown as male parents right next to gynoecious female parents, using bees as the pollinators and harvesting the fruits and seeds together.Therefore, the development of androecious lines is a major goal in cucumber breeding.Currently,more androecious mutants and lines in different genetic backgrounds are needed to achieve a greater breadth of selectable traits for breeding.
In this study, we discovered an androecious mutant in the ethyl methanesulfonate (EMS)-mutagenized library that we generated in the cucumber inbred line ‘406’.We performed a genetic analysis and implemented bulkedsegregant sequencing together with derived cleaved amplified polymorphic sequence (dCAPS) marker verification of this new androecious mutant.These analyses revealed that a nonsynonymous mutation inACS11confers this phenotype.This mutation replaced a serine (Ser) with a phenylalanine (Phe) in ACS11, which abolished its enzymatic activity.This study provides a new germplasm that can be used as an androecious male parent for breeding, and it offers new insights into the catalytic mechanism of the ACS enzyme.
2.Materials and methods
2.1.Plant materials
The northern China cucumber inbred line ‘406’ was used to construct an EMS-mutagenized library.The seeds of inbred line ‘406’ were treated with 1.5% (w/v) EMS (Sigma M0880,USA) for 12 h (Chenet al.2018).The M1plants were selfpollinated, and the androecious linea23was identified in the M2population.The BC1F1populations were produced by crossing ‘406’ anda23, and the BC1F2populations were obtained by self-pollination of BC1F1plants.Two additional F1populations were obtained by crossinga23and406a(aco2mutant), anderez(acs11mutant).Requests for these EMS-induced mutants require a material transfer agreement with Hunan Xingshu Seed Industry Co., Ltd.(Changsha, Hunan, China).Seeds of the cucumber inbred line ‘YB-G’, ‘H203’ and ‘A203’ were obtained from Hunan Xingshu Seed Industry, China.If the proprietary inbred line is requested, then Hunan Xingshu Seed Industry can provide a hybrid, derived from the requested inbred line,at its discretion.All plants were grown in a greenhouse at the Institute of Vegetables and Flowers of the Chinese Academy of Agricultural Sciences in Beijing.
2.2.SNP calling and filtering
The androecious linea23was backcrossed with ‘406’(monoecious) to obtain BC1F1individuals, which were self-pollinated to produce BC1F2seeds.Fifty monoecious BC1F2seedlings were pooled as the monoecious pool(MP pool), and 49 androecious seedlings were pooled as the androecious pool (AP pool).Short reads obtained using the Illumina sequencing platform from each pool were aligned to the cucumber reference genome (inbred line ‘9930’) (Liet al.2019) using Burrows-Wheeler Aligner(BWA) software (Li and Durbin 2009).Variants were identified using bcftools software with “-mv” parameters.Single nucleotide polymorphism (SNP) calling was carried out by directly comparing the sequences of the AP pool with those of the MP pool using a custom Python script.
The output file with all SNPs was uploaded to a filter pipeline to identify candidate genomic regions harboring the mutation responsible for the androecious phenotype.The pipeline included several criteria: (i) a reliable SNP that is bi-allelic; (ii) quality scores for sequencing reads and their mapping to the reference genome should be higher than 20; (iii) the number of uniquely mapped reads should be at least 3 and less than 150 at any SNP position; (iv) the AP pool and ‘406’ should have different genotypes; and (v)‘406’ should be homozygous at the SNP position.
An SNP index was then calculated for both pooled samples as the proportion of reads harboring SNPs that differed from the monoecious parent ‘406’.Typical EMStype mutations (G-to-A or C-to-T transitions) present in at least one pool were extracted.
2.3.Validation of the causative mutation using dCAPS markers
To validate the mutation that results in the androecious phenotype using dCAPS markers, all SNPs on chromosome 2 were analyzed.The dCAPS primers were designed using the online website dCAPS FINDER 2.0(Neff 1998).A restriction endonuclease recognition site was created by introducing a single nucleotide mismatch near the SNP site in the forward primer.The reverse primer was designed using SnapGene.After performing PCR using 10 ng genomic DNA as the template, the resulting PCR products were digested with the appropriate restriction enzyme, followed by electrophoresis on a 4% (w/v) agarose gel at 140 V for 45 min.The PCR conditions were as follows: 34 cycles of 95°C for 30 s,58°C for 30 s, and 72°C for 20 s.Thirty-two androecious plants from the F2population were analyzed with four dCAPS markers.The primers are listed in Appendix A.
2.4.Protein production
The coding sequences of wild-typeACS11,ACS11S301F,and the truncatedACS11form of theerezmutant were cloned into the pET30a plasmid and subjected to sequencing validation.Validated plasmids were individually transformed into theEscherichiacoliBL21 (DE3) strain.Positive colonies were grown in LB medium with 50 mg L–1kanamycin overnight at 37°C, with shaking at 200 r min–1.Each of the overnight cultures were then used to inoculate 500 mL of LB medium containing 50 mg L–1kanamycin, and incubated to an optical density at 600 nm of 0.6 at 37°C, with shaking at 200 r min–1.Isopropyl β-D-1-thiolactopyranoside (IPTG) was then added to a final concentration of 0.05 mmol L–1.The cultures were incubated at 16°C with shaking at 180 r min–1for 16 h,after which the cells were collected by centrifugation.Cell pellets were resuspended in 50 mL lysis buffer (0.3 mol L–1NaCl, 50 mmol L–1KH2PO4, and 10 mmol L–1imidazole(pH 8.0)) containing 1 mg mL–1lysozyme (Amresco, Solon,OH, USA; 0663) and incubated on ice for 4 h.The cells were disrupted by an ultrasonic crusher until the bacterial fluid was clear and translucent, and the supernatant was collected by centrifugation at 10 000×g for 15 min at 4°C.Then, 2 mL of 50% (v/v) Ni-NTA agarose slurry (Hilden Qiagen, Germany; 30210) was added to each sample that had been equilibrated with lysis buffer.The mixture was gently shaken on ice for 30 min and placed on an Econo-Pac column (Bio-Rad Labs, Hercules, California,USA; 7321010).The unbound proteins were washed off by three column volumes of wash buffer (0.3 mol L–1sodium chloride, 50 mmol L–1KH2PO4, and 20 mmol L–1imidazole (pH 8.0)).The fusion proteins were eluted using elution buffer (0.3 mol L–1sodium chloride, 50 mmol L–1KH2PO4, and 250 mmol L–1imidazole (pH 8.0)).Next, using a centrifugal filtration device (Merck Milliken,Darmstadt, Germany; UFC201024), the fusion proteins were dissolved in 1× phosphate buffered saline (PBS,0.14 mol L–1NaCl, 2.7 mmol L–1KCl, 10 mmol L–1Na2HPO4,and 1.8 mmol L–1KH2PO4).Then, the concentration of the purified proteins was determined using a Pierce BCA Protein Assay Kit (ThermoFisher Scientific, MA, USA;23227), and the sample was used for the enzyme activity assays.
2.5.ACS enzyme activity assays
The ACS activity assays were performed as previously described by measuring ethylene production (Bulenset al.2011).Each reaction solution consisting of 200 mmol L–1tricine (pH 8.0), 3.5 μmol L–1pyridoxal-L-phosphate,10 mmol L–1dithiothreitol, and 1.2 mmol L–1S-(5´-adenosyl)-L-methionine chloride was freshly made.The reaction was started by adding 3 μg of purified recombinant protein to 1.6 mL of reaction solution,followed by incubation at 25°C for 2 h while gently shaking.The reaction was terminated by adding 200 μL of 100 mmol L–1HgCl2.Then, 950 μL of reaction mixture and 850 μL of distilled water were mixed in a 10-mL glass bottle and sealed with a lid, and 0.2 mL of a NaOH-NaOCl mixture was injected into the bottle.The NaOH-NaOCl solutions were freshly prepared by mixing two volumes of 5% (w/v) NaOCl and one volume of 6 mol L–1NaOH on ice.The sample was vortexed for 5 s, placed on ice for 4–5 min, and vortexed again for another 5 s to guarantee ethylene release into the upper part of the glass vial.Then,250 μL of gas was extracted from the top of the bottle using a syringe, and the ethylene produced was quantified using an Agilent 6890N gas chromatograph equipped with a flame ionization detector (Agilent Technology, Santa Clara, California, USA).The results are shown as the mean±SE (n=3).
3.Results
3.1.Androecy is conferred by a single nucleotide mutation in ACS11
We identified a mutant line from an EMS-induced mutation library derived from the monoecious line ‘406’.This mutant, which we nameda23, bore only male flowers on the main stem and lateral branches.The BC1F1plants derived from a backcross betweena23and the ‘406’parental line were all monoecious.In addition, the ratio of monoecious to androecious plants fit a 3:1 segregation ratio in the BC1F2population (137 monoecious and 49 androecious,χ2=0.67,P=0.95) (Table 1).We concluded that the androecious phenotype is likely caused by a recessive EMS-induced mutation.
To identify the gene associated with the mutation responsible for the androecious phenotype ina23, we first crosseda23to two other androecious lines,406a(anaco2mutant) anderez(anacs11mutant).The F1plants from thea23×aco2cross were monoecious, while the F1plants from thea23×erezcross were androecious (Fig.1).These results indicated that thea23anderezmutants are allelic,indicating thata23may carry a dysfunctionalACS11.
In parallel, we separately sequenced the pooled genomes (~50× coverage) of 49 androecious and 50 monoecious plants selected from an F2population derived from a backcross betweena23and its parental line ‘406’.The analysis of the sequencing data detected 192 SNPs between the two bulked pools (Appendix B).Of these, we found that SNP2G17753234 mapped to the coding region ofACS11.To confirm the linkage between this SNP and the androecious phenotype, we designed dCAPS markers for SNP2G17753234, two flanking SNPs (SNP2G17211349 and SNP2G18212584),and SNP21397616 (the nearest SNP downstream of SNP2G18212584).From an analysis of 186 seedlings from thea23ב406’ F2population, only SNP2G17753234 showed a perfect co-segregation with the androecious phenotype.Together with the observation that F1plants from thea23×erezcross are androecious, we concluded that SNP2G17753234 is likely to be the causative mutation of the androecy ina23(Fig.1-C; Appendix C).
3.2.Proteins are inactive
The C-to-T mutation corresponding to SNP2G17753234 is predicted to cause an amino acid change from Ser to Phe at position 301 in ACS11 (S301F) (Fig.1-D and E).An alignment between ACS11 from cucumber and related proteins from diverse dicotyledonous plants showed that the S301 residue is located in the conserved domain box 6 (Appendix D).We then predicted the structures of wildtype ACS11 and its ACS11S301Fvariant using the structure of ACS7 from tomato (Solanumlycopersicum) as a prototype.We determined that S301 lies near the pocket to which the ACS coenzyme pyridoxal-5´-phosphate (PLP)binds.In the S301F mutant, hydrophilic Ser is replaced by hydrophobic Phe (Fig.2-A).Due to the larger benzene ring structure of the Phe side chain, the entry of PLP into the catalytic activity pocket may be affected, which in turn might reduce the binding of the substrate S-adenosyl-Lmethionine (SAM) to ACS11.
To investigate whether S301F affects the activity of ACS11, we performed aninvitroassay by measuring ethylene production at different SAM concentrations with recombinant purified ACS11 (Bulenset al.2011).In addition to wild-type ACS11 and the ACS11S301Fvariant,we also included a variant with a premature stop codon matching the mutant ACS11 protein from theerezmutant as a control, as this shorter ACS11 protein exhibits no catalytic activity (Boualemet al.2015).We observed an increase in ethylene production with increasing SAM concentration when incubated with wild-type ACS11, whereas ethylene biosynthesis was very low with both the ACS11S301Fvariant and the ACS11erezvariant (Fig.2-B and C), indicating that the S301F mutation abolishes ACS11 catalytic activity.
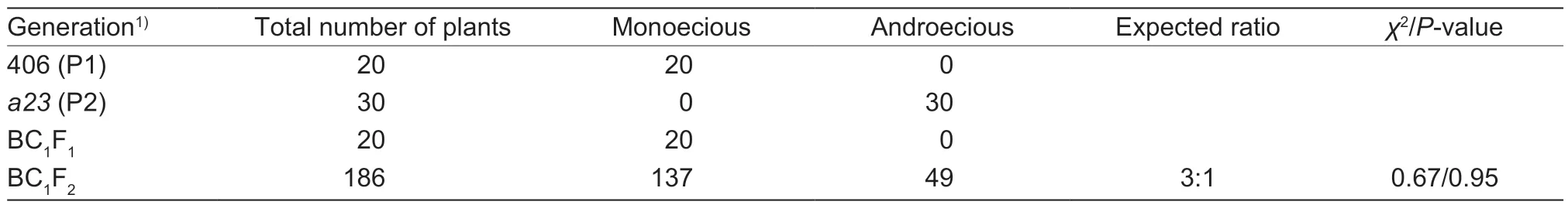
Table 1 The inheritance of sexual phenotype
3.3.Using molecular markers to produce novel male parent and varieties
The ability to cross gynoecious and androecious lines makes it easy to obtain the commercial hybrid F1generation.As the dysfunction of either ACS11 or ACO2 has been shown to confer androecy in cucumber (Boualemet al.2015; Chenet al.2016), we separately crossed the gynoecious1983Gwith both androecious linesa23(acs11mutant) and406a(aco2mutant).The1983G×a23F1was gynoecious, which was consistent with the previous report thatACS1Gis epistatic toACS11, as cucumber plants will produce only female flowers whenACS1Gis present regardless of the genotype atACS11.The1983G×406aF1was monoecious, and the female flower ratio was lower than that of the1983G×a23F1(Fig.3-A).Therefore, we concluded that the androeciousACS11loss-of-function mutants were more suitable for use in breeding as a male parent than theaco2mutants.
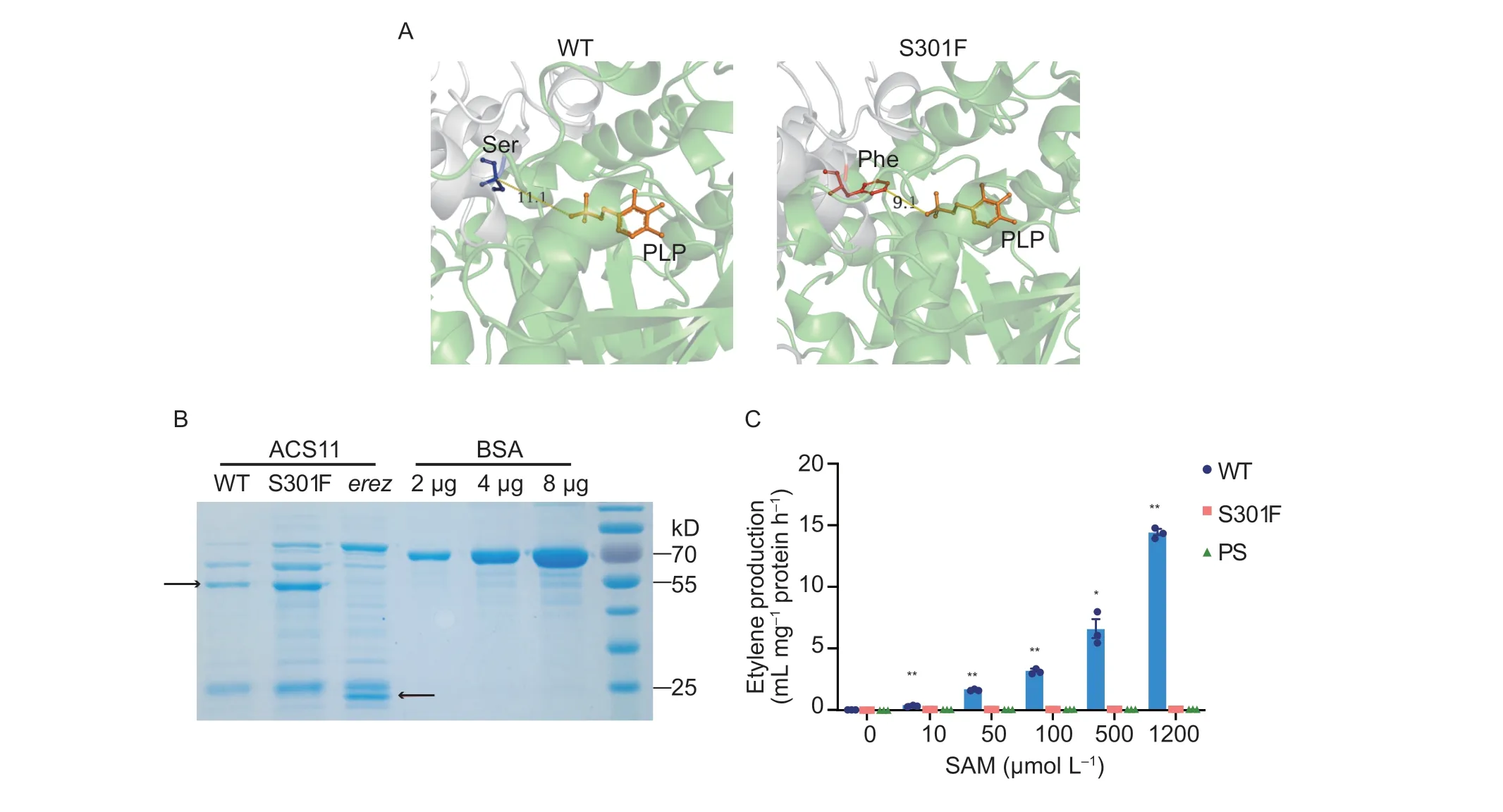
Fig.2 The mutation changed from Ser to Phe at position 301 in CsACS11 (S301F) abolishes enzymatic activity.A, protein tertiary structures of wild-type (WT) and mutant ACS11.Gray and green represent each of the two ACS11 monomers.The orange stick represents the cofactor pyridoxal-5´-phosphate (PLP), the blue stick represents the serine residue in wild-type ACS11, and the red stick represents the phenylalanine substitution in a23.The width of the PLP pocket is indicated (Å).B, Coomassie brilliant blue-stained gel showing the purified recombinant ACS11 proteins produced for the in vitro enzymatic activity assays.PS, ACS11 variant with the premature stop codon present in the erez mutant.The arrows indicate the wild-type and ACS11erez proteins.C,ACS11 enzymatic activity assay, as determined by ethylene production from wild-type ACS11 (blue bars, WT), ACS11S301F (orange bars, S301F), and ACS11erez (green bars, PS) in the presence of various S-adenosyl-L-methionine (SAM) concentrations.Data are mean±SE (n=3).*, P<0.05; **, P<0.01 based on Student’s t-test compared to the wild-type ACS11.A summary of all statistical analyses is provided in Appendix E.
We then used linea23to breed a new androecious male parent line via backcrossing and molecular marker selection (Fig.3-B).The F1seedlings were obtained by crossinga23(P1) and a monoecious line H203 containing elite traits (P2), and the resulting plants were backcrossed to H203 (P2) four times to obtain BC4F1, with selection for theACS11/acs11genotype at each generation.Then the BC4F1(ACS11/acs11genotype) plants were self-crossed to produce BC4F2.Androecious seedlings with theacs11/acs11genotype in the BC4F2generation were identified with molecular markers and Sanger sequencing.Female flowers were induced by ethephon application, and the plants were self-crossed again to obtain BC4F3.The BC4F3plants were all androecious, and their phenotype was the same as H203 except for the sexual morphology;and this line was named A203 (Fig.3-C).We then crossed the gynoecious line YB-G (P1) and the A203 (male parent).The female flower proportion of the resulting F1population was about 76%, and the plants had other elite traits from the male and female parent lines (Fig.3-C).
The stable androecious phenotype of the A203 line(which never produces fruits or seeds, in either spring or autumn, without chemical treatment) ensures seed purity when it is used to produce hybrid commercial varieties.We then tested the outcome of planting one androecious A203 (male parent) among eight gynoecious YB (female parent) seedlings, using bees as the natural pollinators,and harvested the cucumber fruits and hybrid seeds together.Compared to the traditional production of seeds in the isolated net chamber and using manual pollination,the seed yields were almost the same, and the cost was reduced by about 30%, from 56 to 38 USD kg–1(Fig.3-D).
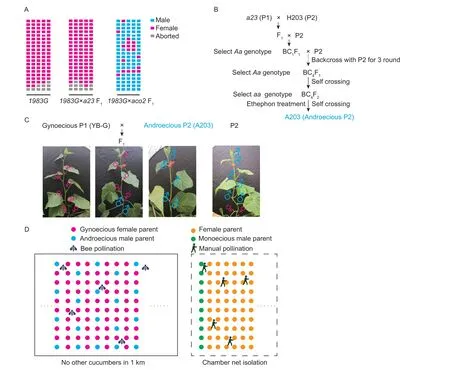
Fig.3 Pipeline for breeding an androecious male parent and a F1 commercial population.A, graphical summary of flower sexual phenotypes of 1983G, 1983G×aco2 F1 and 1983G×a23 F1 plants.Each column represents an individual, and each row represents a node.Flower sex types are shown for 20 nodes.B, pipeline for breeding the androecious male parent using a23 and a monoecious line H203 containing elite target traits.C, pipeline for breeding a commercial F1 generation and representative photographs of sample plants.D, illustrations comparing the production of hybrid seeds using an androecious male parent with bees as pollinators and the traditional method using chamber net isolation and manual pollination.The solid rectangle represents spatial isolation,with no cucumbers of any genotype other than the male and female parent lines in the seed production fields, which are usually at least 1 km away from any other cucumber field.The dotted rectangle represents the net chamber in which the male and female parent lines are planted, and all insects must be killed during the manual pollination.
4.Discussion
Unisexual flowers are an important feature of Cucurbitaceae species.Androecious mutants are very important for studying unisexual flowers and offer substantial advantages in reducing the cost associated with seed production by crossing.Androecious mutants have been reported in melon (Cucumismelo), cucumber,and pumpkin (Cucurbitasp.) (Boualemet al.2009; Chenet al.2016; Garcíaet al.2019).Several mutations inACS11derived from EMS mutagenesis screens cause a transition from monoecy to androecy in melon (Boualemet al.2015).Mutations in ethylene receptors, resulting in ethylene insensitivity, lead to androecy in pumpkin (Garcíaet al.2020).Therefore, the genetic analysis of unisexual flower development underscores the importance of ethylene biosynthesis and signal transduction for female flower formation in cucurbits.
Cucumber has been used as a model system for studying plant sex determination because of its various sexual morphotypes.The distribution and proportions of male and female flowers along the inflorescence stem and sensitivity to environmental factors vary greatly in different cucumber genetic backgrounds.Importantly,their sexual morphotypes cannot be compared directly due to extensive genetic variation, which makes it more difficult to study the regulatory mechanism of unisexual flower development.
Near isogenic lines offer a consistent genetic background, so they are ideal for the in-depth study of specific biological processes.The two androecious mutants derived from EMS-mutagenized ‘406’ were caused by a loss of ACO2 or ACS11 enzymatic activity,respectively.Although the expression domains ofACS11andACO2partially overlap, their expression patterns are different (Boualemet al.2015; Chenet al.2016).In addition, the sexual phenotypes ofwip1acs11andwip1aco2double mutants are quite different (Zhanget al.2021), suggesting that ACS11 and ACO2 might play different roles in controlling the formation of female flowers.ACS family proteins with dual-enzyme activities of ACC synthase and Cβ-S lyase have been reported in recent years (Xuet al.2021).Does ACS11 have other functions besides cooperating with ACO2 in ethylene biosynthesis to promote female flower formation? More detailed research using these isogenic materials could help to answer this question.
The structure of tomato ACS7 was determined in 2001 (Huaiet al.2001).ACS catalyzes the formation of 1-aminocyclopropane-1-carboxylic acid (ACC) as a dimer,with PLP acting as a cofactor.The mutation reported here may affect the entry of the coenzyme by changing the shape and/or size of the pocket, thereby reducing the binding affinity of the substrate and, thus, enzymatic activity.Interestingly, a mutation in melonACS11caused a changed from Ser to Phe at position 295 (S295F), which is also close to the ACS pocket (Boualemet al.2015).These two mutations may therefore abolish ACS activity by a similar mechanism.This study also provides new insights for the study of ACS catalytic mechanisms.
The molecular marker used here was designed according to the causative mutation of the androecious phenotype.Notably, in the process of cultivating the androecious male parent lines, the identification accuracy reached 100%.This would allow androecious male parent material to be planted among gynoecious female parents for pollination by bees, an approach which is also very well suited to mechanized mix harvesting and seed production.Thus, the tools and methods described here can further reduce the cost of seed production and improve seed breeding by integrating vegetable agronomy and mechanical techniques.
5.Conclusion
Here, we discovered a novel androecious mutant in a previously generated EMS mutagenized library.We demonstrated that nonsynonymous mutations in the ethylene biosynthetic geneACS11conferred male flowers through a series of genetic analyses, whole-genome resequencing, and molecular marker-assisted verification.This mutation caused the amino acid at position 301 to change from serine to phenylalanine, which then led to the loss of ACS11 enzymatic activity.In addition, this study provides a new germplasm as the androecious male parent for cucumber breeding, and it provides a new insight into the catalytic mechanism of ACS enzymes.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2018YFD1000803),the National Natural Science Foundation of China(31701933 and 32002036) and the Shandong Provincial Natural Science Foundation, China (ZR2020QC157).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendicesassociated with this paper are available at https://doi.org/10.1016/j.jia.2023.03.003
 Journal of Integrative Agriculture2023年11期
Journal of Integrative Agriculture2023年11期
- Journal of Integrative Agriculture的其它文章
- Germplasm and molecular breeding in horticultural crops
- Development and application of KASP marker for high throughput detection of the seedless trait in grapevine
- QTL analysis of early flowering of female flowers in zucchini(Cucurbita pepo L.)
- Comprehensive analysis of the full-length transcripts and alternative splicing involved in clubroot resistance in Chinese cabbage
- Virucidal activity of MICRO-CHEM PLUS against African swine fever virus
- Effects of the combined application of organic and chemical nitrogen fertilizer on soil aggregate carbon and nitrogen: A 30-year study
