Differential analysis of aqueous humor cytokine levels in patients with macular edema secondary to diabetic retinopathy or retinal vein occlusion
Ke-Ke Hu, Chao-Wei Tian, Man-Hong Li, Tong Wu, Min Gong, Xin-Li Wei, Yu-Ru Du,Yan-Nian Hui, Hong-Jun Du
1Department of Ophthalmology, Eye Institute of PLA, Xijing Hospital, Fourth Military Medical University, Xi'an 710032,Shaanxi Province, China
2Zhongshan School of Medicine, Sun Yat-sen University,Guangzhou 510080, Guangdong Province, China
Abstract● AlM: To evaluate the difference and the correlation between the concentrations of cytokines in the aqueous humor of eyes with macular edema secondary to diabetic retinopathy (DR) or retinal vein occlusion (RVO).
● KEYWORDS: macular edema; diabetic retinopathy;retinal vein occlusion; cytokines; aqueous humor
INTRODUCTION
Diabetic retinopathy (DR) and retinal vein occlusion(RVO) are the main causes of visual impairment and are also two most common retinal vascular diseases, which are increasing yearly with the change of human life-style and aging of the population[1-4].Retinal microvascular or vascular lesions associated with obstruction, macular edema (ME), exudation,non-perfusion area, neovascularization, hemorrhage and fibrosis are the main pathological changes.Patients eventually lose vision due to diabetic ME (DME) or RVO-induced ME(RVO-ME), traction retinal detachment (TRD) and neovascular glaucoma[5-6], of which ME is the most visual threatening.Several studies have shown that although there are differences in the initiating factors and mechanisms of DME and RVOME, there are commonalities at the molecular biological level.For example, growth factors, such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF)and various inflammatory cytokines, including interleukin(IL)-6, IL-8, IL-18, monocyte chemoattractant protein-1(MCP-1) and tumor necrosis factor-α (TNF-α) are elevated in the vitreous and aqueous humor from DME and RVO-ME patients[7-8], suggesting that ischemia and inflammation are involved in the similar pathogenesis of the two diseases.
There have been many attempts to treat DME and RVO-ME by targeting inflammatory mediators or VEGF, and studies have proved the utility of both treatment modalities, while these two modalities worked differently for each disease,which indicated differences between the pathogenesis of DME and RVO-ME.There are several studies on the difference in intraocular cytokine levels of DME and RVO-ME[9], however,the correlations between cytokines and the extent of ME, and whether ischemia (related factors such as VEGF and PDGF)and inflammation (related cytokines such as IL-6, IL-8, IL-18,TNF-α, and MCP-1) play the same role in the pathogenesis of both diseases is inconclusive.In addition, compared to the vitreous, aqueous humor samples have the advantages of being relatively less invasive and less likely to mix with blood cells when collected[10-13].Therefore, in this study, we compared the levels and evaluated the potential implications of ischemia and inflammation related cytokines in the aqueous humor from the eyes of DME and RVO-ME patients.The results of the study have implications for better exploring the pathogenesis of these two diseases, finding reliable clinical test indicators and guiding treatment.
SUBJECTS AND METHODS
Ethical ApprovalThis retrospective case control study was conducted from August 2021 to July 2022 at the Department of Ophthalmology in Xijing Hospital of Fourth Military Medical University.Patients diagnosed with ME associated with DR or RVO were included in the patient groups, and patients with idiopathic macular hole (IMH) were used as the control.Xijing Hospital Ethic Committee approval (approval No.KY20222223-C-1) was obtained for the aqueous humor sample collection.Informed consent was obtained from the subjects.
The criteria required for enrolling patients need to be met: 1)aged from 50 to 80y, male or female; 2) the diagnostic criteria for DR or RVO[14-16]; 3) treatment-naïve in DME or RVOME group, or receiving vitreous surgery for the first time for IMH as control group.The exclusion criteria were as follows:1) advanced cases of DR or RVO with extensive retinal detachment or ischemia; 2) previous laser photocoagulation,or intravitreal injection; 3) a history or presence of ocular inflammation; 4) previous intraocular surgery within 6mo prior to the study; 5) serious systemic diseases under treatment other than diabetes and hypertension; 6) not well controlled systemic conditions that make surgery intolerable.
At baseline, a detailed medical history and complete ophthalmologic examinations for all patients, including visual acuity, slit-lamp biomicroscopy, intraocular pressure (IOP),indirect ophthalmoscopy, B-ultrasonography, optical coherence tomography (OCT), and fluorescein fundus angiography (FFA),as well as systemic evaluation were performed and recorded.Each aqueous humor sample was collected in the operating room by retinal specialist under sterile conditions.Undiluted aqueous humor samples were collected from the affected eye at the beginning of standard three-port vitrectomy or intravitreal injection of anti-VEGF drugs.Approximately 0.1 to 0.2 mL of aqueous humor samples were obtainedviaanterior chamber paracentesis, and discarded if there was obvious blood mixing.These samples were immediately transferred to a sterilized plastic tube (1.5 mL) and were stored at -80℃ until analysis.The established surgical operation was completed in the affected eye, and routine postoperative care and review were performed.Samples were submitted to R&D Systems(Minneapolis, Minnesota, USA) for testing by using Human Premixed Multi-Analyte Kit (Luminex).
All statistical analyses were performed using SPSS 22.0 for Windows.The measurement data was represented as mean values with standard deviations.Fisher's test was used for comparisons of categorical data.Normal distribution was tested using the one-sample Kolmogorov-Smirnov test.The Kruskal-Wallis test was used to compare the measured values of the three groups.In case of normally distributed data, a 2-tailedt-test was conducted to compare 2 groups; otherwise,the Mann-WhitneyUtest were utilized.We used Spearman's rank-order correlation coefficients to examine the relationships among variables.APvalue <0.05 was considered statistically significant.
RESULTS
Baseline CharacteristicsThis study consisted of 38 patients(40 eyes), including 11 patients (13 eyes) in the DME group,16 patients (16 eyes) in the RVO-ME group and 11 patients (11 eyes) in the IMH group.The general clinical characteristics,including age, gender, right or left eye (or both eyes), duration of symptoms, central subfield macular thickness (CSMT) and comorbidities with hypertension did not vary significantly between the three groups (P>0.05; Table 1).
Concentrations of Cytokines in the Aqueous HumorCompared with the control group, the DME group showed significantly higher levels of VEGF, PDGF, IL-6, IL-8, MCP-1 and TNF-α (P=0.000, 0.035, 0.027, 0.001, 0.005, 0.018), and the RVO-ME group showed significantly higher levels of VEGF, PDGF, IL-6, IL-8 and MCP-1 (P=0.013, 0.049, 0.046,0.019, 0.028; Table 2, Figure 1).
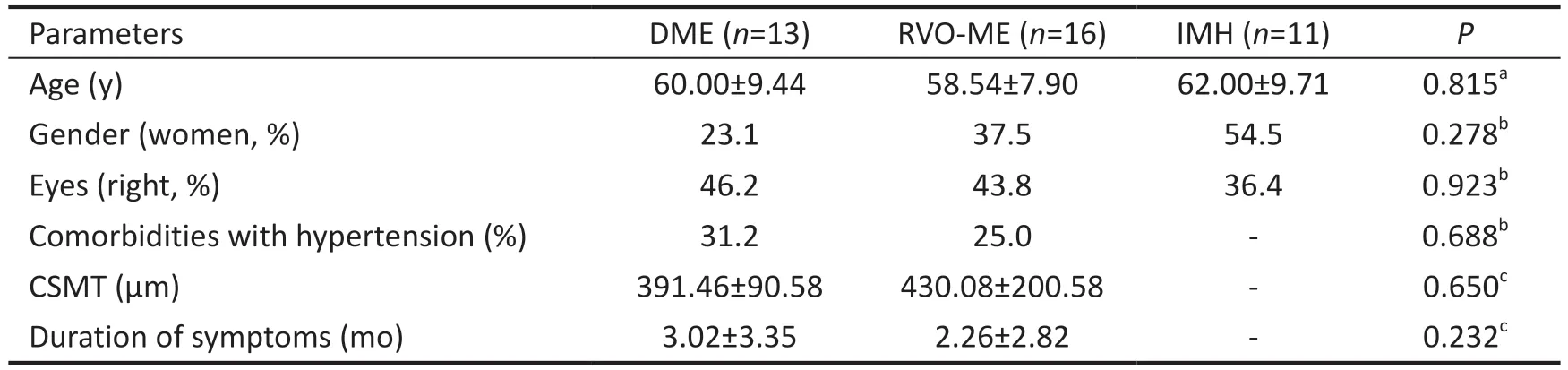
Table 1 Baseline clinical features of the patients in the DME, RVO-ME and control groups
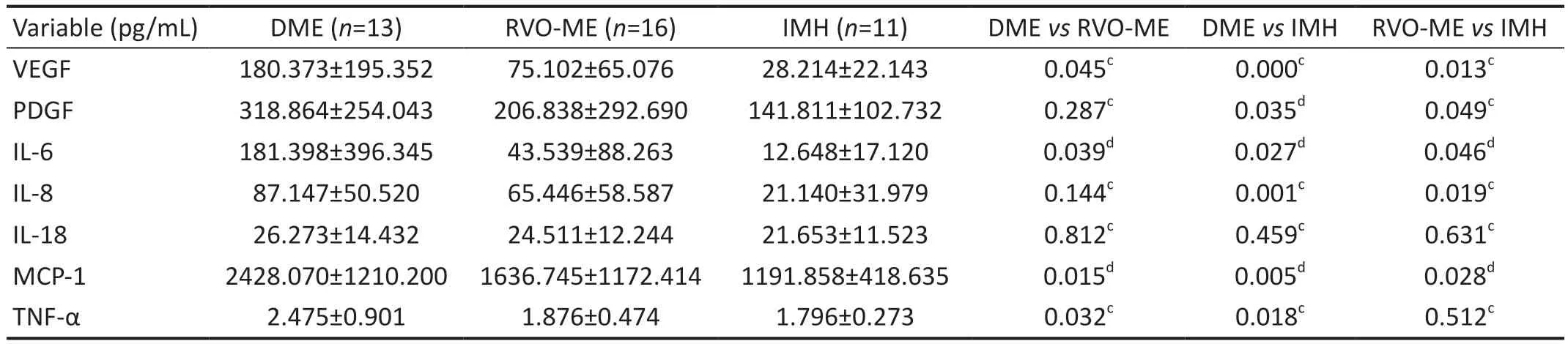
Table 2 Aqueous humor cytokine levels in the DME, RVO-ME and control groups
Further analysis showed that the levels of VEGF, IL-6, MCP-1 and TNF-α were significantly higher in the DME group than in the RVO-ME group (P=0.045, 0.039, 0.015, 0.032; Table 2,Figure 1).
Figure 2 shows representative cases of patient with DME and patient with RVO-ME and cytokine levels in the aqueous humour.
Correlation Analyses of Pro-Inflammatory Factors and ChemokinesIn the DME group, IL-6 level was significantly correlated with IL-8, IL-18, MCP-1 and TNF-α levels(r=0.846, 0.668, 0.742, 0.598,P=0.000, 0.000, 0.000, 0.001).They had significant correlations between the level of IL-8 and the levels of IL-18, MCP-1 and TNF-α (r=0.586, 0.868, 0.681,P=0.002, 0.000, 0.000).Moreover, the levels of IL-18, MCP-1 and TNF-α were significantly correlated (r=0.564, 0.762,P=0.003, 0.000), and also significant correlations between the level of MCP-1 and the levels of TNF-α (r=0.540,P=0.004;Table 3).
There were significant correlations in the RVO-ME group between the level of IL-6 and the levels of IL-8, IL-18, and MCP-1 (r=0.723, 0.533, 0.808,P=0.000, 0.002, 0.000).They had significant correlations between the level of IL-8 and the levels of IL-18, MCP-1 and TNF-α (r=0.436, 0.918, 0.368;P=0.013, 0.000, 0.038).Moreover, the levels of IL-18 and MCP-1 were significantly correlated (r=0.530,P=0.002; Table 4).
DISCUSSION
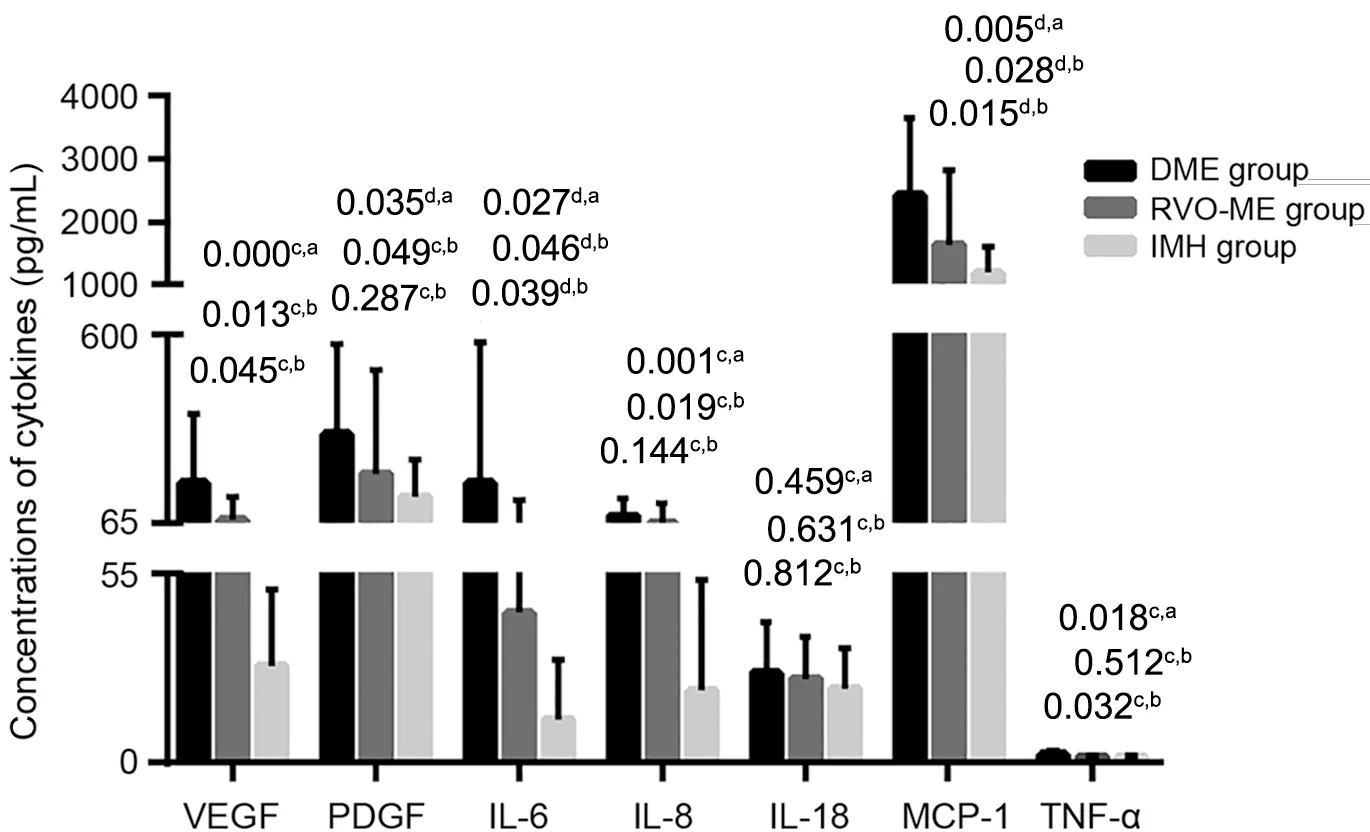
Figure 1 Aqueous humor cytokine levels in the DME and RVO-ME groups cMann-Whitney U test; dIndependent samples t-test.DME:Diabetic macular edema; RVO-ME: Retinal vein occlusion-induced macular edema; IMH: Idiopathic macular hole; VEGF: Vascular endothelial growth factor; PDGF: Platelet-derived growth factor; IL-6:Interleukin-6; IL-8: Interleukin-8; IL-18: Interleukin-18; MCP-1: Monocyte chemoattractant protein-1; TNF-α: Tumor necrosis factor-α.aComparison between RVO-ME group and DME group or IMH group.bComparison between DME group and IMH group.
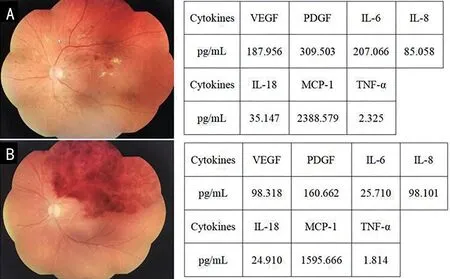
Figure 2 Representative fundus color photograph and cytokine levels A: DME in an aged 56 female with severe NPDR, visual acuity was 20/80;B: RVO-ME in an aged 59 female with branch RVO, visual acuity was 20/100.VEGF: Vascular endothelial growth factor; PDGF: Platelet-derived growth factor; IL-6: Interleukin-6; IL-8: Interleukin-8; IL-18: Interleukin-18; MCP-1: Monocyte chemoattractant protein-1; TNF-α: Tumor necrosis factor-α; NPDR: Non-proliferative diabetic retinopathy.
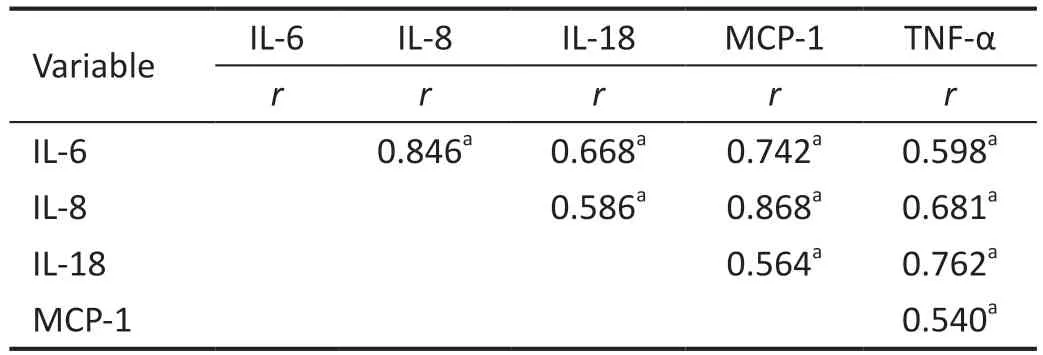
Table 3 Correlations between aqueous humor factors in DME patients
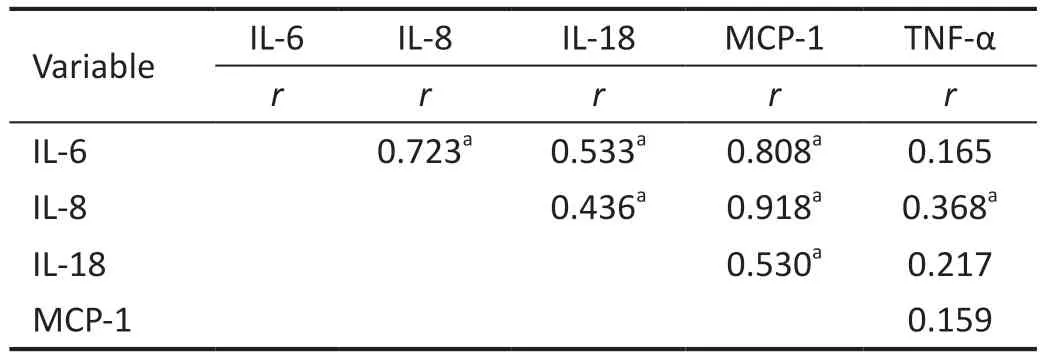
Table 4 Correlations between aqueous humor factors in RVO-ME patients
Ischemia and inflammatory have been confirmed to be involved in the pathogenesis of DME and RVO-ME[15-19]:hypoxia mainly induces the expression of growth factors such as VEGF and PDGF, while inflammation mainly promote the expression of chemokines (such as MCP-1) and inflammatory factors (IL-6, IL-1, and TNF-α).Based on the above understanding, anti-VEGF therapy and anti-inflammatory therapy have also been used as first-line treatment measures for DME and RVO-ME[20].However, it has been found clinically that the same treatment has different responses in different diseases or different patients with the same disease.For example, the study of Yoshimuraet al[2]showed that the effect of intraocular triamcinolone acetonide (TA) injection in RVOME patients was equivalent to that of anti-VEGF in reducing foveal thickness and improving vision, while in DME patients,it showed better effects than anti-VEGF, which suggests that hypoxia and inflammation may play different roles in different diseases.In view of the relative closure of the intraocular environment, the concentrations of the above factors in vitreous or aqueous humor are considered to reflect the degree of hypoxia and inflammation, and guide clinical treatment.In recent years, there have been many similar studies in this field, but they have yielded inconsistent results for a variety of reasons, such as differences in inclusion criteria, disease grouping and sampling methods[21].
In order to deeply understand the role of ischemia and inflammation in the pathogenesis of DME and RVO-ME and to find more reasonable treatment options, we compared the differences in the levels of pro-inflammatory factors (IL-6,IL-8, IL-18, TNF-α), chemokines (MCP-1) and growth factors(VEGF, PDGF) in the aqueous humor of DME and RVOME patients and analyzed the correlations among factors.Our study showed that, the aqueous humor levels of VEGF, PDGF,IL-6, IL-8, and MCP-1 were higher in both DME and RVOME groups compared with the control group, and the levels of TNF-α was higher in the DME group.These results are in accordance with most previous studies[22-27], Gonget al[22]reported that vitreous concentrations of VEGF, PDGF, IL-8, and TNF-α were significantly higher in DME patients than in controls, and Nomaet al[23]reported that vitreous concentrations of VEGF and IL-6 were significantly higher in RVO patients.Our study further confirms the key role of ischemia and inflammation in the above diseases.
In our study, the concentrations of VEGF, IL-6, MCP-1, and TNF-α were significantly elevated in the DME group than those in the RVO-ME group, and aqueous levels of IL-6 in the RVO-ME group were significantly higher than those in the control group.Funatsuet al[28]reported that vitreous levels of IL-6 were significantly elevated in DME patients than those of RVO patients, while there was no significant difference in the concentration of VEGF between the two groups.Contrary to our results, Leeet al's[29]study showed that aqueous levels of IL-6 in the RVO-ME group were not significantly different when compared with controls.In addition, our study found that aqueous levels of IL-8 in DME, RVO-ME patients and TNF-α in RVO-ME patients were higher than in controls,but there were no significant difference.In summary, despite differences in the results of individual cytokines, both the literature and our findings suggest that factors such as ischemia and inflammation are involved in the pathogenesis of DME and RVO-ME, but inflammation may play a more important role in the development of DME.This also suggests that anti-inflammatory therapy should be preferred for DME, or combined with anti-VEGF therapy, especially for those with poor response to anti-VEGF therapy[30-31].
There are complex relationships between growth factors and inflammation-related factors.For example, IL-6 induces the expression of VEGF[32], IL-8 up-regulates the expression of other cytokines and chemokines[33].In patients with DME and RVO-ME, previous studies have shown that vitreous levels of VEGF is significantly correlated with IL-6[24,34].Our study also found significant complex correlations among IL-6, IL-8,IL-18, MCP-1, and TNF-α in aqueous humor of the two diseases, but they were not entirely consistent.This once again indicates the complexity and the difference in the pathogenesis of DME and RVO-ME.Further correlation analysis is important to accurately evaluating the weight of related factors and guiding treatment.
The study has the following limitations: first, this was a single-center retrospective case control study, the significant differences and correlations among the factors need to be verified by prospective randomized studies.Furthermore, we could not control all possible confounding variables that may affect cytokine levels in aqueous humor, such as the stage of disease, RVO classification, and severity of ME.Finally,differences in cytokine levels may be masked by the relatively smaller sample size.
In conclusion, the elevated levels of pro-inflammatory factors, chemokines and growth factors in aqueous humor of DME and RVO-ME patients suggests that ischemia and inflammation are involved in the development of these two diseases.Inflammation may play a more important role in the development of DME when compared with RVO-ME, and therefore anti-inflammation should be the first-line treatment for DME.In addition, the correlation between multiple cytokines also illustrates the complexity of the pathogenesis of these two diseases.In the future, large-sample, multicenter,prospective randomized controlled studies to elucidate the regulation of each cytokine expression, the mechanisms of mutual regulation, and the relationship between them and the severity of the disease are of great significance for elucidating the pathogenesis of disease, finding specific diagnosis, guiding treatment, and evaluating prognosis.
ACKNOWLEDGEMENTS
Foundations:Supported by National Natural Science Foundation of China (No.81470654); Natural Science Foundation of Shaanxi Province (No.2019SF-047).
Conflicts of Interest: Hu KK,None;Tian CW,None;Li MH,None;Wu T,None;Gong M,None;Wei XL,None;Du YR,None;Hui YN,None;Du HJ,None.
 International Journal of Ophthalmology2023年7期
International Journal of Ophthalmology2023年7期
- International Journal of Ophthalmology的其它文章
- Chickenpox followed streaky multifocal choroiditis with prednison treatment in a girl with asthma
- Pneumonia and ocular disease as the primary presentations of Takayasu arteritis: a case report
- Unilateral blurred vision in pediatric patient associated with cavum velum interpositum cyst
- Highly cited publication performance in the ophthalmology category in the Web of Science database:a bibliometric analysis
- Comparison of efficacy of conbercept, aflibercept, and ranibizumab ophthalmic injection in the treatment of macular edema caused by retinal vein occlusion: a Metaanalysis
- Ocular manifestations and quality of life in patients after hematopoietic stem cell transplantation
