Crystal structure and terahertz spectrum studies of complex [Cu2(dmp)2(bdppmapy)I2]
LI Yingyu,GAO Chengjie,HU Fuzhen,LI Zixi,ZHOU Qingli,JIN Qionghua
(1.Department of Chemistry,Capital Normal University,Beijing 100048,China;2.Key Laboratory of Terahertz Optoelectronics,Ministry of Education,Beijing Advanced Innovation Center for Imaging Technology,Department of Physics,Capital Normal University,Beijing 100048,China )
Abstract:Terahertz time-domain spectroscopy (THz-TDS) based on femtosecond laser is a novel technique for coherent far-infrared spectroscopy.The weak interactions between molecules have corresponding absorption peaks in the terahertz band,so the terahertz spectrum can reflect abundant and complicated structural information.In this paper,the intermolecular forces of the copper(I) complex [Cu2(dmp)2(bdppmapy)I2] have been explored using the terahertz technique,and the effect of temperature on the π…π effect and other weak forces has been further characterized by using temperature-dependent terahertz.Temperature-dependent terahertz time-domain spectroscopy can accurately identify the π-stacking of copper complexes,a feature that thermogravimetric analysis and powder X-ray diffraction can not reveal.Given the high sensitivity of terahertz technology,the variations of the THz absorption peaks at different temperatures are used to qualitatively reflect the photoluminescence quantum yields of the cuprous complexes.These results open up a new direction for scientific research through terahertz spectroscopy in highly luminescent materials.
Keywords:terahertz spectrum;weak force;π-stacking;Cu(I) complex
0 Introduction
Intermolecular weak forces are present in practically all chemical reactions,and π-stacking has received a lot of attention[1-4].As a metal with low price and sufficient resource reserve,copper has been used in luminescent metal complex for optical devices and is widely considered as a reasonable substitute for precious metals due to its good efficiency[4].Cu(I) complex is widely used in preparing organic light-emitting devices (OLED)[6-7],memory devices[8],organic photocatalysis[9],and other material fields[10-12].With the development of luminescent copper complexes,Cu(I) complexes such as [Cu(N^N)(P^P)]+/0 (where N^N represents a chelated diamine ligand and P^P is either a chelated diphosphine ligand or two monophosphate ligands) have been extensively studied[13-15].Such weak forces are frequently appraised and defined by X-ray single crystal diffraction.However,the definition of the weak force is difficult for many materials for whose single-crystal diffraction is not possible.In addition,low-energy weak-forces are also difficult to analyze and characterize by other spectroscopic methods.
It was not until 1985 that modern terahertz science and technology began to develop with the rapid development of ultrafast laser,photonics and materials science technology[16].The frequency of the terahertz spectrum is significantly lower than those of infrared light,and the absorption features in the terahertz region can be attributed to weak intermolecular interaction.It has become a powerful tool to detect non-covalent interaction forces and has important strategic value and broad application prospects in medical imaging,safety detection,material detection and other fields[17-19].In fact,identifying all absorption peaks is difficult and unnecessary for substances with complex structures,and only the main peaks need to be identified.This designation relies on repeated comparisons of structures of the starting materials and terminal products.The purpose of delving into terahertz spectra,is to understand the relationship among absorption peaks,structures and properties,which can be used to explain the existing experimental results and guide synthesis.
We previously reported the Cu(I) complex (Fig.1),1,10-phen derivatives were selected as N-ligands.Pyridylphosphine ligands can form stable coordination compounds with transition metals.The formed complex has excellent photophysical properties,and the conjugated planar structure is easy to form π-stacking.In this paper,the reported complex was characterized by terahertz spectroscopy.The purpose is to detect and expose the relationship between weak π-stacking of the complex and the luminescence using terahertz time-domain spectroscopy,these results have been further confirmed by temperature-dependent terahertz spectroscopy.In our previous work,terahertz time-domain spectra were successfully linked to photoluminescence quantum yields via weak forces[20].At the same time,this is the first application of temperature-dependence terahertz technology in complex.
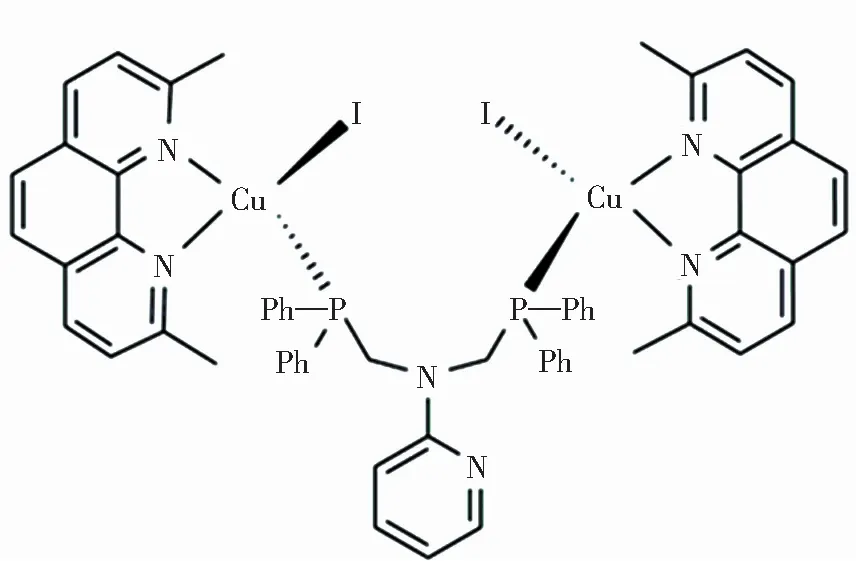
Fig.1 Chemical structure of Cu2(dmp)2(bdppmapy)I2
1 Experimental
1.1 Materials
All commercially available starting materials were used as received,and solvents were used without any purification.The syntheses of the ligand bdppmapy and metal complexes were described in our previous paper[21].
1.2 Measurements
CCDC No.2075018 contained the supplementary crystallographic data for complex.The crystal data were collected by Bruker SMART CCD X-ray single crystal diffractometer and analyzed by SHELEXL program;thermal stability analysis (TGA) was performed on a Labsys NETZSCH TG 209 Setaram;powder X-ray diffraction (PXRD) was performed on a Bruker-D8 ADVANCE diffractometer;terahertz absorption spectra were recorded on a terahertz time-domain device of Capital Normal University of China,effective frequency in the range of 0.2 to 3.0 THz.Terahertz spectra of the complex were measured at 298,313,328,343,358 and 373 K,the sample was recorded with two spectra and repeated determination to confirm the reproducibility of the spectra.Experimental devices for terahertz transmission measurements have been discussed in detail elsewhere[22].
2 Results and discussion
The relationship between the terahertz absorption spectrum and the intermolecular weak force was obtained by observing the terahertz absorption spectrum of the sample.The terahertz time-domain spectrum of the complex was measured at room temperature.The complex contains the diamine ligand,2,9-dimethyl-1,10-phenanthroline (dmp);Cu(I)-based complexes with functionalized dmp have a wide range of applications in luminescence[23].The introduction of methyl groups will hinder the flattening distortion of cuprous coordination center,thereby increasing the photoluminescence quantum yield of the complex.
Complex 1 has the π-stacking effect of the N heterocycle of the dmp ligand to the N heterocycle of the adjacent asymmetric unit dmp ligand.By repeatedly comparing the spatial forces and the terahertz absorption peaks,it can be found that there is only a pair of π…π stacking interaction in the space of complex 1,and the terahertz time-domain spectrum only has a very distinct absorption peak around 1.70 THz (Fig.2),which can be attributed to the N-heterocyclic stacking of the complex.The absorption peaks due to superposition forces are shifted by about 0.4 THz in the high terahertz direction for complex 1,which is because the asymmetric unit of complex 1 is a binuclear neutral molecule.The results show that the absorption peak of the binuclear neutral molecule complex near 1.70 THz belongs to π-stacking between heterocycles,and more similar experiments have been done in our previous study to prove this point[21].
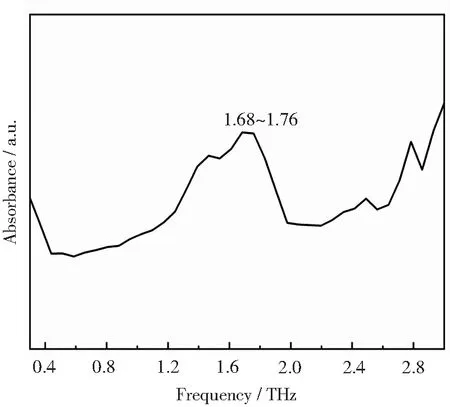
Fig.2 Terahertz absorption spectrum of complex 1 at ambient temperature
When the complex is converted to the excited state by the excitation of light energy,the change of lattice framework,especially the metal coordination center,is an important factor that affecting photophysical properties of the complex[24].This leads us to ponder whether it is possible to offer complex energies similar to that of light-energy,and then terahertz technology was used to detect the transformation of weak force into the lattice in order to create the relationship with complicated photophysical features,particularly the quantum yield.To further investigate the effect of temperature variations on the interlattice stacking of the complexes and on the π-stacking effect and other weak forces,the temperature-dependent terahertz time-domain spectrum of complex 1 for simple stacking structures was investigated in the temperature range of 298 to 373 K (Fig.3(a)).Note that the absorption peak at cal.1.70 THz,is significantly cut with increasing temperature and splits into two peaks at 328 K and higher temperatures,indicating some changes in π…π stacking during the warming process.The above-mentioned reasons have been confirmed by in situ varied temperature single-crystal analysis (Fig.3(b)).Table 1 lists the crystallographic data and other experimental details of complex 1 at 328 K.Table 2 summarizes the bond lengths and bond angles associated with the central Cu(I) coordination of complex 1 at 328 K.The cell volumes increases by 0.67% from ambient temperature to 328 K,with simultaneous increase of the unit cell parametera,b,candβ.The variation of atomic displacement parameters with temperature is universal[25-27].As mentioned earlier,the absorption peak of complex 1 at 1.70 THz is attributed to π-stacking.Single-crystal diffraction data measured at 328 K show an increase in the length of the π-stacking from 0.383 9 to 0.385 0 nm compared to the ambient environment temperature.In addition,the torsion angle between heterocycles increases simultaneously from 1.951° to 2.405° due to enhanced atomic thermal vibration at high temperatures.Both results lead to a weakening of π-stacking,which may be an important reason for splitting the absorption peak.The increase in the intensity of absorption peaks at low terahertz direction may be attributed to the increase in the intensity of lattice vibration,which needs to be proved at a deeper level.At temperatures above 328 K,the crystal decomposition to the point of irretrievable loss of crystallinity is unavoidable and single crystal data cannot be used for illustration and discussion due to the inability to refine.Further developments in spectral simulation techniques such as molecular dynamics simulation are expected to reveal the origin of temperature dependence of terahertz vibrational bands.
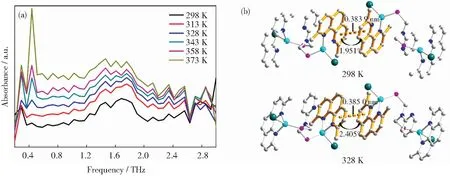
Fig.3 (a) Temperature-dependence of the terahertz absorption spectra of complex 1 between 298 and 373 K in the range of 0.2~3.0 THz;(b) crystal structure view of complex 1 at 298 and 328 K
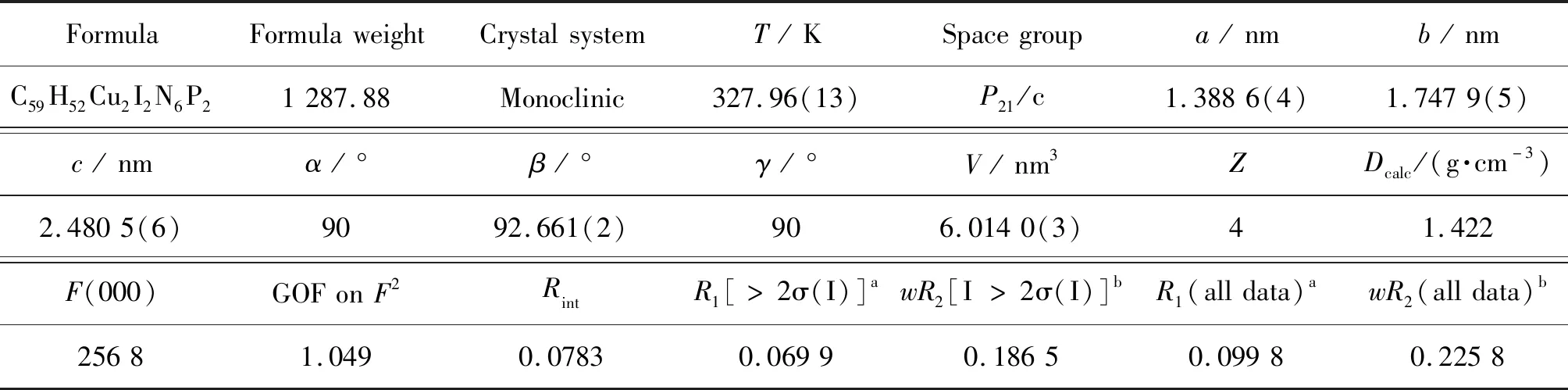
Table 1 Crystallographic data of complex 1 measured at 328 K

Table 2 Selected bond lengths,bond angles and their standard deviations of complex 1 at 328 K
Complex 1 was selected for the study of thermal and chemical stability by TGA and PXRD in the same temperature range.TGA reveals that the quality of complex 1 is almost unchanged in the above temperature range,and is thermally stable up to 537 K under a nitrogen atmosphere (Fig.4(a)).Similarly,its PXRD pattern was measured at 298,313,328,343,358 and 373 K (Fig.4(b)).It can be observed that the patterns of complex 1 at 298 K and higher temperatures can match the simulated patterns.Therefore,conventional methods such as thermogravimetric analysis and powder diffraction cannot be used to study the weak interaction between the lattices within a given temperature range.In other words,the temperature-dependence terahertz spectroscopy technique is a powerful tool for analyzing the variation of weak interactions with temperature.
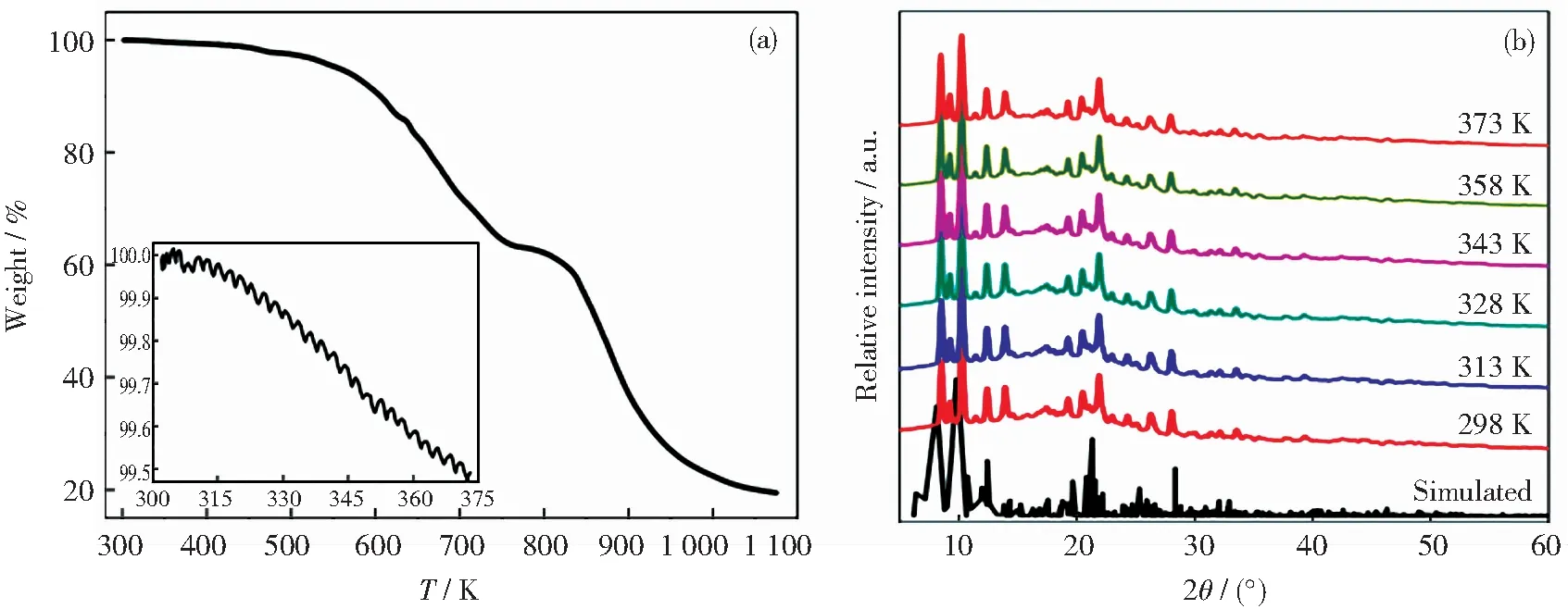
Fig.4 TGA curve (a) and temperature changing PXRD patterns (b) of complex 1
3 Conclusions
In summary,the terahertz time-domain spectrum at room temperature indicates that the absorption peaks at 1.70 THz are associated with π-stacking effects.The π-stacking length and the dihedral angle between the atomic rings creating the stacking force both increase with increasing temperature,according to in situ variable temperature single crystal analysis.In addition,the overall vibration of the lattice skeleton is increased.These conditions both cause a significant change in terahertz absorption peaks.The deformation of compound lattices after energy stimulation can be reflected in the peak shape of heated terahertz time-domain spectra at different temperatures,thereby combining photophysical features with terahertz technology.These findings pave the way for future research into the relationship between weak forces and crystal dynamics.

