Haploidentical hematopoietic stem cell transplantation as promising therapy in the improved survival of pediatric patients with leukemias and myelodysplasias
Ana Clara Carvalho Cardoso Brito, Everton Oliveira Carneiro Ribeiro, Fabricio Freire de Melo
Abstract
Key Words: Haploidentical; Stem cell transplantation; Children; Cancer; Treatment outcome; Prognosis
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (Allo-HSCT) has been shown to be a curative option for children with malignant and non-malignant diseases[1,2].In this type of transplant, the progenitor cells come from genetically distinct donors, who may be related or unrelated and with human leukocyte antigen (HLA) matching or HLA partially matching.The ideal donors for hematopoietic stem cell transplantation are HLA-matched siblings or matched unrelated donors (MUD-HSCTs), but only approximately 30% of patients will have a matched sibling donor and 33% of patients will have a MUDHSCT[1].
Haploidentical HSCT (Haplo-HSCT), a type of allogeneic transplant, represents a promising therapy in this prospect, as this type of transplant is performed with a partially HLA-matched related donor,which is available in 95% of the cases[3].Related donors can be a father, mother, sibling or son.This transplant has a strong antileukemic effect, the graft-versus leukemia (GVL), which contributes to a lower tendency to relapse.However, due to its nature, the occurrence of clinical complications is common, such as complete rejection of the graft, the development of graft-versus-host disease (GvHD)and relapses[2,4].
The main platforms of Haplo-HSCT areex vivografts of depleted T cells (TCD) and T- cell-filled grafts followed by post-transplantation cyclophosphamide (PT-Cy).The first is associated with a great limitation of the development of GvHD, but with slow immune reconstitution and infectious complications.While PT-Cy is associated with excellent immune reconstitution, a low incidence of serious opportunistic infections[3] and it has a more attractive cost-benefit ratio, as it does not require specific technical knowledge.There is also the infusion of unmanipulated grafts with administration of antithymocyte globulin (ATG).This platform consists of activating the donor with granulocyte colonystimulating factor; intensified post-transplant immunosuppression with cyclosporine, methotrexate and mycophenolate mofetil; inclusion of ATG in a combined graft of bone marrow and peripheral blood[5].
Haplo-HSCT has been frequently performed in children with hematological malignancies[6].When considering the highest incidences of childhood malignancies, it is observed that acute lymphocytic leukemia (ALL) is responsible for approximately 70% to 80% of childhood leukemia cases and acute myelogenous leukemia (AML) is responsible for approximately 15% to 20%[7-9].In addition, among other malignancies, myelodysplastic syndrome (MDS) has a very strong interface with the neoplasms mentioned and that, although its incidence is more common in adults, the chance of myelodysplasia evolving to more advanced forms and AML is greater in children.Therefore, many pediatric patients with MDS also are submitted to Haplo-HSCT[2,3].
Regarding the state of the art on allogeneic transplants, it should be noted that there are few publications involving the pediatric public aged up to 18 years[10].In this perspective, we had the interest in exploring the clinical outcomes of pediatric patients with leukemias and myelodysplasias undergoing the Haplo-HSCT.For this, we carried out a systematic review, whose general objective is to identify and summarize the scientific contributions available on haploidentical hematopoietic stem cell transplants performed in the last 10 years in children and adolescents with myeloid and lymphoid leukemias and myelodysplasias, aged up to 18 years.
MATERIALS AND METHODS
We followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)guidelines, which consists of a 27-item checklist and a flow chart for the conduction and reporting of this systematic review[11].
Search strategy
This is a descriptive systematic review, which sought to analyze the scientific contributions available on Haplo-HSCT performed in the last 10 years with selected pediatric audience.To elaborate the guiding question, the strategy PICO-acronym to Patientes, Interventation, Comparation and Outcomes-was used[12].Therefore, the research question that was used to guide the review was: “What is the efficacy and safety of haploidentical hematopoietic stem cell transplantation performed with the pediatric public with leukemias or myelodysplasias?”.
The electronic search for articles was carried out on November 20, 2022 in the Virtual Health Library Brazil (VHL) and on the PubMed website, using the keywords selected according to the classification of Health Sciences Descriptors (DeCS): Cancer, children, transplant, and haploidentical.The Boolean operator “AND” was used.Inclusion criteria were applied, the first being the filter “language-English”,“language-Spanish”, and “language- Portuguese”; and the second, selection of the 10-year period (2012-2022).Forty-one results were found in VHL and 549 in PubMed, totaling 590 articles, all in English.After crossing the bases, 31 repeated articles were discarded (Figure 1).
For the analysis of the 559 articles, the abstracts were read based on the exclusion criteria: Other languages, review studies, case reports, experimental studies, paid articles, other health conditions and age over 18 years old.This step was performed by a pair of reviewers, independently, and all disagreements were resolved by consensus.
It is important to emphasize that articles that included a mixed audience with children and adults over 18 years of age were also discarded, due to the central objective of analyzing the clinical results separately.In addition, paid studies that did not allow the reading of articles through access by the Federated Academic Community of institutional link of the authors of the work were excluded.Finally,it remains to inform that the experimental studies included: Randomized clinical trials, prospective clinical trials, controlled tests and control cases, all with clinical intervention.
The order of exclusion criteria was followed: Types of study, paid articles, health condition and age.The following were discarded at this stage: 59 review articles and meta-analyses, 64 case reports, 112 experimental studies, 117 paid articles without institutional access, 55 articles on other clinical conditions and other hematological neoplastic conditions, 15 studies with mixed age and 19 with age outside the established age range.Qualitative studies, editorials, annals, reports and comments were also excluded.These other types of study, together, totaled 18 articles.
In this perspective, 100 articles were included for full text reading.However, 10 articles were excluded for not providing the full text for free and 02 unavailable studies were also discarded.After the complete reading, 72 articles were excluded for deviating from the eligibility criteria (Figure 1).The final sample included 16 articles.
A new search was performed in other databases, on April 4, 2023.For this, the same descriptors were used, as well as the same Boolean operator, the language inclusion criteria and the 10-year interval used in the previous research.The new search was carried out in the EMBASE and SciELO databases.In the last base, no results were found.At EMBASE, the platform filter “Articles” and “Erratum” was also used to select materials.There were 462 results found.However, with data crossing, 265 articles were excluded due to repetition.
After completing the reading of titles and abstracts, 174 articles were excluded due to the type of study criteria, mixed age, other clinical conditions involved and availability of the article (Figure 1).As a result, 23 articles were read in full, but only 2 studies metal eligibility criteria.In this context, with the results already obtained from the first search, 18 articles were included for analysis in this review.Among them, the types of observational studies involved were: Retrospective, retrospective comparative, and prospective cohort studies.
Data extraction
For data extraction, a spread sheet was prepared in advance for analysis of patients and treatment,which included: (1) Participants: Number of patients, age group, gender; (2) health condition: The type of hematological malignancy; (3) characteristics of the donation: source of cells; (4) intervention:Conditioning regimen; (5) recurrent clinical complications: Types of complications; and (6) clinical outcomes: The overall survival (OS) and event-free survival (EFS) and the main reasons for death of patients.
In all articles, data on the age of the patients were obtained through the available tables, verifying the age range surveyed.In comparative studies, when possible, data were extracted from the public that received the Haplo-HSCT separately.The presentation of the statistical results of OS and EFS will also be directed to the Haplo-HSCT data of the included studies.Thus, OS and EFS rates, which have Haplo-HSCT results separately, will be presented according to their analysis intervals.
Evaluation of the quality of studies
For analysis of the studies, a survey of methodological aspects was carried out, with the authors’ names,journal, year of publication, country where the study was carried out, study design, time of analysis and purpose of the study.The risk of bias of the selected studies was assessed using the Cochrane tool: Risk of Bias In Non-randomized Studies-of Interventions (ROBINS-I)[13].
The ROBINS-I presents seven domains that provide theoretical support for detecting factors that can lead to confounding when analyzing a patient’s outcome, as well as enabling the analysis of possible biases in the selection of participants, performance of interventions, deviations from usual practice,availability of data, assessment of measures and reporting of studies.In this step, two reviewers,independently, assessed each domain and managed to classify each study as low, moderate, serious or critical risk, based on the platform’s guidelines, as can be seen Figure 2.
In order to analyze the clinical results, the factors that influence the prognosis of patients submitted to allogeneic transplants were considered for the detection of the confounding domain[14].These factors were divided into: Pre-transplant, peri-transplant and post-transplant.In pre-transplantation, the disease status, age and sex of the patient, information about donors and source of cells were considered.In the peri-transplantation, it was verified if there was information on the conditioning regimen,prevention of GvHD, the number of cells infused.In the post-transplant period, information on the development of acute and chronic GvHD was considered.
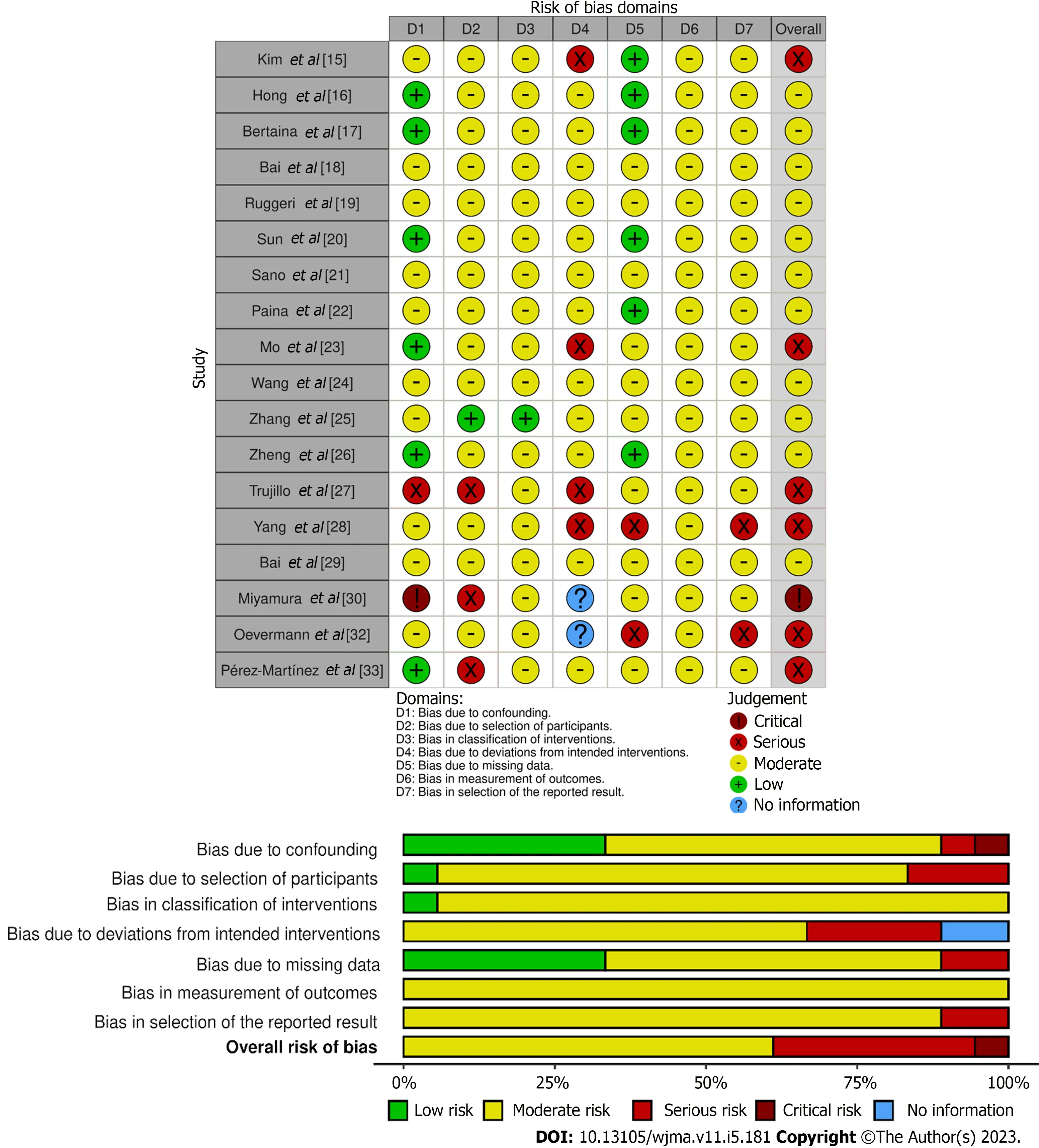
Figure 2 Assessment of the risk of bias of studies in each domain of the tools Revised Cochrane Risk of Bias In Non-randomized Studies-of Interventions (ROBINS-I).
For all factors, the existence of variables that could statistically assess this domain was verified,including: ThePvalue for gender and age of patients, source of cells and donors; the immunophenotyping, white blood cell counting, platelet counting, percentage of blasts, hemoglobin levels, chimerism,cytogenetic techniques and molecular genetics were also observed, the last two when it was necessary.Thus, the indication of the factor and the establishment of the variable for its evaluation were classified as low-risk bias, while the absence of both was considered as critical condition.
RESULTS
Characteristics of the studies
The studies were published between 2014 and 2022, with retrospective and prospective analyses.Thus,in retrospective analyses, the interval between articles was from 1988 to 2021; while in prospective analyses, the public was analyzed between 2011 and 2019.The research was concentrated in seven countries: Korea, China, Italy, Colombia, Spain, Germany and Japan.It is noteworthy that China was responsible for 8 publications (Table 1).
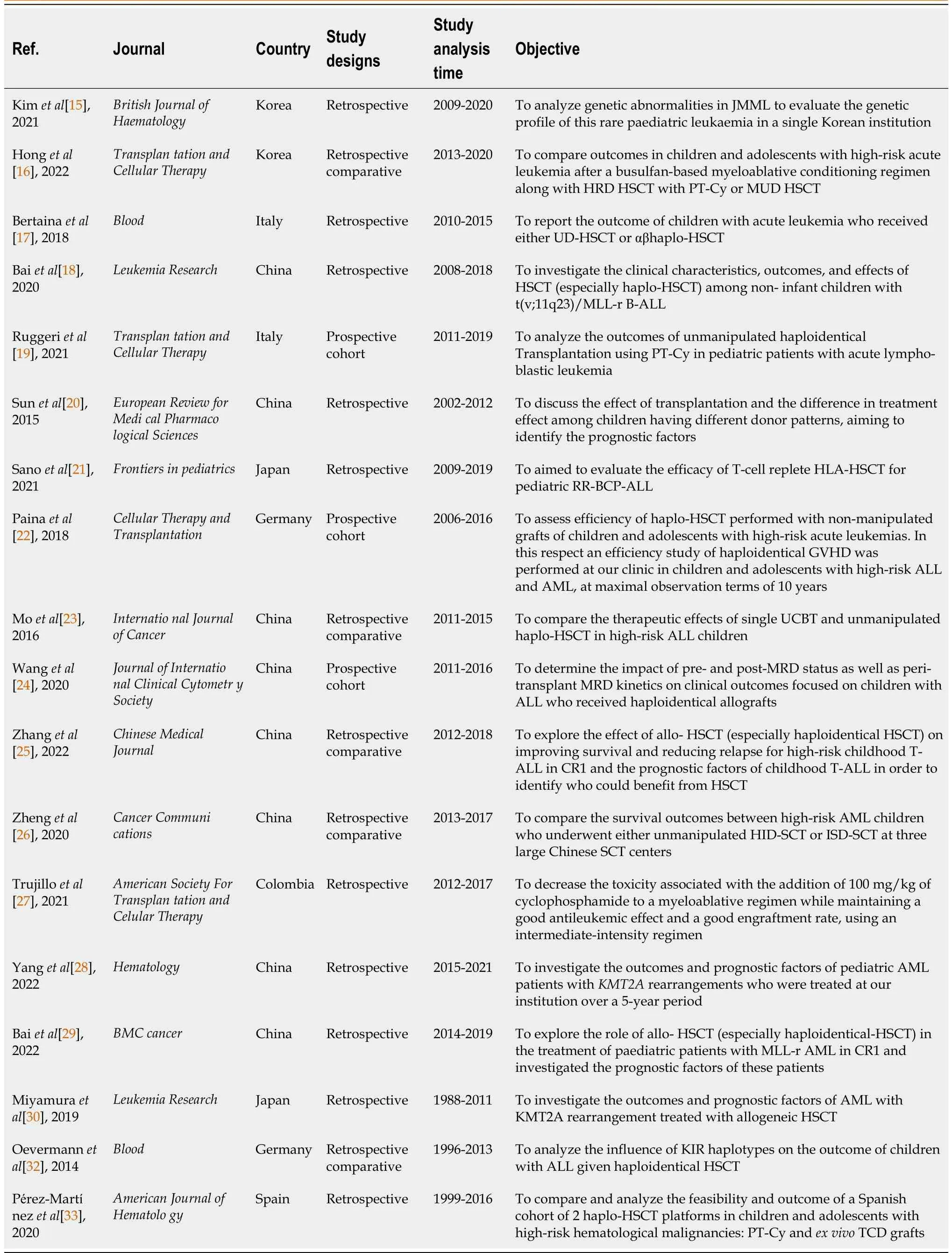
Table 1 Methodological aspects: General characteristics on the studies included
Regarding the risk of bias in the studies, the serious-risk in the analysis of selection bias is due to the fact that some of the aspects of the attributions of the status of the intervention were determined in a way that could have been affected by the knowledge of the previous result combined with the fact that the beginning of the follow-up and the beginning of the intervention did not coincide, so that the interpretation could not be adjusted in the final analysis of the outcomes.While, in the deviations, those studies were indicated that had to switch regimens and co-interventions due to the initial responses of the patients.The changes were not balanced across intervention groups.In addition, the data and reports concern the availability of all information from participants in the interventions and demonstration of multiple analyzes and different subgroups.
Characteristics of the patients
The total sample of all cohorts of the analyzed studies was 1825 patients, considering the entire audience in the comparative studies and information on patient removal due to death before treatment (Table 2).The majority of the sample was composed of males, the highest median age reported was 15 years and the lowest, 1.2 years.The ethnicity of the participants was not a topic well explored by the authors.
Among the reported clinical conditions, the following were observed: Juvenile myelomonocytic leukemia (JMML), ALL, acute myeloid leukemia, chronic myelogenous leukemia, mixed lineage leukemia, mixed-phenotype acute leukemia and NK cell leukemia.In addition, it is verified that 1295 patients underwent Haplo-HSCT, considering the information on the withdrawal of patients from the original cohorts due to another type of donation, such as a MUD-HSCT and matched sibling donor(MSDT) (Table 2).
Regarding the type of conditioning, different types of regimens were observed, with the adoption of the Myeloablative Conditioning Regimen, some with total body irradiation, and also the Reduced Intensity Regimen.In studies that detailed the types of chemotherapy, it was observed that the main drugs used were: Busulfan, Fludarabine, Cytarabine, Cyclophosphamide, Melphalan, and Semustine(Table 3).
In addition, for the prevention of graft disease and mobilization of the BM and peripheral blood stem cells (PBSC), the interventionists of the analyzed studies used different combinations of drugs, therefore,the same study used one or more compounds.As a result, ATG was administered by 12 studies; posttransplant cyclophosphamide, 5; Cyclosporine A, 13; Methotrexate (MTX), 11; Mycophenolate mofetil(MMF), 10; Tacrolimus, 6; granulocyte colony stimulating factor, 6; and Sirolimus, 1.Two studies did not present the names of the drugs used for this purpose.
Outcomes
Recurrence, graft rejection and delayed immune recovery are the major clinical challenges that have been identified.The occurrence of hematological recurrences was observed in most of the studies, with the exception of Kimet al[15], in which this type of clinical complication was not explicitly mentioned.Among the authors who reported, seven studies pointed to extramedullary recurrences (EMR).Honget al[16] identified extramedullary recurrence in the central nervous system, the others did not report the location of the EMR.
Acute graft-versus-host disease (aGvHD) and chronic graft-versus-host disease (cGvHD) were also observed in most of the analyses, with the exception of the study by Bertainaet al[17], in which there were no cases of grade III-IV or visceral aGvHD in the haploidentical transplant cohort, and two other studies that did not discuss this type of complication.
In the total sample of all cohorts of the analyzed studies, the pre-transplant minimal positive residual disease (pre-MRD+) was identified in 288 patients; while 337 patients were MRD negative (pre-MRD-).After transplants, 90 patients were identified with positive MRD (post-MRD+), 19 with reemerging MRD.However, these data are limited, considering the fact that some authors did not present the pretransplant and post-transplant MRD status together, and also not all comparative studies that separated the results of the analyzed cohorts.In addition, eight other articles did not have either status.
It is known that immunosuppression makes the patient susceptible to infections.Therefore, infections are also among the main clinical complications, with viral, bacterial and fungal infections being reported.Among viral infections, cytomegalovirus and adenovirus were the most common.In addition,due to treatment-related toxicity, multiple organ failure, hemorrhagic cystitis, and cerebral and alveolar hemorrhages have been reported.Among organ failure, liver impairment was reported in 05 studies and 02 studies also pointed to the involvement of the gastrointestinal tract and skin.Other causes of complications include sepsis, pneumonia, and cases refractory to treatment.Other types of complications occurred in smaller numbers.
The main causes of death were relapses, graft-versus-host disease, infections and transplant-related complications.Furthermore, non-recurring mortality (NRM) was also presented in most of the studies.However, in the study by Honget al[16], the group that received Haplo-HSCT did not present any cases of NRM.
The OS and EFS rates are indicated in Table 3.It is observed that in the 5-year interval, the lowest EFS and OS rates were 29.5% and 68.0%, respectively.The EFS result of 29.5% was reported in patients with transplants from a KIR A haplotype donor.In this same interval, the highest rates of EFS and OS were 80.1% and 81.0%, respectively.At the 10-year interval, OS rates were 64.7% for patients in a firstcomplete remission (CR1) and second CR (CR2); and 18.1% for patients transplanted beyond remission(Table 3).

Table 2 Characteristics of haploidentical transplants identified in the studies included
It is emphasized that, in the study by Baiet al[18], patients in the chemotherapy regimen cohort who relapsed and opted for haploidentical transplantation had an OS rate of 57.1%.Thus, in CR2, the results were less satisfactory in relation to the results of transplants performed in CR1 in that same study.At the same time, it was observed in the study by Ruggeriet al[19] that patients in CR1, CR2, and CR3 had a 2-year EFS of 65.0%, 44.0%, and 18.8%, respectively.Finally, it is noted that three other studies showed an EFS rate below 50%, with an interval of 2 years in two studies and 3 years in two studies (Table 3).
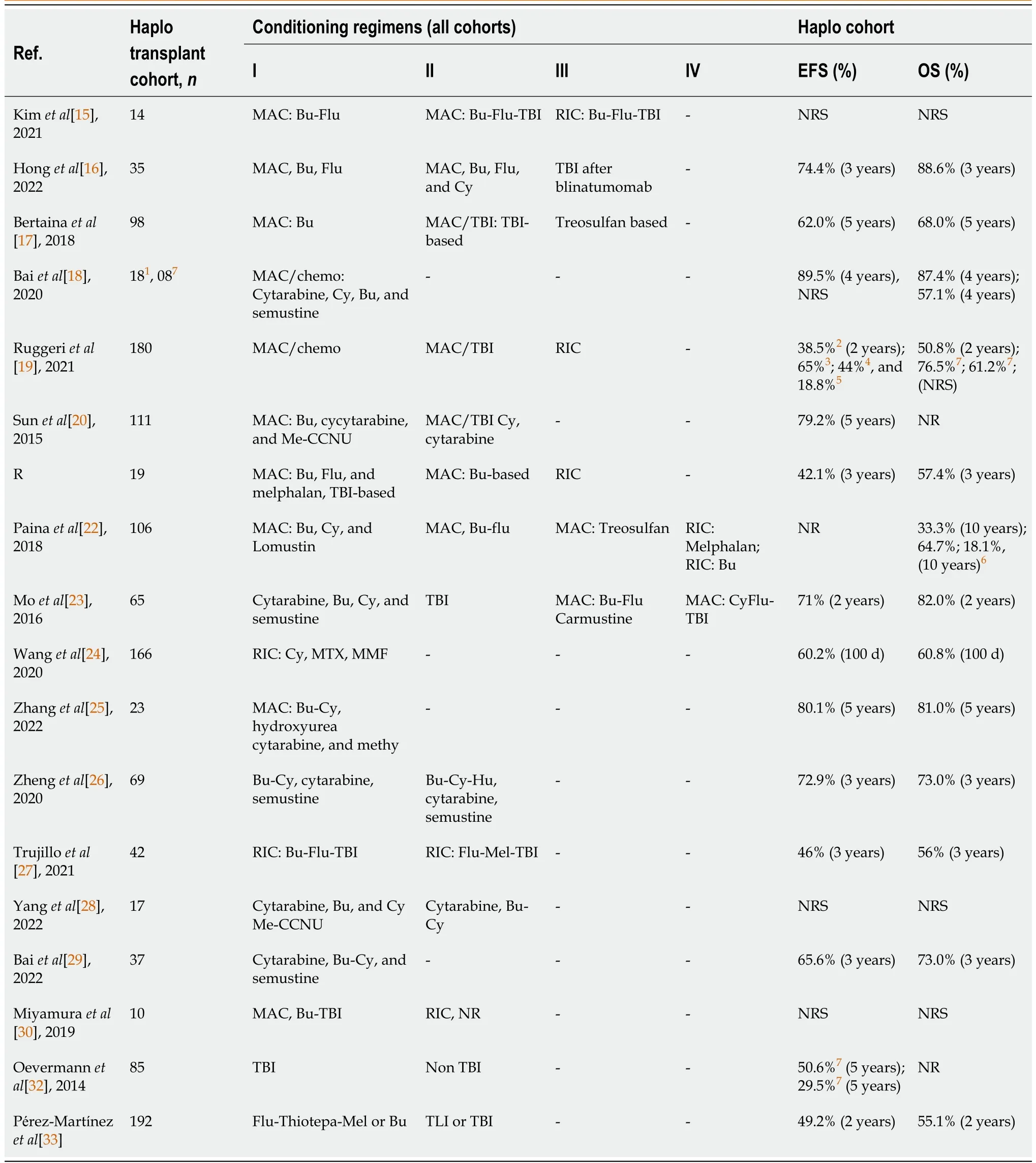
Table 3 Conditioning, event-free survival and overall survival of the analyzed patients
DISCUSSION
Based on the analysis of selected studies, this review presented the clinical results indicated of pediatric patients with leukemias and myelodysplasias younger than 18 years old who underwent Haplo-HSCT.Until the moment, there are few published reports on the use of Haplo-HSCT in selected pediatric populations.In our review, the total sample in the analyzed studies of patients undergoing Haplo-HSCT was 1295 patients, in which both favorable outcomes and poor prognostic factors were observed.Haploidentical transplantation is often indicated in more severe cases and for patients in second remission according to the time and place of disease recurrence[19], however, studies have indicated efficient results for patients treated in first complete remission and with early referral[19,20-22].
Ruggeriet al[19] indicate the importance of disease status as one of the most important prognostic factors influencing the risk of disease recurrence and the probability of EFS and OS.In that study,patients in CR1, CR2, and CR3 had 2-year EFS of 65.0%, 44.0%, and 18.8% and 2- year OS of CR1 and CR2, 76.5% and 61.2%, respectively.These results are consistent with the study by Baiet al[18], in which patients in CR1 had a 4-year OS of 87.4% and patients in CR2 had an OS at the same interval of 57.1%.Thus, both studies indicate the worsening of the patient’s prognosis as the disease progresses and indicate that the results show the feasibility of Haplo-HSCT for patients in CR1 and CR2.In contrast, Moet al[23] found no relationship between pre-transplant disease and EFS, however, the authors point to the low number of patients in non-remission or relapse at the time of transplantation, which limits their analyses.
Pre-transplant MRD status is also indicated as a poor prognostic factor, often related to an increased probability of recurrence[19,23,24].In our review, 288 patients were identified with pre-MRD+ in the analyzed studies.These patients had higher recurrence rates than the pre-MRD- group.However, in the analysis by Zhanget al[25] and Baiet al[18], there was no clear impact predictor at the level of MRD after induction.That is, despite a trend towards lower OS/EFS and higher cumulative incidence of relapse in patients with MRD+ after induction, the results were not statistically significant[18].The authors presented as justifications the limited number of patients in the analysis and the effectiveness of allo-HSCT in the impact of the MRD level after induction[18].
However, in our series, the data extracted on MRD was limited, as not all studies explored this prognostic factor and few authors provided the comparison and follow-up of cohorts with pre-MRD+/pre-MRD- and post-MRD+/post-MRD-.Thus, there is little information on the effects of MRD on outcomes in Haplo-HSCT, although MRD can be configured as a transformative approach in risk stratification[18].
The graftvsleukemia (GVL) effect is related to chronic GvHD[23].Studies indicate that the GVL effect is stronger in patients who receive haploidentical transplantation[23,26].In a multivariate analysis in the study by Moet al[23], mild and moderate cGvHD was associated with a significant improvement in the survival of patients who presented with transplantation, possibly due to the GVL effect, and that,despite the high incidence of cGvHD, there was no significant increase in the risk of NRM in the Haplo-HSCT group.However, the same authors alert to the fact that intense immunosuppressive therapy,which has been correlated with severe cGvHD, can revoke the effector cells of the GVL effect and impair the quality of life of these patients.
When analyzing aGvHD, the study by Bertainaet al[17] was noteworthy.There were no cases of grade III-IV or visceral aGvHD in the haploidentical transplant cohort[17].In this study, the authors performed a multicenter scaled analysis to compare the efficacy of αβhaplo- HSCT and MUD-HSCT in a cohort where the TBI-based conditioning regimen was frequently used.Furthermore, in that study,there was a lower risk of NRM in αβhaplo-HSCT recipients compared to HLA misMUD-HSCTs, the authors attributed to the fact that chronic GvHD was also limited.
Other variables such as age have been identified as prognostic factors in some studies.In the study by Zhanget al[25], children with ALL aged ≥ 10 years was an independent risk factor that affected 5-year OS and EFS rates.Advanced age was also associated with a poor prognosis with a higher risk of extramedullary recurrence, according to Ruggeriet al[19].However, compared to all the data, it is noted that few studies have identified the statistical significance of advanced recipient age.No statistical significance was found for the recipient’s gender either.
The studies Indicate the association between high initial leukocyte count and risk of recurrence[25]and, in Ruggeriet al[19], the use of PBSC was associated with a significantly lower OS, with a higher risk of NRM.While, Trujilloet al[27] indicate that PBSC is associated with a lower number of relapses.In this regard, as the source of graft cells is a modifiable factor, it would be interesting if the source was better researched, considering the characteristics of patients and diseases.
Genetic abnormalities and rearrangements
Cytogenetic abnormalities are important prognostic factors in cases of hematologic malignancies.Thus,understanding these manifestations can contribute to decisions regarding treatment strategies[15,25].In this regard, our study brought, based on retrospective analyses, the presentation of the effects of cytogenetic alterations in high-risk myeloid and lymphoid leukemias, such as rearrangements of mixedlineage leukemia genes, and in myelodysplastic syndromes with rare genetic profiles.
Therapeutic considerations range from observation to allogeneic stem cell transplantation, depending on the genetic subtype.In the case of JMML, a very aggressive form of MDS, Kimet al[15] showed, in their analyses, that allo-HSCT is still presented as the only curative treatment option for most patients and that patients frequently have poor EFS rates.In that study[15], patients with mutations involving RAS pathway genes and somatic mutations in non-receptor protein tyrosine phosphatase type 11 were the most common mutations identified, and had a 5-year EFS of 72.9% and 41.7%, respectively.Among these patients, 14 patients received Haplo-HSCT.The authors drew attention to the fact that cytogenetic changes influence disease progression, rather than the onset of leukemia, which makes it valuable for predicting disease outcomes.
In the same perspective, the presence of cytogenetic abnormalities in 11q23 involving lysine-specific methyltransferase 2A (KMT2A) has been associated with adverse outcomes and higher rates of early death and relapse even after allogeneic hematopoietic stem cell transplantation[28,29].Its occurrence is more common in children than in adults, and the prognostic value influencing outcomes in pediatric AML is associated with the fusion partner gene.The KMT2A/MLLT3 fusion resulting from t(9;11)(p22;q23) KMT2A is the most common rearrangement in children.However, it is the t(6,11) and AF10 translocation partners in t(10,11) that are often associated with poor prognosis[25].
In the study by Miyamuraet al[30], no patient with t(6,11) remained alive in CR and only 1 patient with t(10,11) remained alive in CR, which corroborates the findings in the literature.However, the authors note that the t(9,11) translocation partner was found more frequently in their patients and that their results did not differ significantly from other 11q23 abnormalities.Although the lack of difference in transplant results was justified based on the retrospective analysis and the possible biases generated,this is something that deserves to be further studied.
In this regard, Yanget al[28] noted the high occurrence of KMT2A rearrangements in childhood AML and how the prognosis of children with t(9;11)(p22;q23) remains controversial.Thus, when they performed a retrospective investigation on the outcomes and prognostic factors of pediatric AML patients with KMT2A rearrangements, it was identified that approximately 31.3% of the investigated children had the KMT2A/MLLT3 fusion gene.Some of these children underwent hematopoietic stem cell transplantation, where four received donations from compatible sibling donors and another seventeen received haploidentical transplants.As a result, presented, they had EFS between the two groups ofP= 0.303.Therefore, EFS rates were not statistically significant among patients who received haplo-HSCT and full-matched HSCT, which indicates that, in the absence of a suitable fully matched donor, children with high-risk AML who carry mutations in the KMT2A gene, may accept haploidentical hematopoietic stem cell transplantation.
KIR haplotypes impacts
It has been observed that the presence of donor-derived alloreactive NK cells influences the outcome of haploidentical hematopoietic stem cell transplantation, given that among HLA non-identical donors and recipients, donor NK cells that encounter recipient target cells without an HLA class I allele present in the donor’s HLA genotype can exert antileukemic effects[31].Among these effects are lower rates of relapses, graft failure and GvHD, which contributes to patient survival[31].In this context, it was discussed about the response of patients to treatment considering the influence of killer cell immunoglobulin-like receptors (KIRs) present on NK cells.
The expression of inhibitory KIR receptors is responsible for the alloreactivity of NK cells in allogeneic hematopoietic stem cell transplantation[31,32].Oevermann and other collaborators[32]presented a series of 85 patients with high-risk ALL confirmed by Haplo- HSCT, where 74% of donors had KIR B haplotype and 26.0% of the donors had KIR A haplotype.Patients transplanted from the B haplotype donor had a 5-year EFS of 50.6%, while patients transplanted from the A haplotype donor had 29.5%.
This was also observed in the study by Pérez-Martínezet al[33], where early reconstitution of NK cells was reported on theex vivoTCD platform and related prognoses with the donor KIR B haplotype, while the KIR A haplotype increased the probability of relapse on the PT-Cy andex vivoTCD platforms.In this regard, the authors drew attention to the inclusion of genotyping when choosing donors, with preferential selection of KIR B haplotype donors due to the results observed.
Therapeutic and regimens effects
Discussions about the main platforms of Haplo-HSCT and the type of conditioning regimen that patients are submitted during treatment are points of evaluation between the authors.In the records of Pérez-Martínezet al[33], in an analysis of morbidity and mortality associated with GvHD, considerations were found regardingex vivografts of depleted TCD and grafts filled with T cells followed by PTCy.Thus, although a higher incidence of aGvHD grades I-II was noted in patients treated with the PTCy platform, the results that include OS, EFS and recurrence demonstrated that there are no statistical differences between both grafts in the outcomes analyzed by the authors.The great challenge that remains, on both platforms, is overcoming relapse as the main cause of transplant failure[33].
Treatment platforms have been widely studied.Studies point to the impact of high doses of purification of CD34 cells on GvHD, with a reduction and decrease in cases.However, this technique has a high transplant-related mortality due to the delay in immune reconstitution, which promotes a high risk of infection during the first months after transplantation.In this perspective, another type of purification, which has been widely used, is the partial depletion of T cells, such as αβ, which has shown optimistic results, since this technique maintains some subsets of T cells, such as γδ T cells, NK cells and memory T cells without increasing GvHD.Regarding the PT-Cy approach, which has been shown to be effective in reducing GvHD, there is the advantage of not requiringex vivomanipulation of the graft.However, PT-Cy requires prolonged immunosuppression treatment[19,27,33].
With regard to conditioning, discussions of myeloablative (MAC) and low-intensity (RIC) regimens have been contrasting.Bertaina and other authors[17] pointed to a low incidence of graft failure (2%) in the αβhaplo-HSCT group with the use of a fully MAC conditioning regimen and associated the regimen as a possible explanation for the lower incidences of relapse in the analyzed patients.As in the studies by Ruggeriet al[19], the MAC regimen was associated with significantly longer GvHD/relapse-free survival.While Trujilloet al[27] point out that the MAC regimen is often associated with acute and longterm toxicities, such as secondary malignancies and increased acute mortality, and that the combination of a RIC with haploidentical cells using the PT-Cy platform has a very strong antileukemic effect.From this perspective, studies that analyze MAC and RIC regimens and their contribution to patients’ quality of life are needed.
CONCLUSION
Haplo-HSCT has been shown to be a promising therapeutic option.In recent years, the number of Haplo-HSCT has increased with the pediatric public under the age of 18, however, publications do not keep up with this pace.This review was performed based on retrospective and prospective data; thus,the methodological aspects of the studies may have influenced the analysis.Therefore, randomized clinical trials and meta-analyses should be encouraged in order to confirm the reported findings.
When exploring the published studies, it was observed that prognostic factors such as treatment platforms, cytogenetic abnormalities and disease state exert a strong influence on the clinical outcomes of transplanted patients and other variables can be obtained in order to collaborate with risk stratification and selection approaches of donors.Nevertheless, the information extracted about age and source of stem cells as prognostic factors is insufficient to provide a conclusion, considering the counterpoint of information across the studies presented.
The indication of Haplo-HSCT for patients in first complete remission is evident.Studies have indicated efficient results for patients treated at this stage and with early referral, with significantly important and different survival rates.Thus, it is noted that disease status as one of the most important prognostic factors influencing the risk of disease recurrence and the probability of EFS and OS.
One of the clinical challenges is the delay in immune recovery.This delay depends on the chosen treatment platform, since in grafts with highly purified CD34 cells there is a limitation of cell reconstitution.While on the PT-Cy, reconstitution has greater speed and lower financial cost, but with prolonged immunosuppression treatment, and, in partial T-cell depletion, some T-cell subsets are received without increasing GvHD.In this perspective, there are pros and cons between the treatment platforms, and, therefore, the characteristics of the disease and the patients to be transplanted must be considered.
Relapse, identified as a primary transplant failure, was the most recurrent clinical complication, with many factors contributing to relapse.Among these factors, a limitation of this review was the analysis of MRD status, with pre-MRD+/pre-MRD- and post-MRD+/post- MRD- cohorts.Here, although pre-MRD+ was pointed out as a poor prognostic factor, the numbers were too few for a complete analysis.Thus, studies that seek to identify the effectiveness of Haplo-HSCT in the impact of the MRD level after induction should be encouraged.
In summary, Haplo-HSCT represents a promising therapy, considering the potential number of possible donors and the accommodation and treatment platforms that can be offered.The results obtained show that this type of transplant has a strong antileukemic effect, with generally accepted rates of survival.Overcoming relapse as the first cause of transplant failure is the great clinical challenge.
ARTICLE HIGHLIGHTS


Research objectives
To identify and summarize the scientific contributions available on haploidentical hematopoietic stem cell transplants performed in the last 10 years in children and adolescents with myeloid and lymphoid leukemias and myelodysplasias, aged up to 18 years.
Research methods
This is a descriptive systematic review.The VHL, PubMed, EMBASE and SciELO databases were consulted, but the results were only obtained in the first three.Based on the eligibility criteria, 18 articles were included in this review.For data extraction, the characteristics of the patients and treatment were sought, which included the number of patients, age group, gender, health condition, characteristics of the donation, conditioning regimen and recurrent clinical complications.
Research results
The studies included 1825 patients, most of whom were men, although gender was not an independent factor for the patients’ prognosis.Regarding age, the data are inconclusive, as well as for the source of stem cells.Pre-transplant DRM status and intense immunosuppressive therapy are also factors that impact patient prognosis.The main complications observed were acute graft-versus-host disease,chronic graft-versus-host disease and infections.Clinical challenges are relapse, graft rejection and delayed immune recovery.In general, the studies indicated good results for patients treated in first complete remission and with early referral.
Research conclusions
The indication of Haplo-HSCT for patients in first complete remission is evident.Studies have shown efficient results for patients treated in this phase and with early referral, with significantly important and differentiated survival.In this perspective, considering the potential number of potential donors and the treatment platforms that can be offered, Haplo-HSCT appears to be a promising therapy.Randomized clinical trials and meta-analyses should be performed to confirm the reported findings.
Research perspectives
The pre-MRD+ was pointed out as a poor prognostic factor, as well as age and cell source, but the numbers were too few for a complete analysis.Thus, it is suggested that researchers consider these aspects and include the MRD status, with pre-MRD+/pre-MRD- and post-MRD+/post-MRD- cohorts.The analysis of the influence of the ethnicity of the patients must be done, this will contribute even more to the evaluation of the Haplo-HSCT.
FOOTNOTES
Author contributions:Cardoso Brito ACC, Oliveira Carneiro Ribeiro E, and Freire de Melo F equally contributed to this paper with the conception and design of the study, literature review and analysis, drafting and critical revision and editing, and final approval of the final version; and all authors agree to be accountable for all aspects of the work in ensuring that questions that are related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
PRISMA 2009 Checklist statement:The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Brazil
ORCID number:Ana Clara Carvalho Cardoso Brito 0000-0001-9470-9069; Fabrício Freire de Melo 0000-0002-5680-2753.
S-Editor:Chen YL
L-Editor:A
P-Editor:Cai YX
 World Journal of Meta-Analysis2023年5期
World Journal of Meta-Analysis2023年5期
- World Journal of Meta-Analysis的其它文章
- Evidence relating cigarette, cigar and pipe smoking to lung cancer and chronic obstructive pulmonary disease: Meta-analysis of recent data from three regions
- Diabetes mellitus: An overview of the types, prevalence, comorbidity, complication, genetics, economic implication, and treatment
- Real-world effectiveness of mRNA COVID-19 vaccines in the elderly during the Delta and Omicron variants: Systematic review
- Pulmonary cytomegalovirus infection: A case report and systematic review
- Advances in the mechanism of action of metformin in pituitary tumors
- Vitamin D deficiency among outpatients and hospitalized patients with diabetic foot ulcers: A systematic review and meta-analysis
