Ion-Velocity Imaging Study of Dissociative Charge Exchange Reactions between Ar+ and trans-/cis-Dichloroethylene
Zi-Xin Chen,Jie Hu,Yaya Zhi,Chun-Xiao Wu,Shan Xi Tian
Hefei National Research Center for Physical Sciences at the Microscale,Department of Chemical Physics,University of Science and Technology of China,Hefei 230026,China
Dissociative charge exchange reactions between Ar+ ion and trans-/cis-dichloroethylene(trans-/cis-C2H2Cl2) are investigated with the ion-velocity imaging technique.The dechlorinated product C2H2Cl+ is the predominant,and most of this product show the spatial distribution around the target,implying that the dissociation occurs in the large impact-parameter collision and via the energy resonant charge transfer.Meanwhile,a few C2H2Cl+ locate around the center-of-mass,which is attributed to the fragmentation of intimate association between C2H2Cl2 and Ar+ or in the small impact-parameter collision.The product C2HCl+ exhibits the velocity distribution features similar to those of C2H2Cl+.The rarest product C2HCl2+ shows the distributions around the molecular target,due to the quick dehydrogenation after the energy-resonant charge transfer in the large impact-parameter collision.
Key words: Ion-molecule reaction,Dissociative charge exchange,Velocity map imaging
I.INTRODUCTION
Ion-molecule reaction is one of the most fundamental processes in the astrochemistry,etching plasma,and combustion [1-3].Basic physical chemistry processes,such as charge,energy transfers,and chemical bond cleavage or formation,are frequently involved in the low-energy (several eV) ion-molecule reactions [4].As the doorway of chemical reactions,charge exchange or transfer reactions lead to the molecular ions in different quantum states as well as the molecular dissociative products.Recently,using our own cross-beam velocity map imaging (VMI) apparatus,the strong collision-energy dependences of the charge transfer (CT) reactions between Ar+and small molecules have been revealed[5-8].On the other hand,dissociative charge exchange(DCE) reaction is of another interest and has been studied by Farrar’s and our group [9-12].A long-term view is that DCE should be a cascade of the CT process.However,our previous studies [11,12] indicate that DCE is not always a subsequent event of the CT process.Furthermore,the CT,even its long-distance process,can be accessed in a pathway completely different from photoionization [13].Therefore,some traditional understandings about the ion-molecule reactions,in particular,the CT and DCE processes,need to be revised.More recently,we found that the CT between Ar+andtrans-/cis-dichloroethylene (trans-/cis-C2H2Cl2) was primarily accomplished via the energyresonant process in the large impact-parameter collision [14].
Dichloroethene is an environmental pollutant and a potential human carcinogen [15],having three isomeric forms,iso-C2H2Cl2,cis-C2H2Cl2,andtrans-C2H2Cl2,their dissociation processes were investigated by several groups [16-18].Pakes and coworkers observed the dissociative yields C2H2Cl+and C2H2+in the photoninduced threshold photoelectron photoion coincidence(TPEPICO) study [16],and Bodi and coworkers investigated the fragmentations leading to the products of C2H2Cl+,C2H2Cl2+,C2HCl+,and C2H2+,using their imaging photoelectron-photoion coincidence (iPEPICO) spectrometer [17].With the selected ion flow tube method,Mikhailov and coworkers investigated the ionmolecule reactions at room temperature,and besides the parent ion C2H2Cl2+,the daughter ions C2H2Cl+,C2H2Cl2+,and C2HCl+were found in the collisions with Ar+[18].The dechlorinated ion,C2H2Cl+,is the predominant product in the photoionization process with the photon energies below 16 eV [16,17] or the ionmolecule reaction for the reactant cations with their neutral ionization energies (IE) of 12.5-16 eV [18];on the contrary,above 16 eV,the double-dechlorinated ion C2H2+turns to be the dominant yield [16-18].
Considering that IE(Ar+) equals 15.76 eV,we reasonably refer that the C2H2+ion should not be produced if the translational-to-internal energy transfer is not efficient enough.However,it is unknown what are the predominated products and the dynamics mechanisms of these DCE reactions at the collision energy of several eV.The possible DCE reactions investigated here are listed below:
The released energy for the exothermic reaction (1) is 3.672/3.656 eV for thetrans-/cis-C2H2Cl2isomers according to the photodissociation onset of C2H2Cl+ion[17],the exoergicity of reaction (2) is 3.92/3.95 eV for thetrans-/cis-C2H2Cl2[18],and the released energy for exothermic reaction (3) is about 0.5 eV according to the breakdown curves of dichloroethene [17].In the reactions between Ar+andtrans-/cis-C2H2Cl2at room temperature [18],the C2H2Cl+is the predominant product,the second is C2HCl+,while the minor C2H2Cl2+is almost as rare as the parent ion.Besides the relative abundances of different ionic products,the translational energy and angular distributions can be derived from the VMI measurements [6].In this work,the DCE reactions betweentrans-/cis-C2H2Cl2and Ar+in the center-of-mass collision energy (Ec.m.) range of 2-10 eV are investigated with the VMI technique.
II.EXPERIMENTS
The details of our crossed-beam VMI apparatus are described in Refs.[5,6].Here is a brief introduction.The pulsed reactant ions are generated by the electron-impact ionization.Through controlling the time sequence of the ionization electron pulse and the two repulsion voltage pulses,the ion bunches can be confined well both in space and time domains [6].This reactant ion beam is perpendicular to the incident direction of the continuous supersonic molecular beam in the laboratory coordinate.The molecular beam is cooled via a nozzle with a 30-µm aperture,which is located 23 mm upstream from a collimated skimmer with a 0.51-mm orifice.The reactant ion beam and neutral beam intersect at the center of a field-free region with the volume about 2 mm×2 mm×2 mm.The field-free region is generated by surrounding two circle electrodes and a shield cylinder with the same potential.The product ions of above DCE reactions are produced in this region,and then pushed out by a pulsed electric fields applied on the repeller plate of the VMI lenses together with the scattered reactant ions.The flight direction of the product ions is perpendicular to the ion beam and target beam,and the whole Newton sphere with a clear ion velocity image is detected at the end of the VMI system.The kinetic energy spreads of the target molecules and incident Ar+ions (which is primarily limitation) cause that the energy resolution ΔE/Eof the detected ionic products is about 8% with the translational energy up to 3.5 eV in the laboratory coordinate.Limited by the detector size with a 75-mm diameter,the product ions cannot be detected if the translational energies are higher.The working frequency of our present apparatus increases to 10 kHz,since a delay-line detector (DLD80 from Roentdek) is applied [5].Using the off-line data analyses,a central-sliced ion velocity image can be easily obtained from the whole Newton sphere of ionic products [5].
The high-purity (99.99%) argon gas was used to produce reactant Ar+ions.And the liquid samplestrans-/cis-C2H2Cl2(99.7%) were further purified with several freeze-pump-thawed cycles in this work.As mentioned in our previous study,a statistical ratio of the two spin states (2P3/2:2P1/2) of reactant Ar+ion was about 2:1 in the electron impacts at 30 eV.The kinetic energy (or velocity) and its spread of the reactants were determined before the ion-molecule collision experiments.During the experiments,the reaction chamber was maintained at a steady vacuum condition of 5.6×10-7Torr.At theEc.m.of 3.82,5.38,and 9.33 eV fortrans-C2H2Cl2(2.17 and 4.05 eV forcis-C2H2Cl2),the whole Newton spheres of the product C2H2Cl+,C2HCl+,and C2H2Cl2+ions were recorded by VMI system.It should be noted that the isotopic molecules C2H235Cl2,C2H235Cl37Cl,and C2H237Cl2have the intensity ratio of about 9:6:1,and theseEc.m.values are given for the major isotopic species and the minors are slightly different.Here theEc.m.values are given for the major isotopic one.
III.RESULTS AND DISCUSSION
A.C2H2Cl+ and C2HCl+ from the DCE reactions of Ar+ and trans-C2H2Cl2
The time-sliced (at the equatorial plane of Newton sphere) velocity images of C2H2Cl+and C2HCl+are plotted within the center-of-mass coordinate and shown inFIG.1.The red circles denote the velocity sphere of the parent ion C2H2Cl2+produced in the energy-resonant CT reaction,i.e.,the velocity sphere of the target moleculetrans-C2H2Cl2.In the mass spectra,we identified the35,37Cl-isotopic yields.The time-sliced velocity images of37Cl-products (C2H237Cl+,C2H37Cl+,and C2H235Cl37Cl+ions) are almost the same as the corresponding35Cl-products (C2H235Cl+,C2H35Cl+,and C2H35Cl2+ions),thus we pay more attention to the reactions leading to the35Cl-products and the isotopic effect will not be considered.
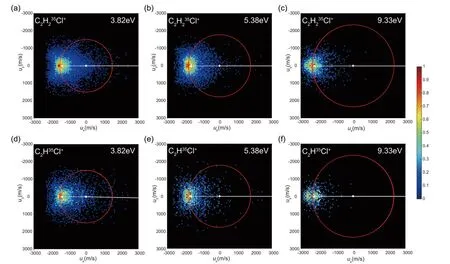
FIG.1 Time-sliced images of C2H235Cl+ (upper) and C2H35Cl+ (bottom) produced in the DCE reactions between Ar+and trans-C2H2Cl2 at the center-of-mass collision energies of 3.82,5.38,and 9.33 eV.The red circles embedded on each image indicate the velocity position of the parent ion C2H235Cl2+produced in the energy-resonant CT from Ar+(2P3/2) to trans-C2H235Cl2.The white arrows represent the velocities of Ar+ (right pointing,backward scattering,here the velocities are too large to show all in the images within the scale) and trans-C2H2Cl2 (left pointing,forward scattering) in the centerof-mass (a white point in the image center) coordinate.
FIG.1shows that most of C2H2Cl+/C2HCl+yields are scattered forward and the velocities spread around the velocity oftrans-C2H2Cl2.These ions are produced by chlorine-/hydrochlorine-elimination of the parent ion which is formed by the resonant CT in the large impact-parameter collision.In this cascade process,the internal energy of the predissociative parent ion is gained from IE(Ar+),i.e.,trans-C2H2Cl2+inE2Au,F2Ag,andG2Bustates [14] is formed via an energy-resonant CT from Ar+totrans-C2H2Cl2.Note that repulsiveE2Au state induces the remarkable elongation of the C-Cl bond and highly results in the fragmentation to C2H2Cl++Cl [14,19].On the other hand,the diffuse distributions of these ionic yields indicate the slow elimination processes,possibly experiencing several molecular rotational periods oftrans-C2H2Cl2+prior to the dissociation.Nevertheless,one can find some ionic signals along the collision axis,even around the center-ofmass at 3.82 eV.The dissociation process in the small impact-parameter collision should be responsible for these yields.The predossociativetrans-C2H2Cl2+may be dragged back due to the charge(Ar+)-induced dipole(trans-C2H2Cl2) attraction before the CT,which is somewhat analogous to that in the DCE reaction of Ar+with O2[11].This intimate collision also looks like the process experiencing a transient complex.
As shown inFIG.2(a) and (b),the collision-energy dependence of the C2H2Cl+/C2HCl+distribution can be found.In contrast to the aggregation of the spatial distributions with theEc.m.value increasing (fromFIG.1(a) to (c) or (d) to (f)),the kinetic energies of C2H2Cl+/C2HCl+(FIG.2(a) and (b)) indicate the expanding distributions.This difference arises from that the image sizes inFIG.1are scaled independently with pixel units while the kinetic energy distributions inFIG.2(a,b) are plotted in eV.The fast fragmentations after the resonant CT (shown with the dashed lines inFIG.2(a,b) result in the highest intensities of C2H2Cl+/C2HCl+.Moreover,the wider profile of the kinetic energy distribution observed at the higherEc.m.value (e.g.,9.33 eV) should be attributed to the more available energy of the slow fragmentations.As for the fast fragmentation,the collision-energy dependent angular distributions of C2H2Cl+/C2HCl+shown inFIG.2(c,d) indicate that such a fast fragmentation could be accelerated as theEc.m.increases.

FIG.2 Energy distributions of the DCE product C2H235Cl+ (a) and C2H35Cl+ (b) from trans-C2H2Cl2 at the center-ofmass collision energies of 3.82,5.38,and 9.33 eV.The products distributing within the range of scattering angle θ=±10°are selected for plotting.The dashed lines denote the energy-resonant CT position (corresponding to the red circles in FIG.1).Angular distributions of the product C2H235Cl+ (c) and C2H35Cl+ (d) in the velocity range of ±500 m/s centering on the energy-resonant CT velocity.
As for the fragment ion C2HCl+,there are two possible channels: one is the loss of HCl,which is thermochemically favored (the dissociative photoionization onset for HCl loss is about 11.9 eV) even in the comparison with the Cl-loss way (the dissociative photoionization onsets for Cl loss are 12.086 and 12.102 eV for thetrans-andcis-C2H2Cl2isomers,respectively) [17];the other is the sequential or concerted Cl and H losses from C2H2Cl2+in high-lying excited states.In the iPEPICO study of three isomers of C2H2Cl2,the breakdown diagram indicated that the latter C2HCl+could be produced only as the photon energy reached 17 eV [17].In the present work,if C2HCl+comes from the sequential dissociation,the translational-to-internal energy transfer must be efficient enough,i.e.,reserving the internal energy about 1.3 eV over the ionization energy IE(Ar+).Then the sequential or concerted dissociation likely makes the heaviest fragment C2HCl+closer to the center of mass.However,the efficient energy transfer is usually accomplished via an intimate collision instead of the long-range interaction,which possibly leads to the diffuser velocity distribution of C2HCl+.Therefore,this sequential or concerted dissociation mechanism should be unfavorable while C2HCl+may be produced by the HCl elimination.The latter mechanism is also applicable to elucidate that the velocity images of C2HCl+show the features almost same as those of C2H2Cl+.
B.C2H2Cl+ and C2HCl+ from the DCE reactions of Ar+and cis-C2H2Cl2
As observed in the case oftrans-C2H2Cl2,C2H2Cl+is also the predominant product of the targetcis-C2H2Cl2.Furthermore,FIG.3shows that the velocity images of C2H2Cl+and C2HCl+fromcis-C2H2Cl2are also similar to each other.Two pathways leading to C2H2Cl+have been proposed fortrans-C2H2Cl2,i.e.,the fast fragmentations leading to the ball-like distribution around the target and the slow fragmentations responsible for the products along the collision axis and around the center of mass.These two pathways are also observed inFIG.3.

FIG.3 Time-sliced images of C2H235Cl+ (upper) and C2H35Cl+ (bottom) produced in the DCE reactions between Ar+and cis-C2H2Cl2 at the center-of-mass collision energies of 2.17 and 4.05 eV.The red circles embedded on each image indicate the velocity position of the parent ion C2H235Cl2+ product in the energy-resonant charge transfer from Ar+(2P3/2) to cis-C2H235Cl2.The white arrows represent the velocities of Ar+ (right pointing,backward scattering,the velocities are too large to show all in the images within the scale at 4.05 eV) and cis-C2H2Cl2 (left pointing,forward scattering) in the centerof-mass (a white point in the image center) coordinate.
The kinetic energy distributions of C2H2Cl+and C2HCl+yields fromcis-C2H2Cl2are plotted inFIG.4(a) and (b).The profile change with the increase ofEc.m.is also analogous to that shown inFIG.2(a)and (b).The kinetic energy distribution of C2H2Cl+at 4.05 eV is much broader than that at 2.17 eV.The angular distributions of these two ionic fragments exhibit inFIG.4(c) and (d),also indicating the trend observed inFIG.2(c) and (d).Therefore,we can conclude that there are the same DCE dynamics fortrans-C2H2Cl2andcis-C2H2Cl2.
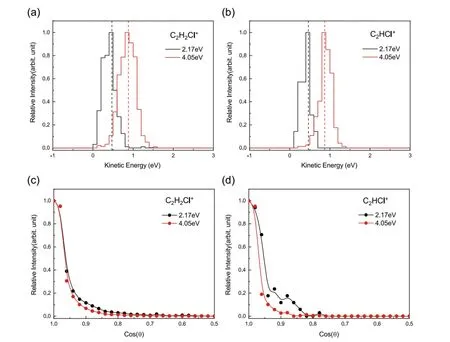
FIG.4 Energy distributions of the DCE product C2H235Cl+ (a) and C2H35Cl+ (b) from cis-C2H2Cl2 at the center-of-mass collision energies of 2.17 and 4.05 eV.The products distributing within the range of scattering angle θ=±10° are selected for plotting.The dashed lines denote the energy-resonant CT position (corresponding to the red circles in FIG.3).Angular distributions of the product C2H235Cl+ (c) and C2H35Cl+ (d) in the velocity range of ±500 m/s centering on the energyresonant CT velocity.
In our work,the branching ratios of C2H2Cl+to C2HCl+fragments in the DCE reaction of Ar+withtrans-C2H2Cl2andcis-C2H2Cl2at several electron volts are about 3,having no significant differences from those in the ion-molecule reactions at thermal collision energy carried out by Mikhailov and coworkers [18] and also those in the dissociative photoionization process done with iPEPICO [17].
C.C2HCl2+ from the DCE reactions of Ar+ and trans-C2H2Cl2
Among the DCE reactions of dichloroethene,the Helimination is highly unfavorable and only the images of C2HCl2+were recorded fortrans-C2H2Cl2and shown inFIG.5.Since the H atomic mass is too small to influence the momentum of the much heavier C2HCl2+,C2HCl2+shows the velocity distribution or image which is almost the same as the parent cation C2H2Cl2+formed via a CT process (FIG.1in Ref.[14]).Again,the energy-resonant CT in the large impact-parameter collision plays an important role in the dehydrogenated process.The angular distributions inFIG.6show the similar forward-scattering feature,except for that at 3.11 eV.This abnormal case was also observed in the pure CT process [14],possibly owing to the rainbow scattering with the large impact parameter in the classical scattering theory [20].

FIG.5 Time-sliced images of C2H35Cl2+ produced in the DCE reactions between Ar+ and trans-C2H2Cl2 at the center-ofmass collision energies of 2.13 eV (a),3.11 eV (b),4.27 eV (c),and 9.33 eV (d).The red circles,white arrows and white point embedded on each image indicate the same as FIG.1.
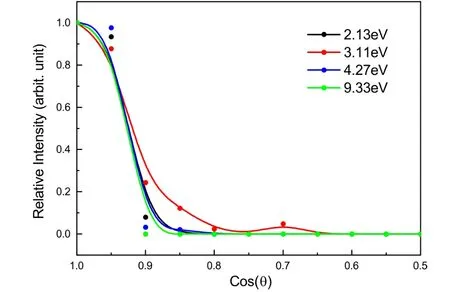
FIG.6 Angular distributions of the product C2H35Cl2+ in the DCE reactions between Ar+ and trans-C2H2Cl2 at the center-of-mass collision energies of 2.13 eV,3.11 eV,4.27 eV,and 9.33 eV.
IV.CONCLUSION
Here we report the experiment study of the DCE reactions between Ar+andtrans-/cis-C2H2Cl2in 2-10 eV collision energy range,using our crossed-beam VMI apparatus.Three dissociation channels such as the Cl-,HCl-and H-eliminations have been investigated.The predominated ionic yield C2H2Cl+can be generated through two pathways: the major dissociation pathway through the energy-resonant process in the large impactparameter collision and the minor one experiencing the strong interaction process in the small impact-parameter collision.The latter leads to the distribution along the collision axis near the center-of-mass with strong energy dependence.C2HCl+is proposed to be the yield by the HCl elimination instead of the Cl and H losses.The mechanism about the energy-resonant CT in the large impact-parameter collision is also essential in the production of C2H2Cl2+.Moreover,we find that isomer effect is negligible in the DCE reaction of Ar+withtrans-C2H2Cl2andcis-C2H2Cl2.
V.ACKNOWLEDGMENTS
This work is supported by the National Natural Science Foundation of China (No.22003062 and No.21625301) and the Chinese Academy of Sciences(No.YZ201565).
 CHINESE JOURNAL OF CHEMICAL PHYSICS2023年5期
CHINESE JOURNAL OF CHEMICAL PHYSICS2023年5期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Minimum-Modified Debye-Hückel Theory for Size-Asymmetric Electrolyte Solutions with Moderate Concentrations
- Quantum Dynamics Calculations on Isotope Effects of Hydrogen Transfer Isomerization in Formic Acid Dimer
- Design Strategy of Infrared 4-Hydroxybenzylidene-imidazolinone-Type Chromophores based on Intramolecular Charge Transfer: a Theoretical Perspective
- Photothermal Catalytic Selective Oxidation of Isobutane to Methacrylic Acid over Keggin-Type Heteropolyacid
- Controllable Modulation of Morphology and Property of CsPbCl3 Perovskite Microcrystals by Vapor Deposition Method
- On-the-Fly Nonadiabatic Dynamics of Caffeic Acid Sunscreen Compound
