黄芪-当归配伍对小鼠动脉粥样硬化模型血管损伤的影响*
李菀榆, 龙清吟, 傅馨莹, 马露, 谭维, 李艳玲,徐顺洲, 张伟, 邓常清
黄芪-当归配伍对小鼠动脉粥样硬化模型血管损伤的影响*
李菀榆, 龙清吟, 傅馨莹, 马露, 谭维, 李艳玲,徐顺洲, 张伟△, 邓常清△
(湖南中医药大学中西医结合学院,中西医结合心脑疾病防治湖南省重点实验室,湖南 长沙 410208)
从血管壁细胞凋亡和焦亡方面来探讨黄芪-当归配伍对小鼠动脉粥样硬化(atherosclerosis, AS)模型血管壁细胞损伤的影响。将雄性载脂蛋白E基因敲除(apolipoprotein E gene knockout,-/-)小鼠随机分为模型组、黄芪-当归配伍低剂量组、黄芪-当归配伍中剂量组、黄芪-当归配伍高剂量组和阿托伐他汀组,以同周龄雄性C57BL/6J小鼠为对照组,每组5只。除对照组外均给予高脂饲料喂养12周构建AS模型,模型成功后灌胃给予药物。HE染色观察主动脉内膜增生厚度;全自动生化分析仪检测总胆固醇(total cholesterol, TC)、甘油三酯(triglyceride, TG)、低密度脂蛋白胆固醇(low-density lipoprotein cholesterol, LDL-c)及高密度脂蛋白胆固醇(high-density lipoprotein cholesterol, HDL-c)水平;ELISA法检测血浆白细胞介素1β(interleukin-1β, IL-1β)和IL-18含量;免疫荧光法、RT-qPCR法及Western blot法检测血管壁细胞凋亡和焦亡相关因子B细胞淋巴瘤蛋白2(B-cell lymphoma-2, Bcl-2)、Bcl-2关联X蛋白(Bcl-2-associated X protein, Bax)、活化的胱天蛋白酶3(cleaved caspase-3)、核苷酸结合寡聚结构域样受体蛋白3(nucleotide-binding oligomerization domain-like receptor protein 3, NLRP3)、cleaved caspase-1和gasdermin D-N(GSDMD-N)的mRNA及蛋白表达,评价药物对AS病变血管壁细胞损伤的作用。高脂喂养可成功建立AS模型。与对照组比较,模型组血浆TC、TG和LDL-c含量显著升高(<0.01),HDL-c含量显著降低(<0.01),血管内膜增厚(<0.01),血浆IL-1β和IL-18含量显著增加(<0.01),血管壁细胞Bax、cleaved caspase-3、NLRP3、cleaved caspase-1和GSDMD-N表达增加(<0.01),Bcl-2表达降低(<0.01)。与模型组比较,黄芪-当归配伍可降低血浆TC、TG和LDL-c含量(<0.05),升高血浆HDL-c含量(<0.05),减轻血管内膜增生程度(<0.01),降低血浆IL-1β和IL-18含量(<0.05),抑制血管壁细胞Bax、cleaved caspase-3、NLRP3、cleaved caspase-1和GSDMD-N表达(<0.01),上调血管壁细胞Bcl-2表达(<0.01)。以黄芪-当归配伍中剂量作用最显著。黄芪-当归配伍能延缓小鼠AS病变,减轻机体炎症反应,抑制小鼠血管壁细胞凋亡和焦亡,其机制与抑制血管壁细胞凋亡和焦亡相关因子激活有关。
动脉粥样硬化;黄芪-当归配伍;-/-小鼠;细胞凋亡;细胞焦亡
心血管疾病(cardiovascular disease, CVD)是严重危害人类健康的疾病[1]。动脉粥样硬化(atherosclerosis,AS)是心血管疾病的病理基础,其发病机制与脂质代谢紊乱、氧化应激和血管壁免疫炎症反应、血管壁细胞损伤等存在联系[2-3]。通过抑制AS的血管壁损伤,对维护血管稳态、抑制斑块形成、促进斑块稳定具有重要意义。近年来,免疫炎症反应和血管壁细胞损伤(细胞凋亡、细胞焦亡)被证实贯穿于AS发生发展的始终,是AS的重要病理生理机制,已成为防治AS的新切入点[4-7]。目前通过调控细胞凋亡、焦亡和免疫炎症反应来防治AS的药物大多处于实验阶段,单一靶点的过度抑制往往有导致免疫功能下降等风险,而中医药多成分、多靶点的作用特点可有效调控细胞凋亡、焦亡和免疫炎症反应,已成为中医药防治心血管疾病新的治疗方向。黄芪、当归作为益气活血的经典药对,可调节血脂、抗氧化、保护血管内皮细胞等,具有良好的抗动脉粥样硬化作用[8]。课题组前期研究表明,黄芪和当归在一定比例范围配伍时具有抑制血管内膜增生及血管局部炎性反应的作用,其中以芪归1∶1配伍的作用为佳[9-10]。由于AS是心脑血管疾病的重要病理基础,而血管壁细胞损伤和免疫炎症反应是AS发生发展的重要病理生理机制,因此,我们推测益气活血药对黄芪-当归配伍可能通过影响血管壁炎症反应、调节血管壁细胞凋亡和焦亡,发挥改善血管病变的作用。因此,本研究利用载脂蛋白E基因敲除(apolipoprotein E gene knockout,-/-)小鼠AS模型,从细胞凋亡和焦亡角度研究了黄芪-当归配伍(-combination, AA)对小鼠AS血管壁损伤的影响,以进一步明确其改善AS血管病变的作用机制,为其临床合理应用提供实验依据。
材料和方法
1 实验材料
1.1动物4~6周龄SPF级雄性ApoE-/-小鼠,体质量18~22 g,每组5只,共25只;4~6周龄SPF级雄性C57BL/6J小鼠,体质量18~22 g,共5只;购自北京华阜康生物科技股份有限公司,动物合格证号为SCXK(京)2019-0008。动物均饲养于湖南中医药大学SPF级实验动物中心,环境昼夜交替12 h,温度(22±2) ℃,湿度50%±10%。动物实验经湖南中医药大学实验动物伦理委员会批准(伦理审核批号为LL2022010401)。
1.2动物饲料高脂饲料(3%胆固醇、0.5%胆酸钠、2%丙基硫氧嘧啶、5%白糖、10%猪油、5%牛奶、76.3%普通饲料)购于北京科奥协力饲料有限公司,许可证号为SCXK(京)2019-0003。普通饲料购于湖南嘉泰实验动物有限公司,许可证号为SCXK(湘)2020-0006。
1.3药物根据我们以往的研究结果[9],本研究黄芪和当归以1∶1配伍,分为低、中、高3个剂量组。中药饮片购自湖南中医药大学第一附属医院并由左亚杰教授鉴定,黄芪为豆科植物蒙古黄芪(Fisch.) Bge. Var. mongholicus (Bge.) Hsiao或膜荚黄芪(Fisch.) Bge.的干燥根,当归为伞形科植物当归(Oliv.) Diels的干燥根。阿托伐他汀(atorvastatin, ATV)片(批号H20133127)购自浙江乐普药业股份有限公司。
1.4主要试剂苏木素染液、伊红染色液购自北京中杉金桥生物技术有限公司;白细胞介素1β(interleukin-1β, IL-1β)和IL-18 ELISA试剂盒购自上海茁彩生物科技股份有限公司;B细胞淋巴瘤蛋白2(B-cell lymphoma-2, Bcl-2)兔抗小鼠多克隆抗体和Bcl-2关联X蛋白(Bcl-2-associated X protein, Bax)兔抗小鼠多克隆抗体购自武汉三鹰生物技术有限公司;cleaved caspase-3兔抗小鼠多克隆抗体和核苷酸结合寡聚结构域样受体蛋白3(nucleotide-binding oligomerization domain-like receptor protein 3, NLRP3)兔抗小鼠多克隆抗体购自江苏亲科生物研究中心有限公司;caspase-1兔抗小鼠多克隆抗体和gasdermin D (GSDMD)兔抗小鼠多克隆抗体购自Cell Signaling Technology;山羊抗免IgG和BCA蛋白测定试剂盒购自武汉伊莱瑞特生物科技有限公司;RNA提取试剂盒购自北京天根生化科技有限公司;逆转录试剂盒和PCR扩增试剂盒购自上海近岸科技有限公司。
1.5仪器全自动生化分析仪(型号Chemray 800,深圳雷杜生命科技有限公司);多功能酶标仪(型号Cytation3,BioTek);Gel DocXR+凝胶成像系统(型号Chemi DocTM XRS+)、Mini-PROTEAN Tetra型电泳槽(型号1658001)和Mini Trans-Blot型转印槽(型号1703939)均为Bio-Rad产品。
2 方法
2.1药物制备参考课题组前期方法[11],取黄芪和当归1∶1配伍药材,分别加8倍和6倍量水浸泡后,煎煮2次(2和1.5 h),合并2次滤液,参考课题组前期给药剂量[9-10],将药液以旋转蒸发仪浓缩成低剂量浓度为0.23 g/mL(生药),中剂量浓度为0.46 g/mL(生药),高剂量浓度为0.92 g/mL(生药)。ATV用时以生理盐水制备成混悬液使用。
2.2动物分组及处理-/-小鼠以高脂饲料喂养12周,建立AS动物模型[12]。C57BL/6J小鼠作为对照组,共5只,普通饲料喂养12周。AS模型制备成功后,采用随机数表法分组,分为模型(model)组、黄芪-当归配伍低剂量(low-dose AA, AA-L)组、黄芪-当归配伍中剂量(medium-dose AA, AA-M)组、黄芪-当归配伍高剂量(high-dose AA, AA-H)组和ATV组,每组各5只。对照(control)组继续喂养普通饲料,并灌胃等量生理盐水;其余各组继续予高脂饲料喂养,同时灌胃药物,每天一次,连续4周,给药体积为0.01 mL·g-1·d-1。动物分组和给药剂量见表1。
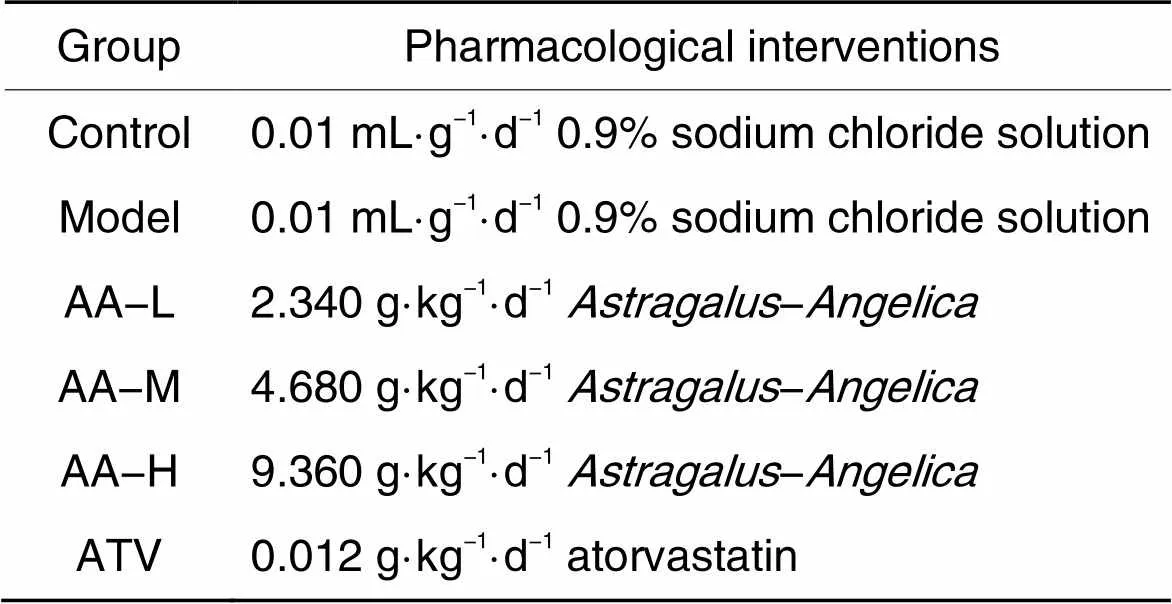
表1 动物分组及药物干预
2.3标本采集实验完成后用2%戊巴比妥钠腹腔注射麻醉小鼠。腹主动脉采血置含EDTA-K2抗凝剂的试管中混匀,4 ℃、900×离心15 min,取上层血浆-80 ℃保存待测。然后血管内灌注生理盐水至流出液清亮,取主动脉根部至髂总动脉分支处血管,用4%多聚甲醛固定主动脉弓部,余下部分-80 ℃保存。
2.4HE染色法评价小鼠主动脉病变石蜡切片,按说明书HE染色,显微镜下采集图片。用显微图像分析软件(ImageJ)测量后,参考课题组前期方法[13]及文献方法[14]计算主动脉内膜面积(intimal area, IA)、内膜增生率(hyperplasia rate of intimal area, HRIA)和内膜厚度增生率(hyperplasia rate of intimal thickness, HRIT)。
2.5血脂水平检测用全自动生化分析仪检测血浆总胆固醇(total cholesterol, TC)、甘油三酯(triglyceride, TG)、低密度脂蛋白胆固醇(low-density lipoprotein cholesterol, LDL-c)及高密度脂蛋白胆固醇(high-density lipoprotein cholesterol, HDL-c)含量。操作按说明书。
2.6ELISA法检测小鼠血浆IL-1β和IL-18含量操作按说明书指引进行。
2.7免疫荧光法检测主动脉凋亡和焦亡相关蛋白cleaved caspase-3和NLRP3表达主动脉固定后,进行组织免疫荧光染色:石蜡切片脱蜡至水,抗原修复后封闭,加入cleaved caspase-3(1∶200)和NLRP3(1∶2 000)Ⅰ抗4 ℃孵育过夜,Ⅱ抗避光室温孵育50 min,DAPI避光室温孵育10 min复染细胞核,封片。荧光显微镜观察,采用ImageJ软件1.5.3分析cleaved caspase-3和NLRP3的荧光强度,以单位面积荧光强度表示cleaved caspase-3和NLRP3的相对表达强度。
2.8RT-qPCR法检测主动脉中Bax、Bcl-2及NLRP3 mRNA表达提取小鼠主动脉总RNA并测其浓度和纯度,逆转录成cDNA,采用SYBR法进行实时荧光定量PCR测定。引物由Sangon Biotech设计和合成,详细序列见表2。
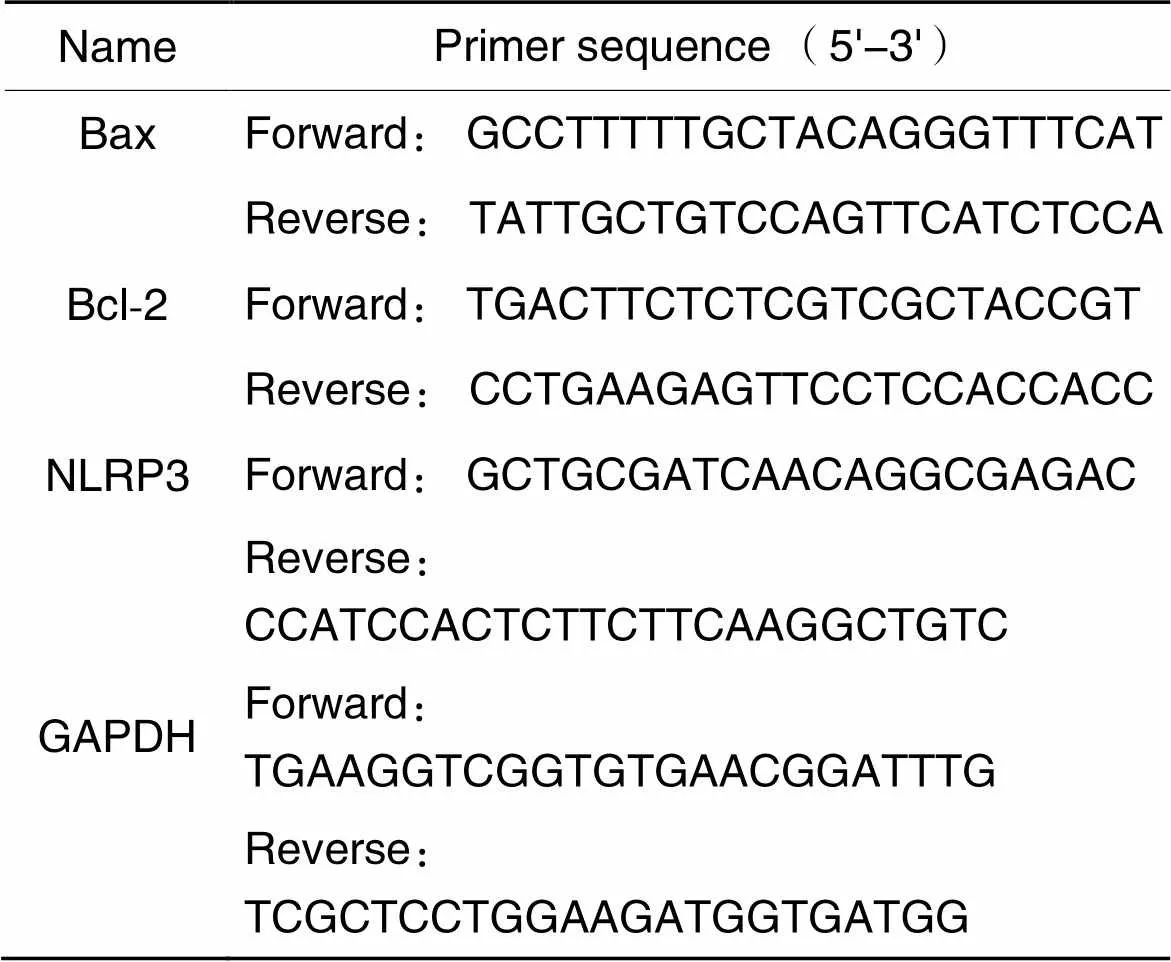
表2 引物序列
2.9Western blot法检测主动脉Bax、Bcl-2、cleaved caspase-3、NLRP3、cleaved caspase-1及GSDMD-N蛋白表达提取主动脉蛋白,BCA法测其浓度,变性后经电泳,转膜,封闭,分别加入β-actin(1∶8 000)、Bax(1∶4 000)、Bcl-2(1∶2 000)、cleaved caspase-3(1∶1 000)、NLRP3(1∶1 000)、caspase-1(1∶1 000)、GSDMD(1∶1 000)Ⅰ抗,4 ℃孵育过夜;Ⅱ抗Goat Anti-Rabbit IgG(1∶10 000)37 ℃孵育60 min;用Image Lab 4.0软件对蛋白条带进行相对光密度()定量分析。cleaved caspase-1以cleaved caspase-1/pro-caspase-1的比值进行定量,GSDMD-N以GSDMD-N/GSDMD的比值进行定量,其余以目的蛋白/内参β-actin的比值对目的蛋白表达进行定量。
3 统计学处理
实验数据以均数±标准差(mean±SD)表示,用SPSS 23.0软件统计分析。先进行正态性检验和方差齐性检验,如为正态分布、方差齐,先进行单因素方差分析,再以LSD法进行组间两两比较;方差不齐者用Dunnett's T3检验。以<0.05为差异有统计学意义。
结果
1 黄芪-当归配伍对主动脉病变的影响
正常对照组主动脉内膜完整,中层平滑肌细胞排列整齐,内膜下未见泡沫细胞;模型组主动脉内膜增厚,平滑肌细胞增殖且排列紊乱,内膜下脂质沉积并见大量泡沫细胞,IA、HRIA和HRIT显著增加(<0.01);黄芪-当归配伍干预后可减轻主动脉内膜增生程度、泡沫细胞沉积及脂质沉积,与模型组比较,芪归低、中、高剂量组IA、HRIA及HRIT降低(<0.05),见图1。
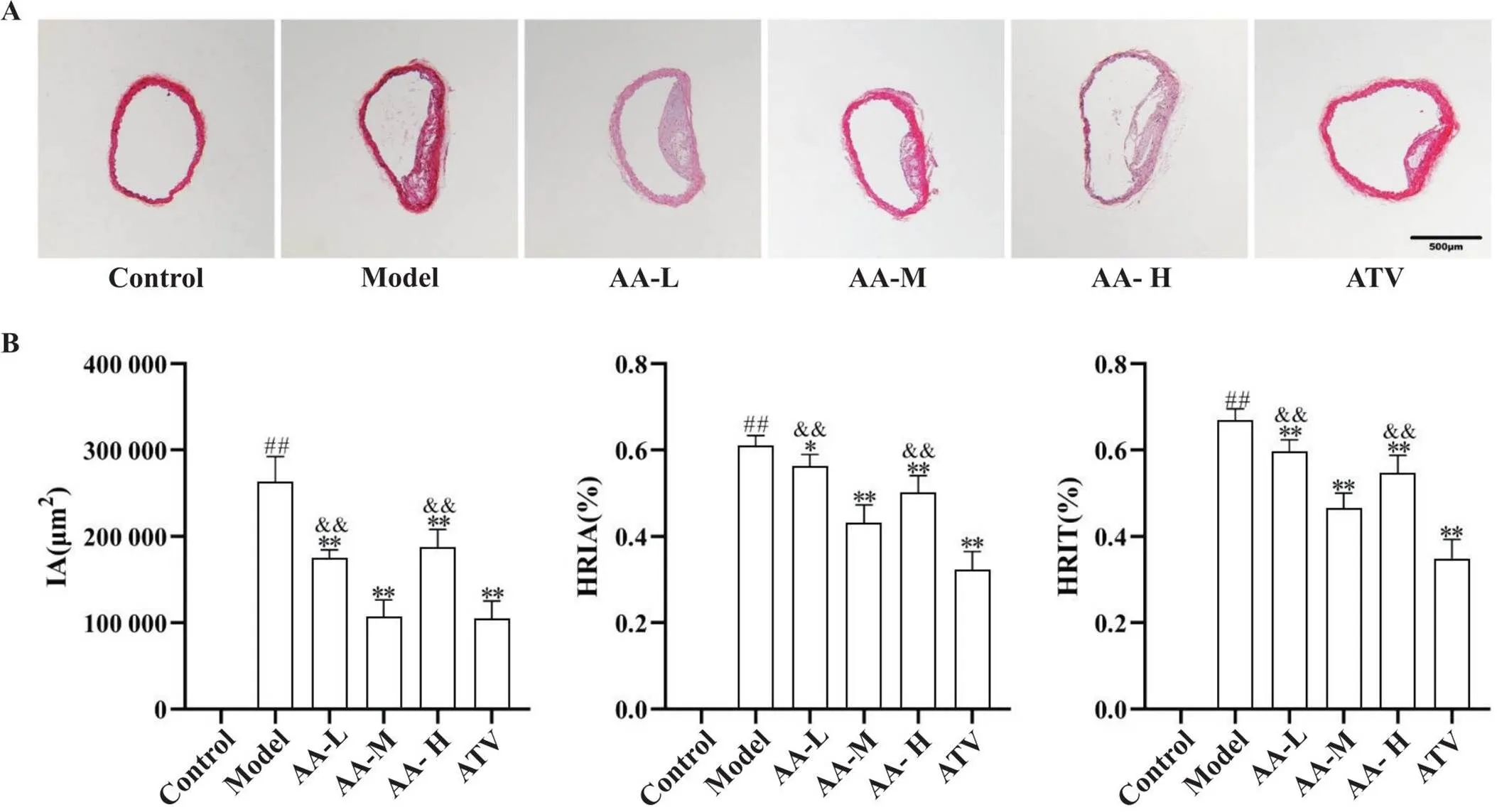
Figure 1. Comparison of HE staining of aorta and aortic IA, HRIA and HRIT. A: aortic HE staining (scale bar=500 µm); B: aortic IA, HRIA and HRIT. Compared with model group, IA,HRIA and HRIT were reduced in low-, medium- and high-dose Astragalus-Angelica(AA-L, AA-M and AA-H) groups and atorvastatin (ATV) group. Mean±SD. n=5. ##P<0.01 vs control group;*P<0.05,**P<0.01 vs model group;&&P<0.01 vs AA-M group.
2 黄芪-当归配伍对血脂的影响
与正常对照组比较,模型组血浆TC、TG和LDL-c含量均显著升高(<0.01),HDL-c含量显著降低(<0.01);与模型组比较,黄芪-当归配伍中剂量组和阿托伐他汀组TC、TG和LDL-c均显著降低(<0.05),黄芪-当归配伍中、高剂量组HDL-c显著增加(<0.05),见图2。
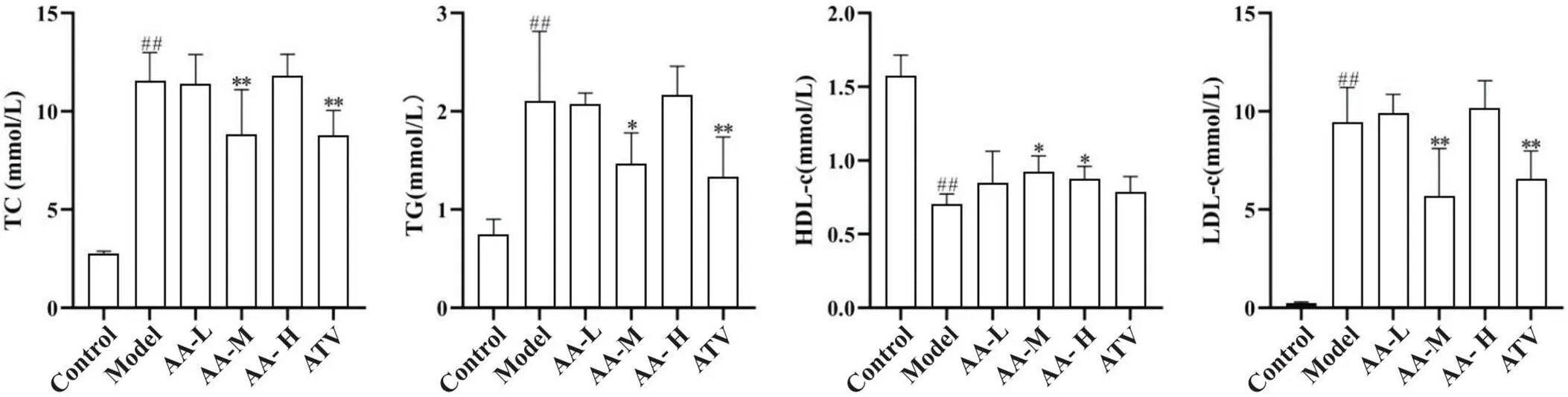
Figure 2. Comparison of plasma TC, TG, HDL-c and LDL-c levels among groups. Compared with model group, TC, TG and LDL-c were significantly lower in medium-dose Astragalus-Angelica(AA-M) and atorvastatin (ATV) groups, and HDL-c was significantly increased in AA-M and high-dose Astragalus-Angelica(AA-H) groups. Mean±SD. n=5. ##P<0.01 vs control group;*P<0.05,**P<0.01 vs model group.
3 黄芪-当归配伍对血浆IL-1β和IL-18的影响
与正常对照组比较,模型组血浆IL-1β和IL-18含量显著增加(<0.01);与模型组比较,黄芪-当归配伍低、中、高剂量组和阿托伐他汀组血浆IL-1β含量均降低(<0.01),黄芪-当归配伍低、中剂量组和阿托伐他汀组血浆IL-18含量降低(<0.05);黄芪-当归配伍中剂量组血浆IL-1β含量显著低于芪归低、高剂量组(<0.01),黄芪-当归配伍中剂量组血浆IL-18含量显著低于黄芪-当归配伍低剂量组(<0.05),见图3。

Figure 3. Comparison of plasma IL-1β and IL-18 levels among groups. Compared with model group, plasma IL-1β level was reduced in low-, medium- and high-dose Astragalus-Angelica(AA-L, AA-M and AA-H) groups and atorvastatin (ATV) group, and plasma IL-18 level was reduced in AA-L, AA-M and ATV groups. Mean±SD. n=5. ##P<0.01 vs control group;*P<0.05,**P<0.01 vs model group;&P<0.05,&&P<0.01 vs AA-M group.
4 黄芪-当归配伍对主动脉细胞凋亡相关因子表达的影响
为进一步明确黄芪-当归配伍改善AS血管损伤的作用,本次用免疫荧光法测定了主动脉细胞凋亡标志物cleaved caspase-3的表达,结果见图4。与正常对照组比较,模型组cleaved caspase-3表达显著增加(<0.01);与模型组比较,黄芪-当归配伍低、中、高剂量组和阿托伐他汀组cleaved caspase-3表达减少(<0.01);黄芪-当归配伍中剂量组cleaved caspase-3表达低于黄芪-当归低、高剂量组(<0.01)。
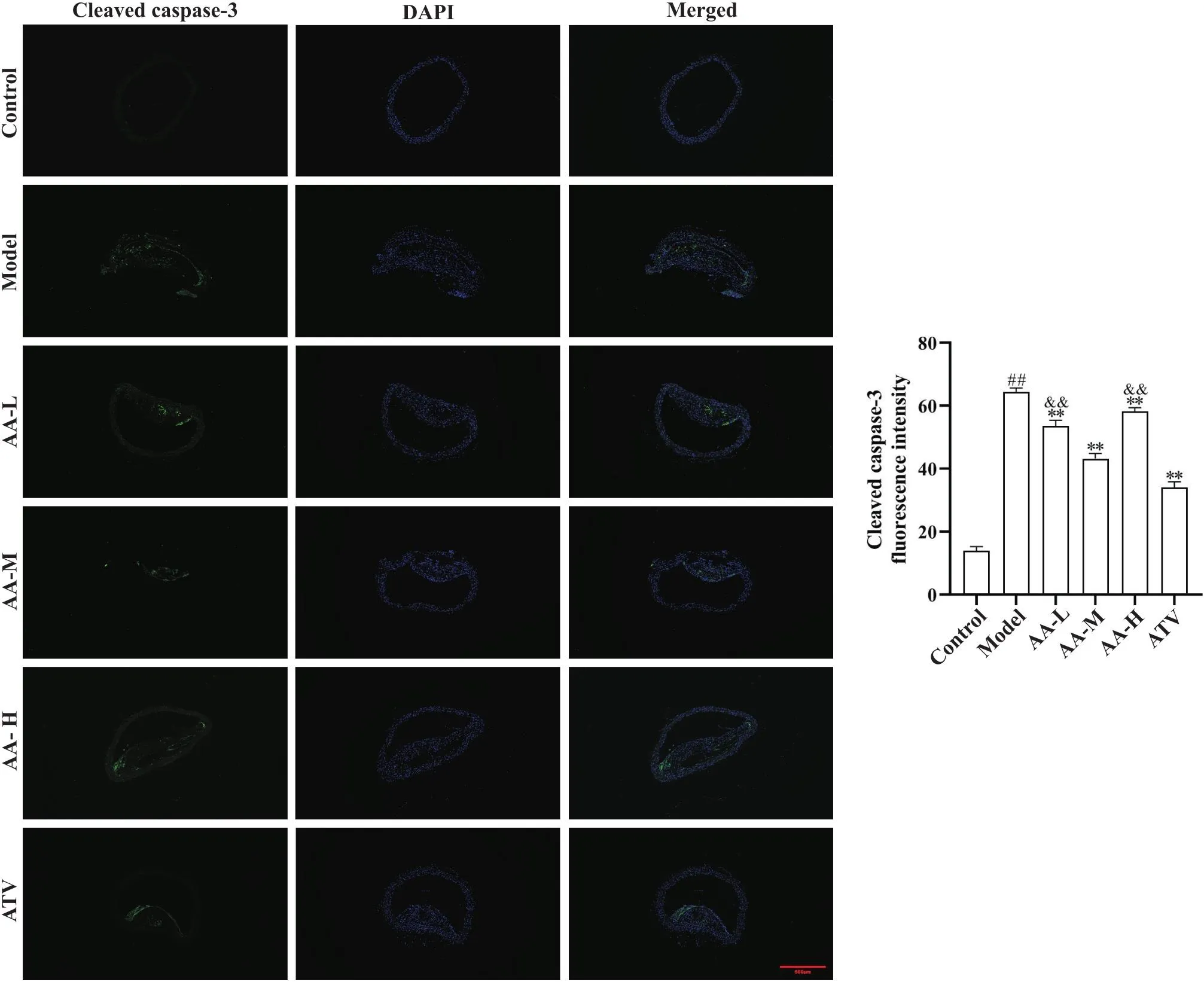
Figure 4. Comparison of aortic cleaved caspase-3 expression in each group. Aortic cleaved caspase-3 immunofluorescence expression (scale bar=500 µm) and cleaved caspase-3 average fluorescence intensity. Cleaved caspase-3 expression was reduced in low-, medium- and high-dose Astragalus-Angelica(AA-L, AA-M and AA-H) groups and atorvastatin (ATV) group compared with model group. Mean±SD. n=5. ##P<0.01 vs control group;**P<0.01 vs model group;&&P<0.01 vs AA-M group.
此外,检测主动脉细胞凋亡相关因子Bax和Bcl-2的mRNA表达后发现,与正常对照组比较,模型组Bax的mRNA表达显著增加(<0.01),Bcl-2的mRNA表达显著降低(<0.01);与模型组比较,黄芪-当归配伍低、中、高剂量组和阿托伐他汀组主动脉Bax的mRNA表达均降低(<0.01),黄芪-当归配伍配伍低、中剂量组和阿托伐他汀组主动脉Bcl-2的mRNA表达增加(<0.01);黄芪-当归配伍中剂量组对Bax和Bcl-2的效应强于黄芪-当归配伍低、高剂量组(<0.01),见图5。
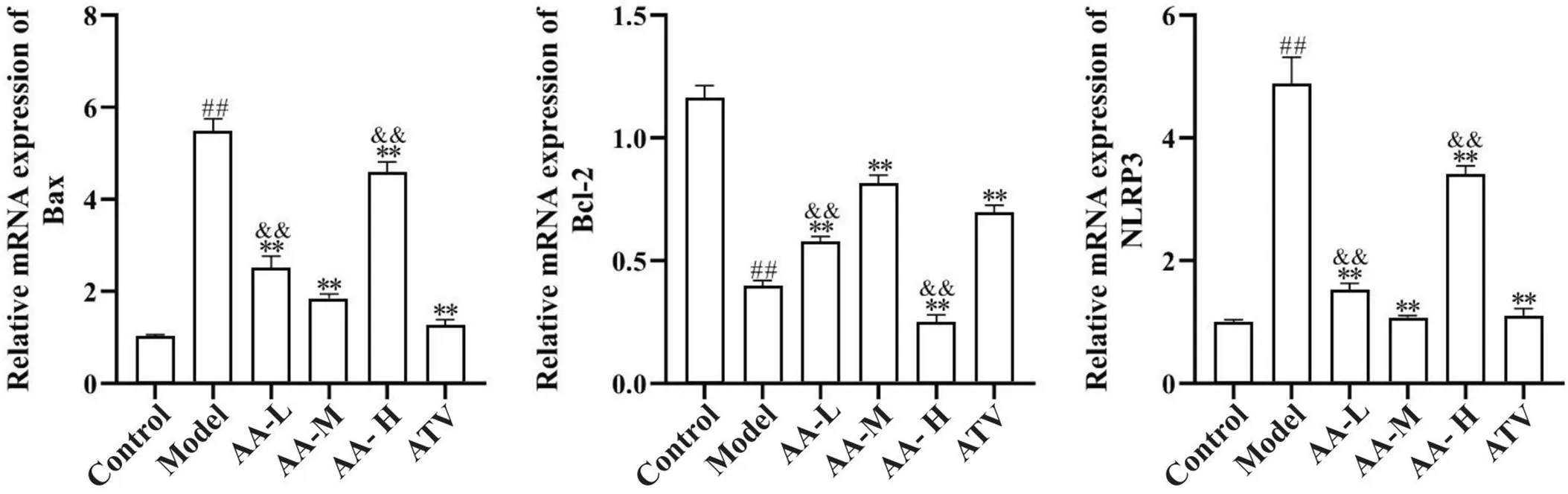
Figure 5. Comparison of aortic Bax, Bcl-2 and NLRP3 mRNA expression among groups. Compared with model group, the mRNA expression of aortic Bax and NLRP3 was decreased in low-, medium- and high-dose Astragalus-Angelica(AA-L, AA-M and AA-H) groups and atorvastatin (ATV) group, and the mRNA expression of aortic Bcl-2 was increased in AA-L, AA-M and ATV groups. Mean±SD. n=5. ##P<0.01 vs control group;**P<0.01 vs model group;&&P<0.01 vs AA-M group.
主动脉细胞凋亡相关因子Bax、Bcl-2和cleaved caspase-3的蛋白表达,结果显示,与正常对照组比较,模型组主动脉Bax和cleaved caspase-3蛋白表达均显著增加(<0.01),Bcl-2蛋白表达显著降低(<0.01);与模型组比较,黄芪-当归配伍低、中、高剂量组和阿托伐他汀组主动脉Bax和cleaved caspase-3蛋白表达均降低(<0.01),黄芪-当归配伍低、中剂量组和阿托伐他汀组Bcl-2蛋白表达增加(<0.01);黄芪-当归配伍中剂量组对Bax、Bcl-2和cleaved caspase-3蛋白表达的效应强于黄芪-当归配伍低、高剂量组(<0.01),见图6。
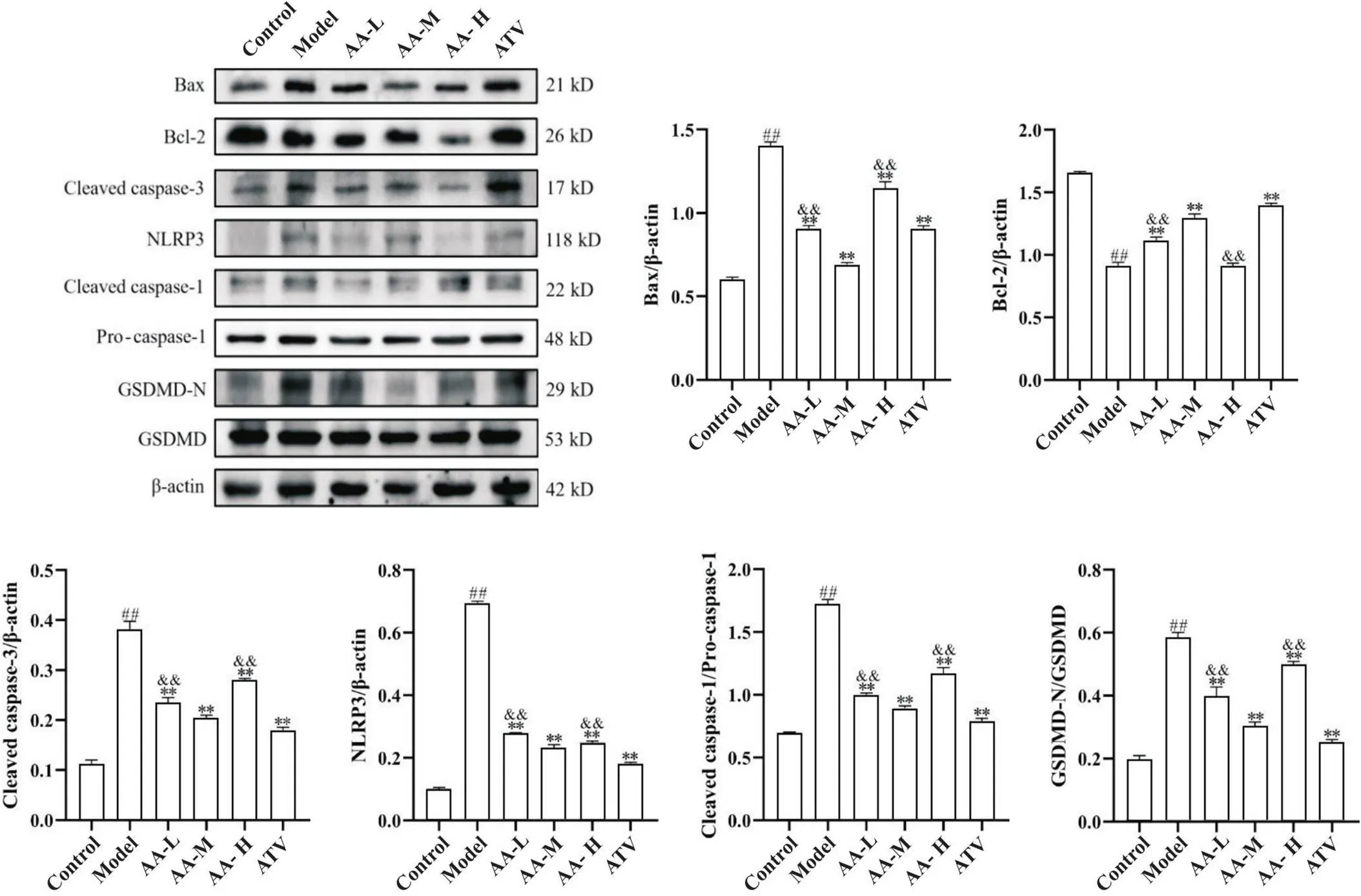
Figure 6. Comparison of aortic expression of apoptosis- and pyroptosis-related proteins among groups. Representative Western blot images and corresponding quantitative data were shown. Compared with model group, aortic protein expression of Bax, cleaved caspase-3, NLRP3, cleaved caspase-1 and GSDMD-N was decreased in low-, medium- and high-dose Astragalus-Angelica(AA-L, AA-M and AA-H) groups and atorvastatin (ATV) group, and Bcl-2 protein expression was increased in AA-L, AA-M and ATV groups. Mean±SD. n=5. ##P<0.01 vs control group;**P<0.01 vs model group;&&P<0.01 vs AA-M group.
5 黄芪-当归配伍对主动脉壁细胞焦亡相关因子表达的影响
为进一步探讨黄芪-当归配伍改善AS血管损伤的作用,我们用免疫荧光法测定了主动脉壁细胞焦亡标志物NLRP3的表达。与正常对照组比较,模型组NLRP3表达显著增加(<0.01);与模型组比较,黄芪-当归配伍低、中、高剂量组和阿托伐他汀组NLRP3表达减少(<0.01);黄芪-当归配伍中剂量组NLRP3表达低于黄芪-当归配伍低、高剂量组(<0.01),见图7。
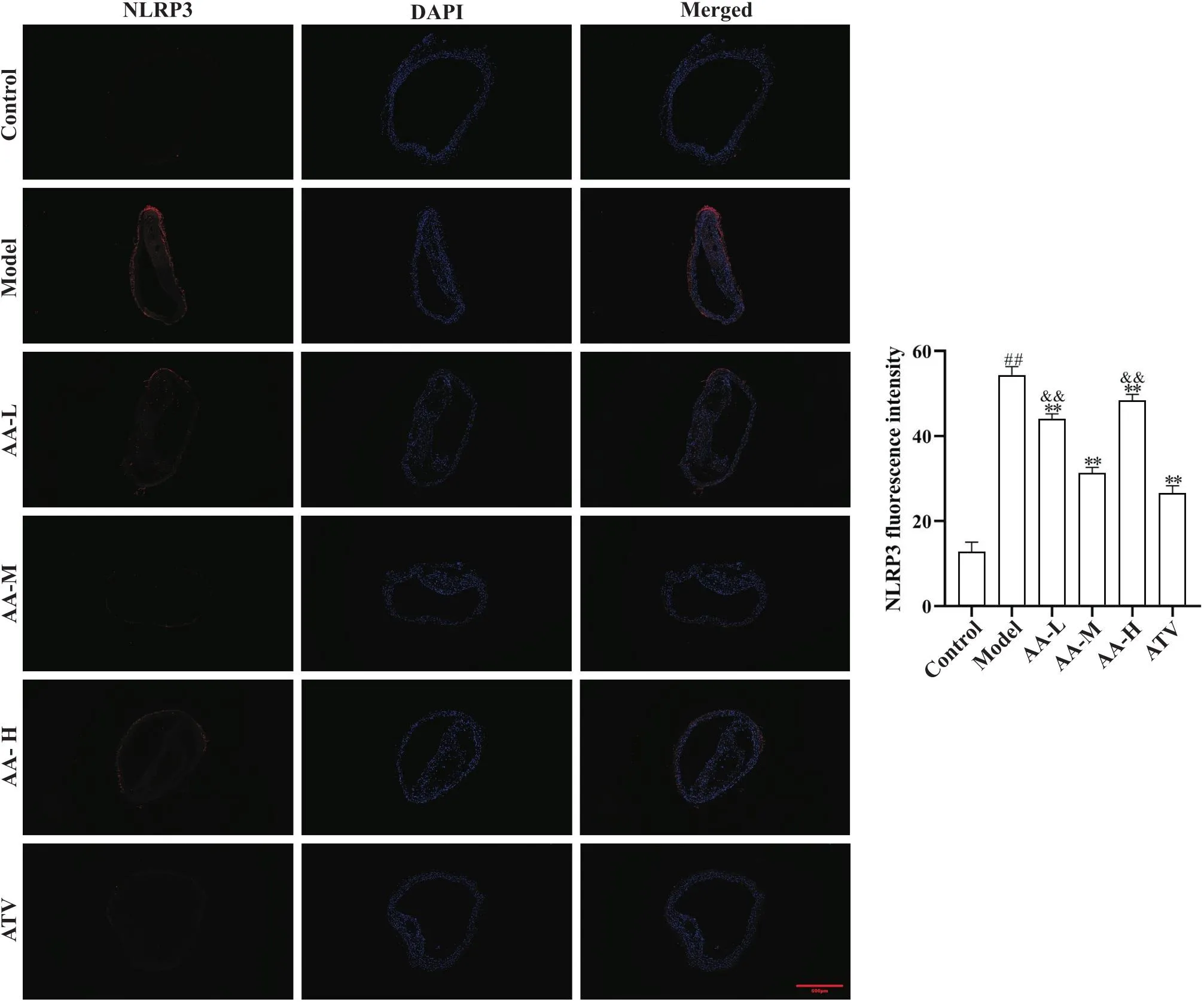
Figure 7. Comparison of aortic NLRP3 expression in each group. Aortic NLRP3 immunofluorescence expression (scale bar=500 µm) and aortic NLRP3 average fluorescence intensity. NLRP3 expression was reduced in low-, medium- and high-dose Astragalus-Angelica(AA-L, AA-M and AA-H) groups and atorvastatin (ATV) group compared with model group. Mean±SD. n=5. ##P<0.01 vs control group;**P<0.01 vs model group;&&P<0.01 vs AA-M group.
与正常对照组比较,模型组主动脉NLRP3的mRNA表达显著增加(<0.01);与模型组比较,黄芪-当归配伍低、中、高剂量组和阿托伐他汀组NLRP3的mRNA表达均降低(<0.01);黄芪-当归配伍中剂量组对NLRP3的效应强于黄芪-当归配伍低、高剂量组(<0.01),见图5。
此外,与正常对照组比较,模型组主动脉NLRP3、cleaved caspase-1和GSDMD-N蛋白表达均显著增加(<0.01);与模型组比较,黄芪-当归配伍低、中、高剂量组和阿托伐他汀组NLRP3、cleaved caspase-1和GSDMD-N蛋白表达均降低(<0.01);黄芪-当归配伍中剂量组对NLRP3、cleaved caspase-1和GSDMD-N的效应强于黄芪-当归配伍低、高剂量组(<0.01),见图6。
讨论
中医认为,心血管疾病病机为本虚标实,气虚为本,血瘀为标。气虚血瘀是AS的重要病机,益气活血法是治疗AS的主要治则之一[15]。黄芪和当归配伍是常用的益气活血药对,我们以往的研究表明,芪归配伍可抑制血管内皮损伤诱导的血管内膜增生,且与抑制血管平滑肌细胞增殖、炎症反应和细胞外基质沉积等有关[10, 16]。表明芪归配伍具有良好的改善血管功能的作用。
本研究显示AS模型组血浆TC、TG和LDL-c含量均升高,HDL-c含量降低,主动脉内膜增厚,同时血浆炎症因子IL-1β和IL-18增加,表明小鼠AS时出现AS病变,血管功能障碍,同时伴体内炎症状态。经黄芪-当归配伍治疗后,血脂改善,血管内膜增生病变减轻,血浆炎症因子水平降低。这表明黄芪-当归配伍可改善小鼠动脉粥样硬化的血管病变和体内炎症状态。
血管壁细胞凋亡和焦亡是AS发生发展的重要病理生理机制[4-7]。AS的发生发展包括血管壁脂质沉积、炎症、结构变化和细胞死亡[17-20]。凋亡和焦亡是细胞两种程序性死亡方式,在AS中发挥着重要的作用。Sarai等[21]的研究表明,在AS中细胞焦亡标志物caspase-1和细胞凋亡标志物caspase-3表达同时增高,并伴随巨噬细胞的死亡,给予caspase-1或caspase-3特异性抑制剂均可抑制巨噬细胞死亡。这提示凋亡与焦亡在AS病变中共存,通过抑制细胞死亡引起的免疫炎症反应可达到缓解AS的目的。
血管壁细胞凋亡通过影响血栓形成、斑块破裂等,参与AS的发生与进展。本研究表明,模型组主动脉凋亡标志物cleaved caspase-3和促凋亡因子Bax表达增加,抗凋亡因子Bcl-2表达降低,提示血管壁细胞凋亡增强,促进了血管细胞损伤。黄芪-当归配伍可降低主动脉cleaved caspase-3和Bax表达,增加Bcl-2表达。表明黄芪-当归配伍可减轻小鼠AS病变的血管细胞凋亡,其作用可能是黄芪-当归配伍可促进抗凋亡因子表达、抑制促凋亡因子表达,抑制了血管细胞凋亡,达到改善血管损伤的作用。近期研究证明,细胞焦亡可加重斑块的不稳定性,导致斑块破裂和血栓形成[22],抑制细胞焦亡可减轻AS的病理损伤[23]。本研究表明,模型组主动脉细胞焦亡标志物NLRP3、cleaved caspase-1和GSDMD-N表达均显著增加,表明AS病变时血管壁细胞焦亡活性增强,并引起血管局部炎症反应,使血管细胞损伤增加,血管功能障碍。黄芪-当归配伍均降低主动脉NLRP3、cleaved caspase-1和GSDMD-N表达,表明黄芪-当归配伍可抑制小鼠AS病变的血管细胞焦亡,减轻血管壁炎症反应,从而达到改善血管功能、抑制AS血管病变发生发展的作用。
综上所述,本研究揭示了益气活血中药黄芪-当归配伍可减轻小鼠AS血管病变,其作用与抑制血管壁细胞凋亡和焦亡、减轻炎症反应有关。这为以细胞凋亡和焦亡为靶点研究中医药缓解AS血管功能障碍提供了研究思路,也为进一步揭示黄芪-当归配伍改善AS血管功能、防治AS病变提供了科学依据。但黄芪-当归配伍通过抑制血管细胞凋亡和焦亡改善血管功能的作用机制尚未完全明确,其作用是以抑制血管壁细胞凋亡为主还是以抑制细胞焦亡为主,仍需深入研究。
[1]中国心血管健康与疾病报告编写组. 中国心血管健康与疾病报告2020概要[J]. 中国循环杂志, 2021, 36(6):521-545.
China cardiovascular health and disease report writing group. Report on cardiovascular health and diseases burden in China: an updated summary of 2020[J]. Chin Circ J, 2021, 36(6):521-545.
[2] Jukema RA, Ahmed TAN, Tardif JC. Does low-density lipoprotein cholesterol induce inflammation? If so, does it matter? Current insights and future perspectives for novel therapies[J]. BMC Med, 2019, 17(1):197.
[3] Chiva-Blanch G, Badimon L. Cross-talk between lipoproteins and inflammation: the role of microvesicles[J]. J Clin Med, 2019, 8(12):2059-2070.
[4] Raggi P, Genest J, Giles JT, et al. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions[J]. Atherosclerosis, 2018, 276:98-108.
[5]张健美,景永帅,张丹参. 动脉粥样硬化的发病机制及治疗[J]. 中国药理学与毒理学杂志, 2019, 33(6):472-473.
Zhang JM, Jing YS, Zhang DS. Pathogenesis and treatment of atherosclerosis[J]. Chin J Pharmacol Toxicol, 2019, 33(6):472-473.
[6]唐朝君,王蕾,朱力. 血小板参与动脉粥样硬化斑块形成的分子途径[J]. 中国分子心脏病学杂志, 2020, 20(4):3437-3444.
Tang ZJ, Wang L, Zhu L. The molecular mechanism of platelets in atherosclerotic plaque formation[J]. Mol Cardiol Chin, 2020, 20(4):3437-3444.
[7]谷依檬,汤紫薇,吴艳艳,等. Inflammasomes/caspases通路与细胞焦亡、细胞凋亡在动脉粥样硬化中的不同作用[J]. 中国中医基础医学杂志, 2022, 28(8):1378-1382.
Gu YM, Tang ZW, Wu YY, et al. Inflammasomes/caspases pathway and the different roles of cell scorching and apoptosis in atherosclerosis[J]. Chin J Basic Chin Med Tradit Chin Med, 2022, 28(8):1378-1382.
[8]龚廷栋,黄水清. 当归补血汤有效组分抗动脉粥样硬化配伍比例的基础研究[J]. 中药新药与临床药理, 2017, 28(4):468-472.
Gong TD, Huang SQ. Fundamental research on combination proportion of effective components with anti-atherosclerosis effects in Danggui Buxue Decoction[J]. Tradit Chin Drug Res Clin Pharmacol, 2017, 28(4):468-472.
[9]彭熙炜,阎卉芳,黄娟,等. 黄芪-当归不同配伍比例对大鼠血管内膜增生的影响[J]. 中国中医药信息杂志, 2017, 24(6):56-59.
Peng XW, Yan HF, Huang J, et al. Effects of different compatibility ratios ofandon vascular intimal hyperplasia in rats[J]. Chin J Inf Tradit Chin Med, 2017, 24(6):56-59.
[10] 彭熙炜,阎卉芳,黄娟,等. 黄芪-当归配伍对大鼠血管内膜增生模型炎性反应的影响[J]. 中华中医药杂志, 2019,34(2):580-584.
Peng XW, Yan HF, Huang J, et al. Effects of the compatibility ofandon the inflammatory response of rats with intima hyperplasia[J]. Chin J Tradit Chin Med Pharm, 2019, 34(2):580-584.
[11]傅馨莹,杨仁义,孙正骥,等. 补阳还五汤苷类组分调控动脉粥样硬化炎性反应及脂质代谢的作用机制[J]. 中草药, 2021, 52(14):4221-4231.
Fu XY, Yang RY, Sun ZJ, et al. Mechanism of glycosides of Buyang Huanwu Decoction on regulating atherosclerotic inflammation and lipid metabolism[J]. Chin Tradit Herb Drugs, 2021, 52(14):4221-4231.
[12] Park SJ, Kim B, Choi S, et al. Imaging inflammation using an activated macrophage probe with Slc18b1 as the activation-selective gating target[J]. Nat Commun, 2019, 10(1):1111.
[13] Wu L, Zhang W, Li H, et al. Inhibition of aortic intimal hyperplasia and cell cycle protein and extracellular matrix protein expressions by BuYang HuanWu Decoction[J]. J Ethnopharmacol, 2009, 125(3):423-435.
[14] 缪静,周鑫斌,毛威,等. 丹蒌片对基因敲除小鼠动脉粥样硬化的疗效研究[J]. 中国中西医结合杂志, 2020, 40(11):1367-1372.
Miao J, Zhou XB, Mao W, et al. Effects of Danlou tablet on atherosclerosis in-gene knockout mice[J]. Chin J Integ Tradit West Med, 2020, 40(11):1367-1372.
[15]韩文博,孙爱军,李红梅,等. 动脉粥样硬化疾病“因风致变”病机理论探讨[J]. 北京中医药大学学报, 2020, 43(11):965-968.
Han WB, Sun AJ, Li HM, et al. Discussion on pathogenesis of "wind-induced" atherosclerosis[J]. J Beijing Univ Tradit Chin Med, 2020, 43(11):965-968.
[16] Yan H, Peng X, Xu H, et al. Inhibition of aortic intimal hyperplasia and vascular smooth muscle proliferation and extracellular matrix protein expressions by-combination[J]. Evid Based Complement Alternat Med, 2018, 2018:1508637.
[17] Hopkins PN. Molecular biology of atherosclerosis[J]. Physiol Rev, 2013, 93(3):1317-1542.
[18] Uryga AK, Bennett MR. Ageing induced vascular smooth muscle cell senescence in atherosclerosis[J]. J Physiol, 2016, 594(8):2115-2124.
[19] Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis[J]. Circ Res, 2016, 118(4):692-702.
[20] Bennett M, Yu H, Clarke M. Signalling from dead cells drives inflammation and vessel remodelling[J]. Vascul Pharmacol, 2012, 56(5/6):187-192.
[21] Sarai M, Hartung D, Petrov A, et al. Broad and specific caspase inhibitor-induced acute repression of apoptosis in atherosclerotic lesions evaluated by radiolabeled annexin A5 imaging[J]. J Am Coll Cardiol, 2007, 50(24):2305-2312.
[22] Xu YJ, Zheng L, Hu YW, et al. Pyroptosis and its relationship to atherosclerosis[J]. Clin Chim Acta, 2018, 476: 28-37.
[23] Ji N, Qi Z,Wang Y, et al. Pyroptosis: A new regulating mechanism in cardiovascular disease[J]. J Inflamm Res, 2021, 14:2647-2666.
Effect of-combination on vascular injury in a mouse model of atherosclerosis
LI Wanyu, LONG Qingyin, FU Xinying, MA Lu, TAN Wei, LI Yanling, XU Shunzhou, ZHANG Wei△, DENG Changqing△
(,,,410208,)
To investigate the effect of-combination on cell injury in the vascular wall of mouse atherosclerosis (AS) model from perspectives of cell apoptosis and pyroptosis.Male apolipoprotein E gene knockout (-/-) mice were randomly divided into model group, low-dose-group, medium-dose-group, high-dose-group and atorvastatin group, with 5 mice in each group. The same number of male C57BL/6J mice at the same week of age were used as control. Except for the control group, all other animals were fed with high-fat feed for 12 weeks to construct AS model, and drugs were given by gavage after successful modeling. Then, the thickness of aortic intimal hyperplasia was observed by HE staining. The total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-c) and high-density lipoprotein cholesterol (HDL-c) levels were measured by a fully-automated biochemical analyzer, and the plasma interleukin-1β (IL-1β) and IL-18 levels were measured by ELISA. The mRNA and protein expression levels of cell apoptosis- and pyroptosis-related factors, including B-cell lymphoma-2 (Bcl-2), Bcl-2-associated X protein (Bax), cleaved caspase-3, nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3), cleaved caspase-1 and gasdermin D-N (GSDMD-N), were detected by immunofluorescence, RT-qPCR and Western blot assays. The combined results were used to evaluate the effect of target drugs on vascular wall cell injury in AS lesions.High-fat feeding induced AS model successfully. Compared with control group, the mice in model group showed increased plasma TC, TG and LDL-c levels (<0.01), decreased HDL-c level (<0.01), thickened intima (<0.01), increased plasma IL-1β and IL-18 levels (<0.01), and elevated expression levels of Bax, cleaved caspase-3, NLRP3, cleaved caspase-1 and GSDMD-N, and reduced expression of Bcl-2 in the vascular wall (<0.01). Compared with model group, treatment with-combination down-regulated plasma TC, TG and LDL-c levels (<0.05), up-regulated the plasma HDL-c level (<0.05), reduced the degree of intimal hyperplasia (<0.01), decreased plasma IL-1β and IL-18 levels (<0.05), inhibited the expression of Bax, cleaved caspase-3, NLRP3, cleaved caspase-1 and GSDMD-N, and promoted the expression of Bcl-2 in the vascular wall (<0.01). Overall, medium-dose-exhibited the most significant effect.Combination ofandcan delay the progress of AS lesions, alleviate inflammatory responses, and inhibit the apoptosis and pyrosis of vascular wall cells in mice, and its mechanism is related to the inhibition of activation of apoptosis- and pyroptosis-related factors in vascular wall cells.
atherosclerosis;-combination;-/-mice; apoptosis; pyroptosis
R363.2; R282.71
A
10.3969/j.issn.1000-4718.2023.09.007
1000-4718(2023)09-1586-10
2023-06-02
2023-08-29
国家自然科学基金资助项目(No. 81874406; No. 82174218);湖南省自然科学杰出青年基金项目(No. 2020JJ2024)
张伟 Tel: 0731-88458201; E-mail: zhangwei1979@hnucm.edu.cn;邓常清 Tel: 0731-88458201; E-mail: dchangq@sohu.com
(责任编辑:余小慧,李淑媛)

