Chickenpox followed streaky multifocal choroiditis with prednison treatment in a girl with asthma
Chun-Li Chen, Zhi-Han Zhang, Yi-Zhe Cheng, Yang Zhang, Xiao-Yan Peng
1Beijing Tongren Eye Center, Beijing Tongren Hospital,Capital Medical University, Beijing 100005, China
2Beijing Institute of Ophthalmology, Beijing 100010, China
3Beijing Ophthalmology and Visual Science Key Laboratory,Beijing 100005, China
Dear Editor,
We report a case of streaky multifocal choroiditis(SMFC) followed by chickenpox in a girl with asthma under prednison treatment.To our knowledge, this is the first case report of an SMFC followed by chickenpox in a patient with asthma.A 15-year-old female with a complaint of fixed shadow in the front of her right eye for six months, showed a medical history of asthma for 5y being controlled by long-term budesonide and formoterol fumarate powder inhalation (4-5 times per day), corrected refractive error for 2y and denied similar family history.A diagnosis of right eye punctate inner choroidopathy (PIC) was considered at another hospital.The same symptoms showed up repeatedly during the observation period.To get further diagnosis and treatment, she came to us.The vision acuity noted visio oculus dexter (VOD) 0.06 and visio oculus sinister (VOS) 0.12, corrected visual acuity (CVA)was VOD -6.00/-1.25×3=1.0, VOS -4.00/-1.50×177=1.0.The intraocular pressure (IOP) was 15.5 mm Hg in the right eye and 16.2 mm Hg in the left eye, and the axial lengths of the right and left eye are 25.04 mm and 24.75 mm respectively.The right eye showed negative manifestations in the cornea,the reaction in the anterior chamber, lens, and vitreous haze with around 100 cells in the vitreous body (Figure 1).For the fundus of this eye, the boundary, as well as the color of the disc, remained normal.However, three yellow-white lesions at the temporal part of the fovea could be seen, accompanied by curvilinear yellow-white streaks (Schlaegel line) with pigmentation at the temporal and nasal part of the disc.These curvilinear streaks, with various lengths composed of multiple small round-shape lesions, were radially distributed (Figure 2).Autofluorescence (AF) present hypo-autofluorescences at the disc, three lesions at the posterior pole, and three curvilinear streaks at the nasal mid-peripheral part (Figure 3).There was no obvious abnormality in the left eye.Optical coherence tomography (OCT, VG100; SVision Imaging, Ltd., Luoyang,China) of the right eye show a hyperreflective nodule at the retinal pigment epithelial (RPE) layer inferior to the superior temporal arch, a hyperreflective material rupturing RPEBruch's membrane at the nasal part of the fovea, and also an arc-shaped change at the outer layer accompanied by sheetlike ruptured RPE-Bruchs membrane at the superior nasal part.The patient failed to do fluorescent angiography and indocyanine green angiography examinations due to asthma.Infectious diseases were excluded through improved systemic examination.The final diagnosis of the right eye is SMFC, and binocular refractive error.This patient was given a retrobulbar injection of 20 mg triamcinolone acetonide (TA), along with 60 mg prednison orally, following a 10 mg weekly reduction for two weeks and then a 5 mg weekly reduction for two weeks.This patient was followed up one month later.
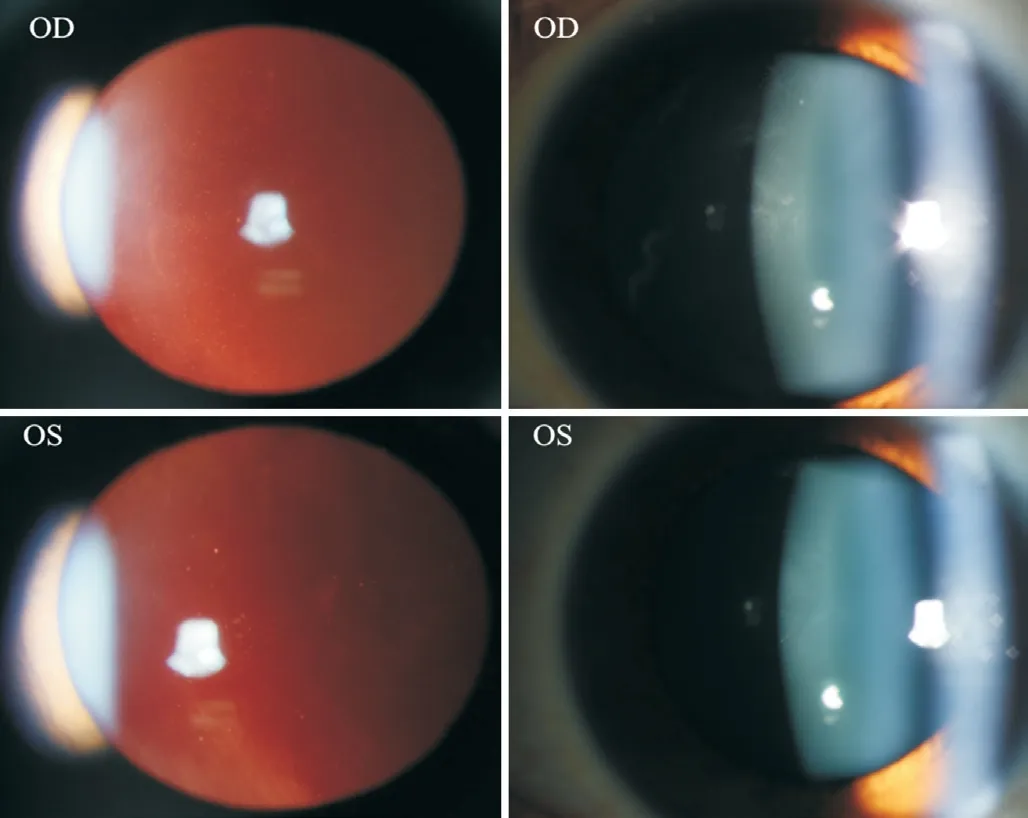
Figure 1 Vitreous haze with around 100 cells in the vitreous body in the right eye and no cells in the left eye.
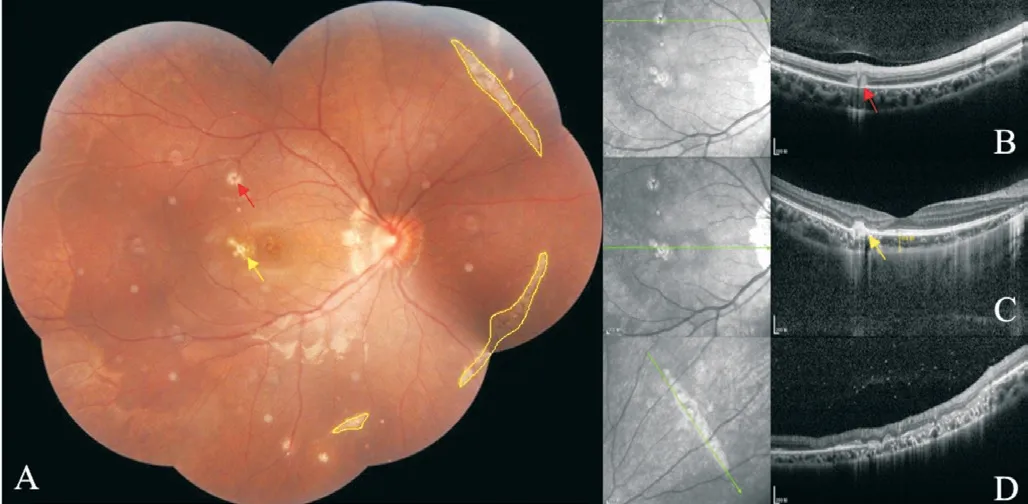
Figure 2 Fudus photograph (A) and OCT (B-D) show curvilinear streaks with various lengths composed of multiple small roundshape lesions were radially distributed The fundus shows active(red arrow) and old (yellow arrow) yellow-white lesions on the nasal part of the disc, posterior pole, and mid-periphery of the right eye.And three curvilinear streaks with multiple dots of yellow-white lesions were seen in the nasal mid-periphery fundus (the superior nasal one was 5.57 mm, 6.41 mm for the inferior nasal one, and 1.55 mm for the inferior one), some of which are accompanied by pigment changes.
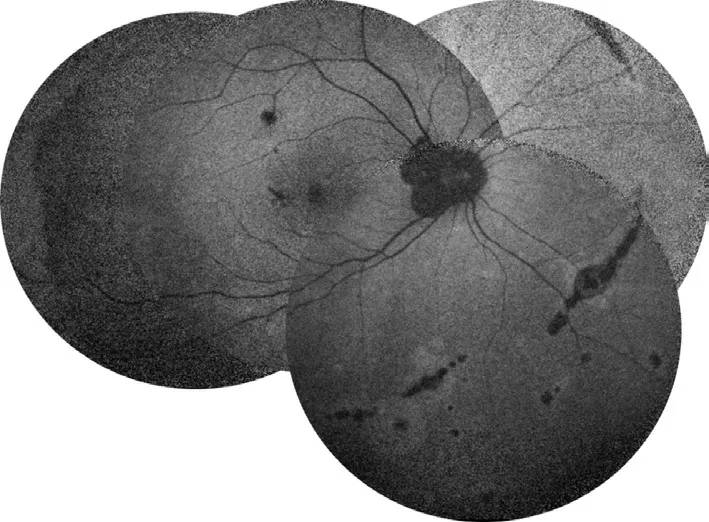
Figure 3 Autofluorescence shows hypofluorescences at the nasal part of the disc, three lesions at the posterior pole, and three curvilinear streaks at the nasal mid-peripheral part, accompanied by patchy hyperfluorescences at the margin.
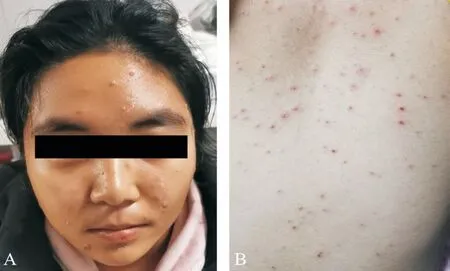
Figure 4 Red papules, blisters, and papules scattered with large miliary grains were shown on the face and trunk, and some of the blisters were scabbed.
On the fourth day of oral prednison therapy, miliary red papules, vesicles, and papules were scattered on her face and trunk, and some vesicles are crusted on the surface.She was diagnosed with chickenpox by a dermatologist (Figure 4), thus steroid therapy was suspended immediately.Although she claimed a history of two doses of herpes zoster virus (HVZ)vaccination, steroid therapy made her a high-risk HVZ patient.This patient should be applied with HVZ immunoglobulin or acyclovir or so forth, according to the guidelines.However,she was restricted by COVID-19 epidemic prevention and control and failed to come to our clinic review in time.The patient's systemic chickenpox subsided and some scabs formed two weeks later.The patient returned to our clinic two months later and complained of a new dark shadow in the front of her right eye.Ophthalmoscope examination found a new active lesion at the temporal part of the fovea in the right eye.The active lesion showed hypofluorescence in the central and hyper fluorescence around it.These yellow-white dots showed unclear edema boundaries on the fundus.OCT showed “hump-like”hyperreflective substances on RPE, at which the retina was thicker than it was first diagnosed (Figure 5).OCTA showed blood-flow signals in the lesion, indicating choroidal neovascularization (CNV; Figure 6).The result of perimetry was correspondence with the patient's symptoms(Figure 7).Taking account of the patient's general condition, a 20 mg TA retrobulbar injection was given, yet the recurrence of the lesion occurred two weeks later.In consideration of new and old lesions recurring repeatedly, as well as the short distance of 1000 μm to the fovea may endanger central vision,an intraocular injection of dexamethasone intravitreal implant combining oral methotrexate 10 mg per week was given.Close attention was paid to the patient's IOP.The patient was followed up for 1y, showing stabled current lesions, no new active lesions (Figure 8), and normal IOP.
DISCUSSION
Multifocal choroiditis (MFC) is commonly seen in young myopic women, most of whom suffer binocular lesions located in the posterior pole and/or peripheral fundus and complain of photopsia, blurred vision, and visual field defects, accompanied by inflammation in the anterior chamber or vitreous body[1-4].About 27%-46% of patients with MFC develop CNV[5-7].Patients usually denied a history of systematic diseases or eye diseases[8-9].MFC and PIC are considered to be the most related white dot syndrome because of their clinical manifestations, visual field defects and flashing sensation,scar appearance, binocular involvement tendency, and the incidence of CNV[10].There is a major controversy on whether PIC and MFC are a pedigree of the same disease[11-12].A study comparing MFC and PIC found no difference between them,which may be limited by the small sample size[12].In 2020,Gilbertet al[11]conducted a study on 343 eyes and found that MFC and PIC have different demographic and clinical characteristics, and show phenotype stability for five years,indicating them being two different diseases.PIC lesions are mostly located in the posterior pole, with small lesions and no inflammation in the anterior chamber or vitreous body, most of them occur in young myopic women around 20-30 years old with an average of -7.50 D refractive error.Meanwhile,MFC lesions are mostly located in the posterior pole and/or mid-peripheral fundus with larger lesions, most of them occur in women around 40y with an average of -2.75 D refractive error.Also, the incidence of epiretinal membrane (ERM) in MFC patients is higher than that in PIC patients, indicating a higher prevalence of intraocular inflammation of MFC.The clinical inflammation of MFC is related to the infiltration of B lymphocytes, especially in CNV, which has not been observed in PIC[13].Based on the distribution and inflammatory status of the lesions, the diagnosis was more in favor of MFC though there were discrepant aspects of both MFC and PIC.MFC with streaky lesions is mostly seen in young high myopia patients with a mean age of 17y and an average of -8.75 D refractive error, and the eye with faster growth in myopia is more prone to develop.The streaky lesions are mainly located in the midperiphery near the equator and are mostly accompanied by pigmentary changes.Approximately 88.9% of patients have concurrent CNV, either single or multiple lesions, located mainly in the parafovea.Thus, we propose the new term,SMFC, as a subtype of MFC[14].
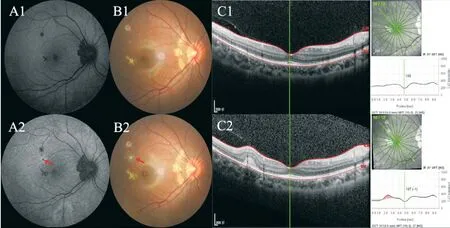
Figure 5 The yellow-white dots on the fundus and on OCT A1-C1: Initially, autofluorescence showed small dots with hypofluorescence corresponding to the new active lesion, fundus photography showed small yellow-white lesions with clear borders, and no obvious abnormality in OCT; A2-C2: Active lesions (red arrow) were visible at the 2-month follow-up, autofluorescence showed hypofluorescence in central and hyperfluorescence surrounding, and fundus photography showed yellow-white dots with unclear borders and mild edema around the lesion.OCT showed “hump-like”hyperreflective substances on RPE, where the thickness of the retina was thicker than it was first diagnosed.OCT:Optical coherence tomography; RPE: Retinal pigment epithelial.
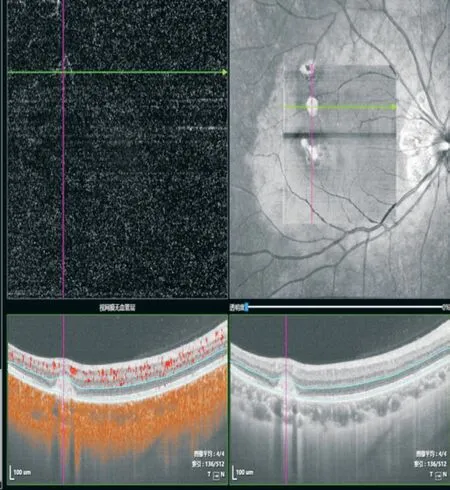
Figure 6 OCTA showed blood-flow signals in the active lesion,indicating CNV.
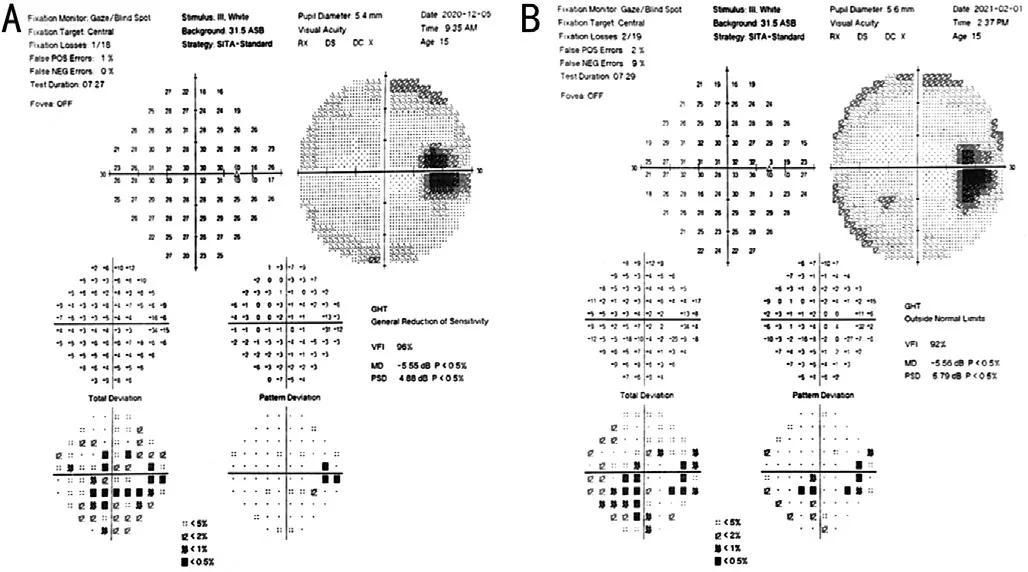
Figure 7 The result of perimetry was correspondence with the patient’s symptom A: In the beginning, perimetry showed three temporal scotomas in the right eye; B: Increased scotomas two months later without treatment.
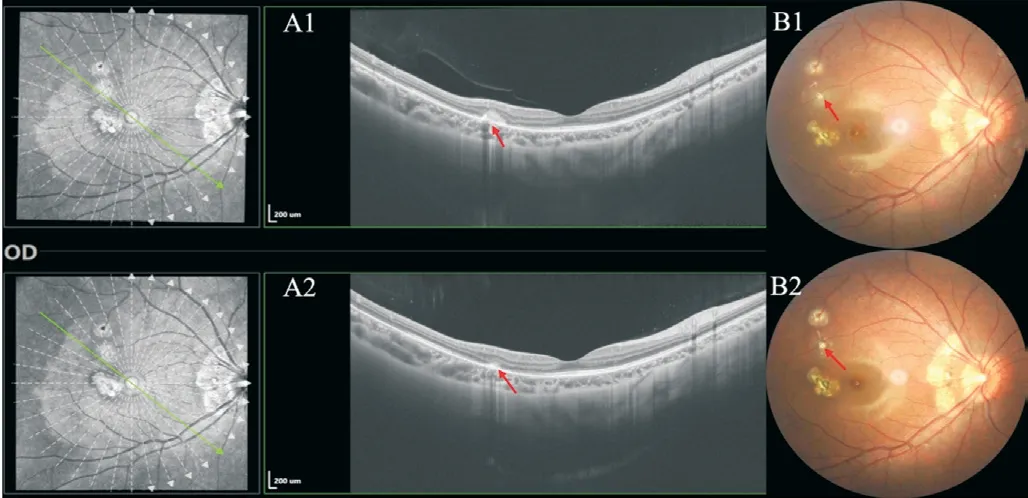
Figure 8 The patient was followed up for 1y, showing stabled current lesions, and no new active lesions During the last followup, OCT showed a significant reduction of hyperreflective substances on RPE.A clear and stable boundary of the original active lesions is shown in the fundus photograph.
Although the cause of MFC remains unclear, there is evidence showing that it is related to autoimmunity and viruses.Previous studies report that MFC is associated with a variety of viruses, such as Epstein-Barr virus[15-20], COVID-19 virus[21],Zika virus[22-23], yellow fever virus[24], Powassan virus[25], and West Nile virus[26-29](Table 1).Animal model studies have also shown that the Zika virus and COVID-19 can affect the retina, with Müller cells and RPE cells being the main host cells[30-31].This indicates a closer relationship between the virus and the pathogenesis of MFC.Choroidal capillaries and the choroid are rich in some potential antigen-presenting cells and the main part of the lesion involves RPE.Thus RPE can be induced to express major histocompatibility complex class II molecules, suggesting that it may interact with T lymphocytes.High-density T lymphocytes, B lymphocytes,and macrophages will penetrate the choroid stimulating the synthesis of cytokines, thereby altering the subsequent immune response.Most researchers believe that the combined effect of genetic and environmental factors may be the main cause of disease[32].One possible mechanism of streaks formation could be the growth of the ocular axis, which occurs through the generation of additional stress in the equatorial and posterior equatorial regions, leading to a decrease in RPE density and retinal thinning.Another mechanism could be that blood-borne microorganisms or immune factors preferentially deposit in this area (watershed zone of the anterior and posterior ciliary arteries), leading to a response of the RPE, resulting in curvilinear scars or streaks[33].
Our patient developed chickenpox after taking the steroid for four days, indicating that the chickenpox virus is more likely to invade and develop when the body's resistance is weak.Varicella zoster virus (VZV) infection activates cellular immunity in the body, actively proliferated T cells may interact with histocompatibility complex class II molecules expressed by RPE, leading to the occurrence of new active lesions.Varicella is an acute respiratory infection caused by the VZV and is characterized by mild systemic symptoms and a rash of macules, papules, blisters, and crusts that appear in batches on the mucous membranes of the skin.The susceptible people are mainly students and children.In practice, a history of previous varicella infection or immune response to vaccination is usually evaluated by detecting the corresponding antibodies in the serum.However, sometimes negative antibodies are detected in the patient's blood, which does not necessarily mean that the patient is susceptible to varicella and that the patient has the appropriate cellular immunity.Serologic methods for VZVIgG antibody titers are 4-fold or more elevated in the recovery phase compared to the acute phase, and the VZV-IgG affinity test is a method to distinguish primary VZV infection from a previous infection but has not been widely used.VZV-IgM can provide evidence of recent active VZV infection, but cannot distinguish primary infection from reinfection or latent reactivation.A negative VZV-IgM result should not be used to exclude the possibility of diagnosis, nor should a positive IgM in the absence of a rash be used to confirm the diagnosis.The application of hormones can attenuate or inhibit antigen and antibody responses, affecting antigen processing and antibody formation; it also inhibits complement production and reduces the body's antiviral capacity, thereby inhibiting reticuloendothelial cell interferon production and its activity decreasing or eliminating the ability of interferon to fight the virus, prompting varicella virus to multiply, spread and spread throughout the body[33-34].
The proportion of MFC receiving any type of treatment is higher than that of PIC.The main treatment includes local or systemic steroids and immunosuppressive therapy and symptomatic treatment.Indications for immunosuppressive therapy include intraocular inflammation, new chorioretinopathy,recurrent or secondary CNV, vision loss and the appearance of new visual symptoms, and evidence of the progression of intraretinal lesions or subretinal fluid on clinical imaging.It has been reported that systemic steroids and immunosuppressive therapy have good therapeutic effects[12,34-36], but there are also reports of refractory cases with poor response to glucocorticoids and immunosuppressive therapy[37].The inconsistent treatment response may be attributed to the course and severity of different diseases, different treatment start times, and dosing schedules.Steroid therapy can make the inflammatory hyper-reflective substances on OCT quickly subside, and the abnormal structure of the outer retina can be restored.For recurrent eyes, the use of systemic steroids and/or immunosuppressive maintenance therapy can also prevent recurrence and stabilize the disease, which is proven to be effective[13].Also, oral steroids for MFC may help to reduce the risk of CNV[38].MFC patients with linear streaks should be treated with sufficient systemic steroids and immunomodulators to prevent the development of the disease[39].
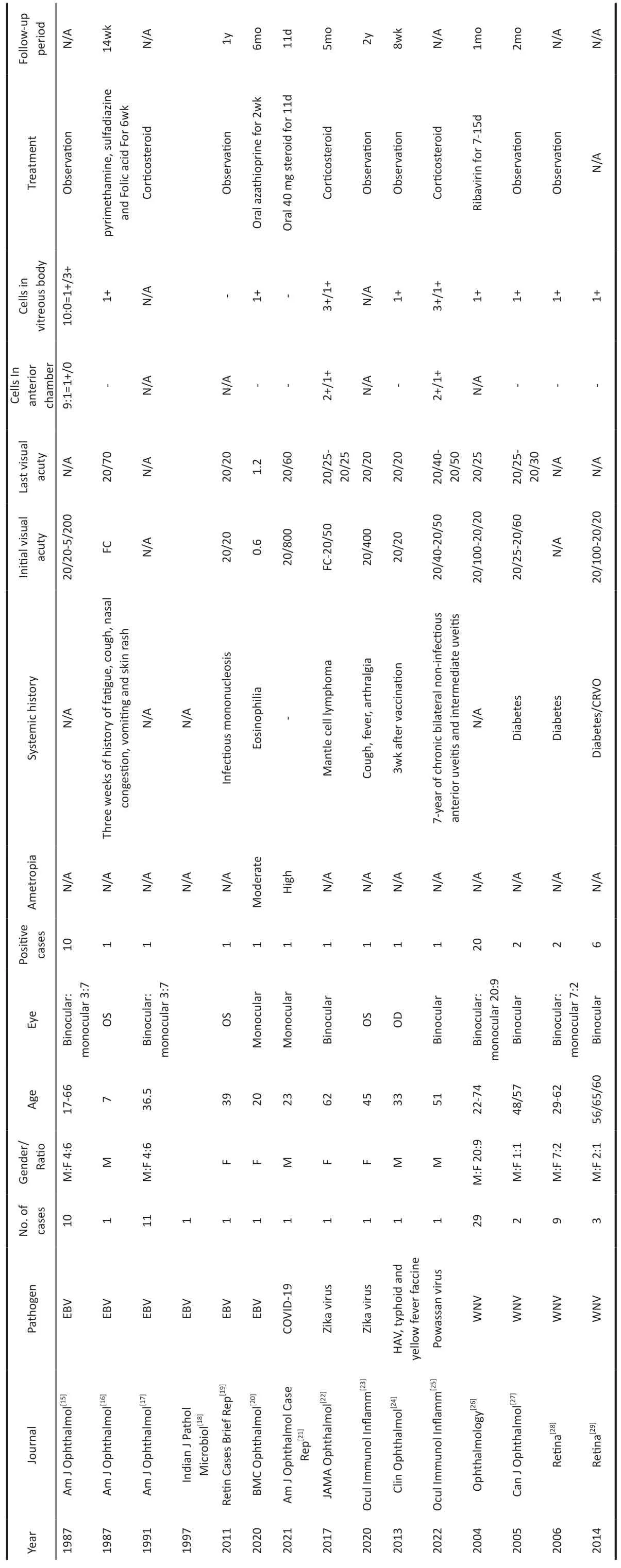
Table 1 Previous studies report of MFC being associated with various viruses
ACKNOWLEDGEMENTS
Authors’contributions:Chen CL: Conceptualization,Writing original draft.Zhang ZH: Writing original draft/editing, visualization.Cheng YZ: Writing, editing.Zhang Y: Visualization.Peng XY: Treatment guidance, writing,reviewing.All authors have read and approved the manuscript.
Conflicts of Interest:Chen CL,None;Zhang ZH,None;Cheng YZ,None;Zhang Y,None;Peng XY,None.
 International Journal of Ophthalmology2023年7期
International Journal of Ophthalmology2023年7期
- International Journal of Ophthalmology的其它文章
- Pneumonia and ocular disease as the primary presentations of Takayasu arteritis: a case report
- Unilateral blurred vision in pediatric patient associated with cavum velum interpositum cyst
- Highly cited publication performance in the ophthalmology category in the Web of Science database:a bibliometric analysis
- Comparison of efficacy of conbercept, aflibercept, and ranibizumab ophthalmic injection in the treatment of macular edema caused by retinal vein occlusion: a Metaanalysis
- Ocular manifestations and quality of life in patients after hematopoietic stem cell transplantation
- Clinical features, radiological imaging, and treatment strategies of nonmetallic intraorbital foreign bodies: a retrospective analysis
