Nusinersen for spinal muscular atrophy types II and III:a retrospective single-center study in South Korea
Hui Jin Shin · Ji-Hoon Na · Hyunjoo Lee · Young-Mock Lee
Abstract Background This study investigated the efficacy and safety of nusinersen,an antisense oligonucleotide,in patients with spinal muscular atrophy (SMA) types II (OMIM:253,550) or III (OMIM:253,400),including those with severe scoliosis or requiring respiratory support via mechanical ventilation.Methods Data from 40 patients with genetically confirmed SMA who were treated with nusinersen at our institute from March 2019 to April 2022 were retrospectively analyzed.Of these,30 patients with an age of onset < 3 years and not on permanent ventilation were selected.Clinical and genetic characteristics were investigated,and motor function was evaluated based on the Hammersmith Functional Motor Scale-Expanded (HFMSE) score.Results The mean age of symptom onset was 1.2 years.Most patients were diagnosed with SMA type II (27/30,90%).Nusinersen was administered via computed tomography-guided or direct intrathecal injection in 87% (26/30) and 13% (4/30) of the patients,respectively.At the 6-,14-,22-,and 26-month follow-ups,72%,71%,88%,and 86% of patients showed motor improvement,respectively,with mean changes in HFMSE scores of 2.10,2.88,4.21,and 5.29,respectively.Multivariable analysis showed that the use of noninvasive ventilation was associated with poorer outcomes of motor function.Conclusions Patients with SMA type II or III who received nusinersen treatment showed significant improvement in motor function.A longer treatment duration led to a higher number of patients with improved motor function.No significant side effects of nusinersen were observed.Patients with SMA,even those with severe scoliosis or on respiratory support,can be safely treated using nusinersen.
Keywords Hammersmith Functional Motor Scale Expanded · Motor function · Nusinersen · South Korea · Spinal muscular atrophy
Introduction
Spinal muscular atrophy (SMA) is an autosomal recessive neuromuscular disease caused by homozygous deletion or mutation of the survival motor neuron 1(SMN1)gene.SMN1(OMIM:600,354) undergoes RNA splicing to become a functional SMN protein,whereasSMN2(OMIM:601,627),which has a single-nucleotide transition of C-to-T-and an exonic splicing suppressor in exon 7,leads to exon 7 exclusion in mostSMN2mRNA transcripts and results in the production of an incomplete SMN protein [1,2].The SMN protein is expressed in many cells of the body,especially in the spinal cord [1].Low levels of SMN proteins are associated with cell death in the central nervous system,especially in spinal motor neurons [2].The low level of SMN protein in patients with SMA causes degeneration of motor neurons in the anterior horn cells in the spinal cord,which results in progressive muscle atrophy,weakness,and paralysis.
Clinical characteristics of SMA include symmetric motor difficulties or regression and proximal motor weakness,which extends from the lower extremities to the upper extremities,axial muscles,and finally the pulmonary and mastication muscles [3].Scoliosis and contracture of the knee,elbow,and ankle joints are frequently observed in patients with SMA [4].SMA is classified into five types according to the age of symptom onset and maximal functional status achieved.The severity of clinical symptoms in SMA is known to correlate with the number of SMN 2 copies [5–7].
Currently,several therapeutic agents are available that targetSMN1andSMN2[8].Onasemnogene abeparvovec-xioi is an adenovirus-associated vector-based intravenous drug carrying a functional copy of the humanSMN1gene.Risdiplam is an orally administered drug composed of a small molecule that modifiesSMN2pre-messenger RNA splicing to increase the amount of functional SMN protein [9].Nusinersen is a modified antisense oligonucleotide (ASO) intrathecal drug that targets the intronic splicing silencer N1 (ISS-N1) site to increaseSMN2exon 7 inclusion,modulating the premRNA splicing ofSMN2,a paralog ofSMN1,to generate a functional SMN protein [2].
Nusinersen is the first drug approved by the FDA,and previous studies have shown that it is effective in ameliorating motor function in both pediatric and adult patients with SMA types I,II,III,and IV [3,4,10–14].A recent meta-analysis showed that nusinersen may show better treatment outcomes when administered to SMA patients at a younger age or with a higher functional status [4].This retrospective single-center study investigated the efficacy and safety of nusinersen in a broad spectrum of patients with SMA types II (OMIM:253,550) or III (OMIM:253,400),both children and adults,including those with severe scoliosis or on respiratory support via mechanical ventilation.
Methods
This retrospective single-center study included patients with genetically confirmed SMA treated with intrathecal nusinersen at our institute between March 2019 and April 2022.The study was conducted in accordance with the ethical standards of the Institutional Review Board of our hospital (3-2022-0111) and with the Helsinki Declaration of 1964,as revised in 2000.The board waived the requirement for informed consent.
Patient selection
The inclusion criteria of patients for this study were as follows:(1) homozygous deletion ofSMN1,confirmed by genetic testing;(2) clinical characteristics that correspond to SMA types II and III,and (3) age of symptom onset below three years.The exclusion criteria of patients for this study were as follows:(1) no definite genetic testing;(2) diagnosis of other congenital structural anomalies;(3) diagnosis of neurological/neuromucular disorders;(4) existence of infection,and (5) history of other medications intended for the treatment of SMA.
Genetic testing
Multiplex ligation-dependent probe amplification (MLPA) was utilized to quantify copy-number changes in theSMN1andSMN2genes.MLPA was performed utilizing an SALSA SMN region test kit.O021,from MRC-Holland (NL).In this kit,there were specific probes forSMN1exons 7 and 8,SMN2exons 7 and 8,NAIPand other gene sequences within the 5q region.A homozygous deletion in exons 7 and 8 of theSMN1gene on chromosome 5q13.2 was identified.Copy numbers of exons 7 and 8 of theSMN2gene were also recorded.
Clinical characteristics of spinal muscular atrophy
SMA types II and III were classified based on the age of onset and maximum motor milestones achieved.In SMA type II,symptoms manifested during 6–18 months,and sitting without support was possible at some point during the patients’ lives.Patients with SMA type III developed symptoms after 18 months of age and were able to stand independently and walk at some point in their lives [15,16].
Administration of nusinersen
Nusinersen was administered via direct intrathecal injection or computed tomography (CT)-guided intrathecal injection.Regardless of patient age or body weight,the dose of nusinersen was 12 mg (5 mL).Patients were administered four loading doses:the first three doses at 14-day intervals and the fourth dose 30 days after the third dose.Subsequently,the maintenance doses of nusinersen were administered every four months (Table 1).

Table 1 Regimen of nusinersen administration
Direct intrathecal injection via lumbar puncture was performed by a pediatrician or child neurologist in a separate treatment room,accompanied by another doctor or physician assistant in case sedation was required and to closely monitor for complications during the procedure.CT-guided intrathecal injection was administered inpatients with severe scoliosis (scoliosis Cobb angle > 50° with vertebral rotation) or a history of previous spinal surgery.The injections were administered at the interventional CT suite by interventional radiologists with at least five years of experience in CT-guided procedures.
Treatment outcome
The primary endpoint was improvement in motor function,measured as the change in the Hammersmith Functional Motor Scale-Expanded (HFMSE) score from baseline.All physical therapists involved in the assessment underwent a comprehensive training session and were experienced in both pediatric and adult patient evaluations.Baseline motor function assessments were performed before administering nusinersen injection.In this study,the patient group with no motor function improvement at the six-month follow-up was compared with the group with significant improvement in HFMSE scores.Motor function outcomes were assessed at 6,14,22,and 26 months after treatment.
The safety and tolerability of nusinersen were assessed by the incidence of adverse events,changes in clinical laboratory parameters,vital signs,neurological examination,and additional visits to the emergency room or admission.These events were reviewed using electronic medical records or questionnaires.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics version 26.0 (IBM,Armonk,NY,USA).Chi-square and independent t tests were performed to identify clinically significant characteristics in patients with SMA.Fisher’s exact test and Pearson’s correlation coefficient were used for further evaluation.The Wilcoxon signed-rank test was performed to verify the significance of the change in HFMSE scores.The board waived the requirement for informed consent.
Results
Study patients
The data of a total of 40 patients with genetically confirmed SMA who were treated with nusinersen at our hospital from March 2019 to April 2022 were retrospectively analyzed.Of these,30 patients who met the inclusion criteria were selected.
Clinical characteristics
Regarding the 30 patients,the mean age at the last clinic visit was 24.85 years;the mean age of symptom onset was 1.2 years,and the mean age at the start of treatment was 22.9 years.The most common initial symptom was hypotonia (13/30,43%),followed by delayed development (12/30,40%) and gait disturbance (5/30,17%).Twenty-seven patients were diagnosed with SMA type II (27/30,90%);three patients were diagnosed with SMA type III (3/30,10%),and nine of these patients had a family history of SMA (9/30,30%).Genetic testing showed that all patients had homozygous deletion ofSMN1,28 patients had three copies ofSMN 2exon 7 (28/30,93%),and 29 had three copies ofSMN 2exon 8 (29/30,97%) (Table 2).
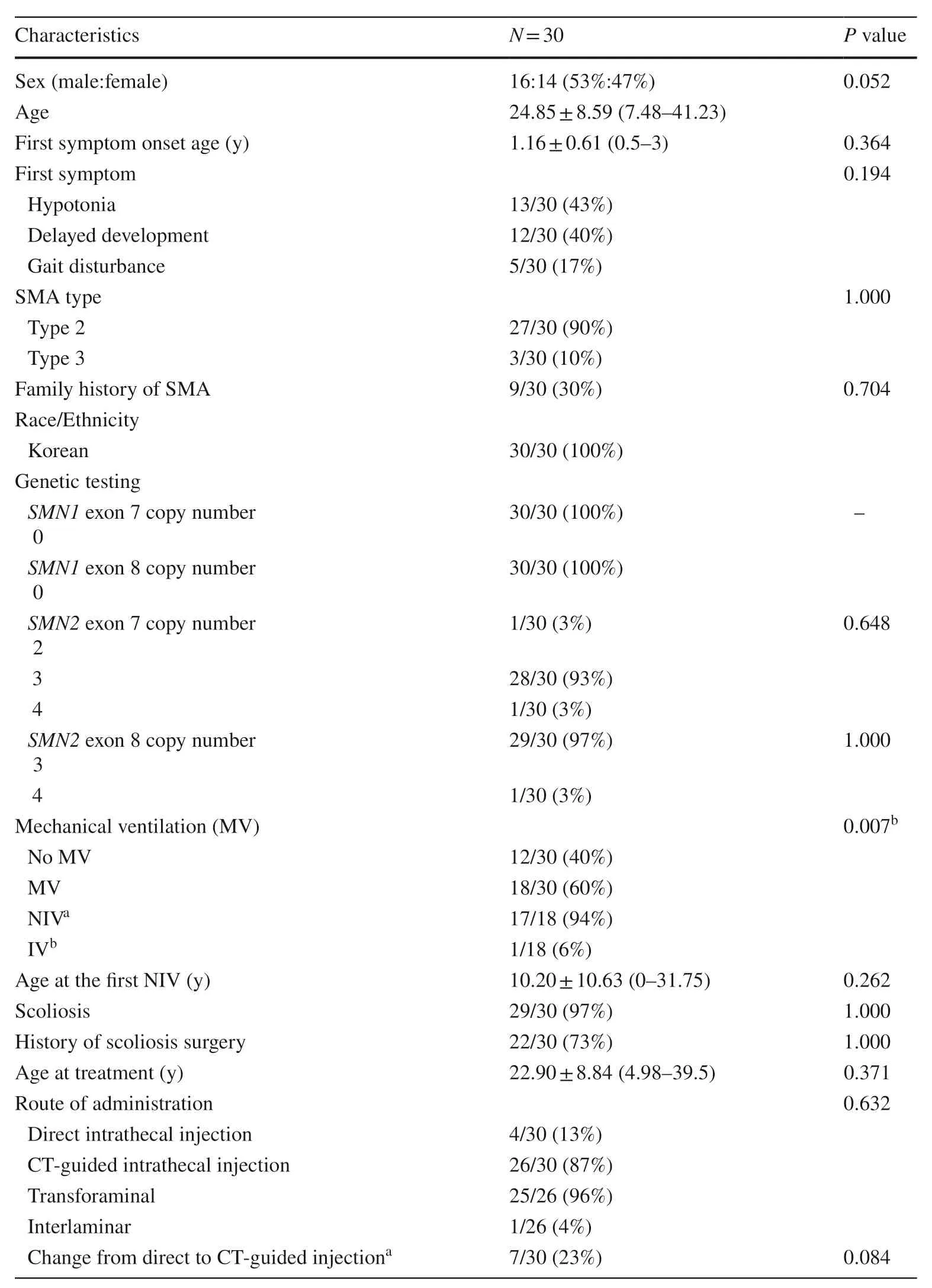
Table 2 General characteristics of patients
More than half of the patients (18/30,60%) required mechanical ventilation,which included noninvasive ventilation with bilevel positive airway pressure or a mechanical ventilator or invasive ventilation via tracheostomy,for less than 16 hours per day without definite oxygen dependency.Comorbidities are demonstrated in detail in Table 3.Statistical analysis showed that the use of mechanical ventilation was significantly associated with poorer motor function outcomes at six months after treatment (Table 2).Details regarding the clinical characteristics and genetic testing for each patient are shown in Tables 4 and 5.
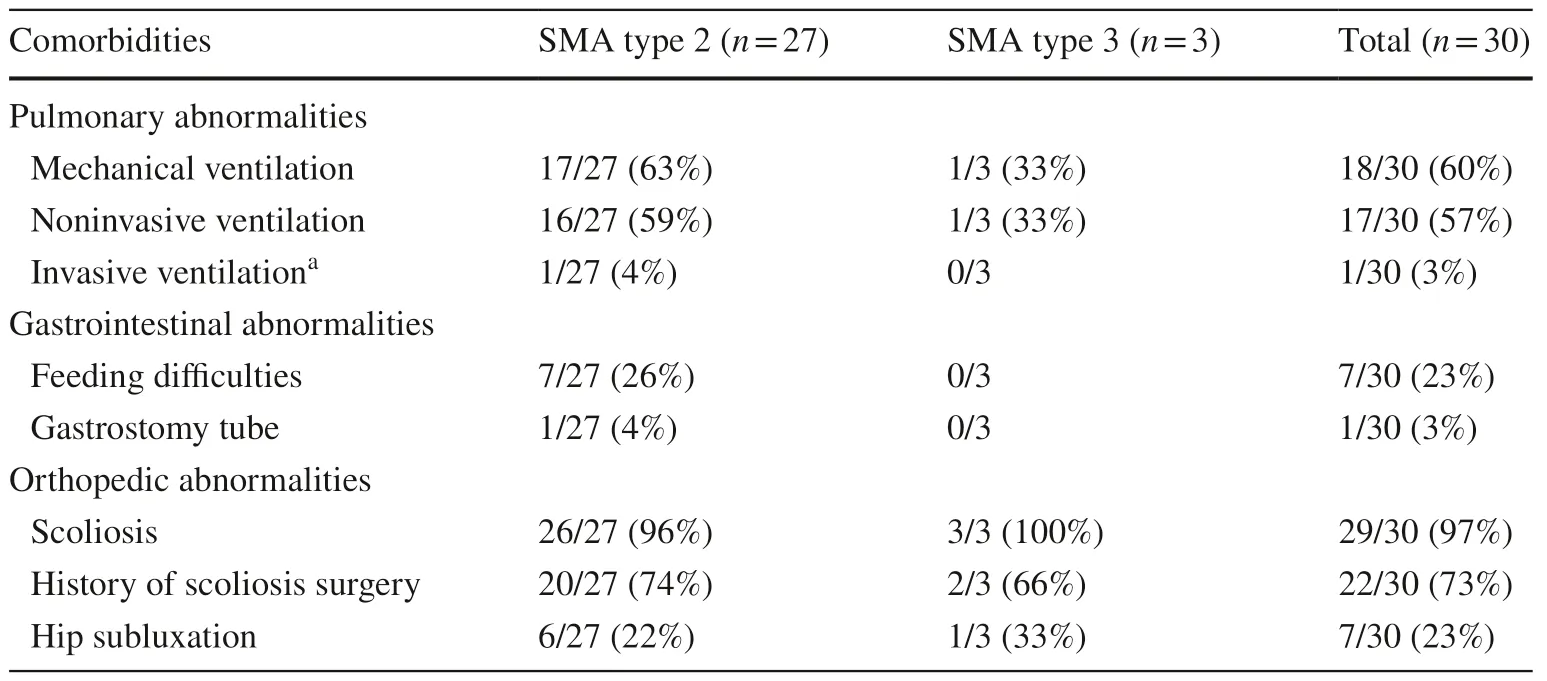
Table 3 Comorbidities according to SMA type
Scoliosis was manifested in 97% (29/30) of the patients,and scoliosis surgery was performed in 73% (22/30) of the patients.Nusinersen was administered via direct intrathecal injection in 13% of the patients (4/30) and via CT-guided intrathecal injection in 87% (26/30) of the patients.Seven patients were switched from direct to CT-guided intrathecal injection during treatment because of severe scoliosis,history of scoliosis surgery,or difficulty in lumbar puncture (Table 2).Regarding CT-guided intrathecal injection,the transforaminal approach was applied in 96% (25/26) of the patients.In the remaining patient,the interlaminar approach was applied after the second injection of nusinersen because of failure to attempt the transforaminal approach (Table 2).
Treatment progress
At the six-month follow-up,72% (21/29) of the patients showed improvement in the HFMSE score with a mean interval change of 2.10 (P< 0.001).At the 14-month follow-up,71% (20/28) showed motor improvement with an HFMSE score that was 2.88 points higher than baseline (P< 0.001).At the 22-month follow-up,88% (21/24) showed improved outcomes with a mean interval change of 4.21 (P< 0.001).At the 26-month follow-up,86% (12/14) of patients showed better HFMSE scores,with a mean increase of 5.29 (P=0.001) (Table 4,Fig.1).We observed that a longer treatment duration led to a higher proportionof patients with improved motor function (Fig.2).Table 5 shows the treatment outcome for each individual patient.
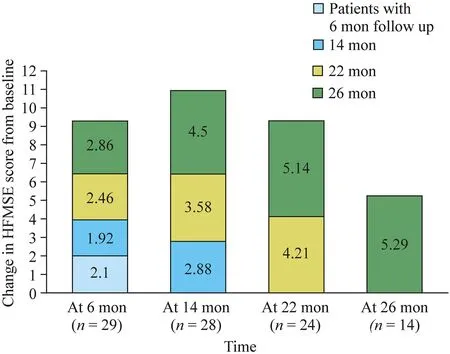
Fig.1 Change in Hammersmith Functional Motor Scale-Expanded (HFMSE) score from baseline
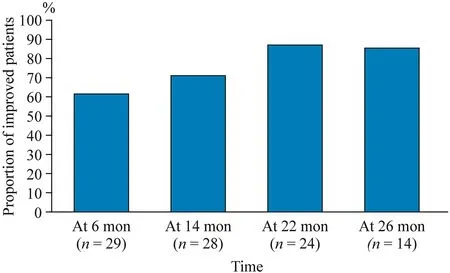
Fig.2 Proportion of improved patients according to time
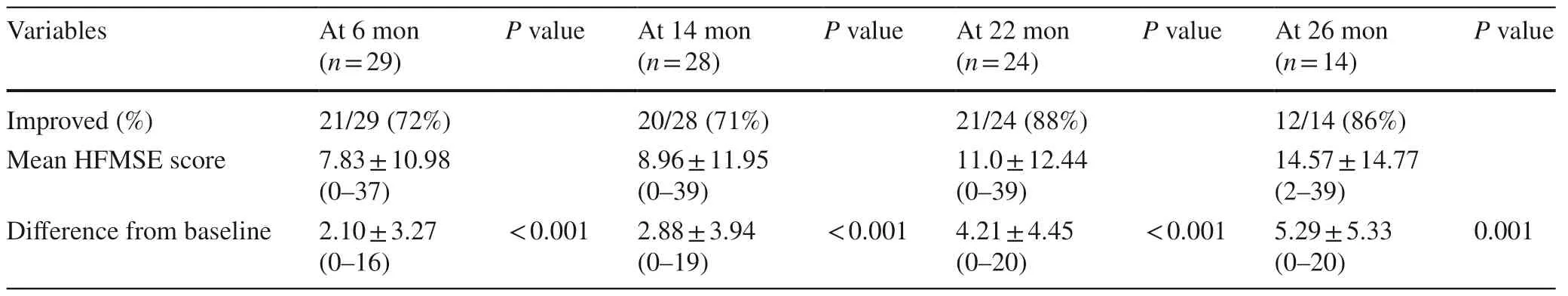
Table 4 Change in HFMSE scores from baseline
Adverse events
Adverse events included complications related to the lumbar puncture procedure.All events resolved within 1–2 days.These adverse events included lower back pain,headache,sensory abnormalities,limb pain,and nausea or vomiting after lumbar puncture (Table 6).
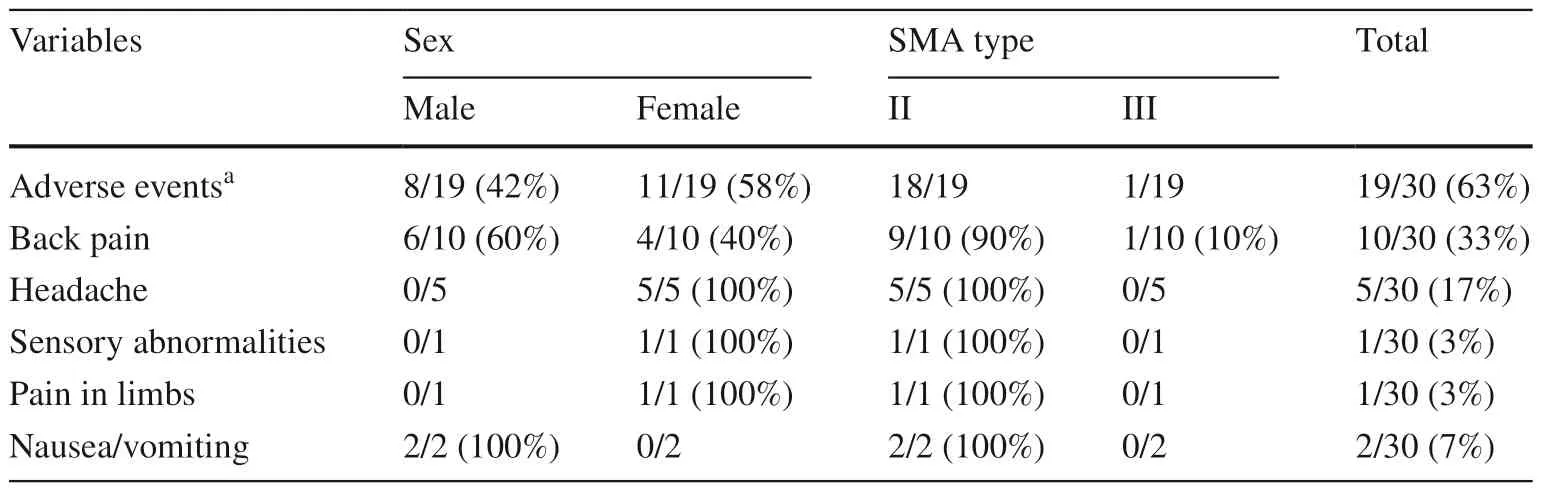
Table 6 Adverse events after administration of nusinersen
Discussion
Nusinersen is an 18-mer 2’-MOE phosphonothioate antisense oligonucleotide (ASO) that binds to a specific target sequence on theSMN2pre-mRNA to take the place of heterogeneous ribonucleoproteins (hnRNPs) at the intronic splice silencing site-1 (ISS-1) between exons 7 and 8.ISS-1 has a strong inhibitory effect onSMN2exon 7 inclusion.Nusinersen binds to ISS-1 and blocks this inhibitory effect,enabling more complete translation of SMN protein fromSMN2[17].
This is the first observational study in Korea that shows the efficacy and safety of nusinersen in a comprehensive group of patients,including both children and adults,diagnosed with SMA type II or III,with comorbidities such as severe scoliosis or requiring respiratory support via mechanical ventilation.Despite these risk factors,patients diagnosed with SMA type II or III presented with significant progress in the HFMSE score.
A recent meta-analysis confirmed that nusinersen is an effective treatment for SMA patients,which significantly increases the HFMSE score [4].Our study also revealed that more than 80% of SMA patients showed improvement in motor function according to the HFMSE score.It is important to note that a longer treatment duration led to a higher proportion of patients with improved motor function.On the other hand,SMA type or age group was not significantly associated with favorable outcomes,which was consistent with prior studies [4].
Prior literature has shown that SMA type I patients,even those on mechanical ventilation or those treated with gastrostomy,could also show improvement in motor function [18,19].Ergenekon et al.reviewed 52 SMA type I patients,including those on mechanical ventilation,and reported that all patients presented with significant improvement in motor function [18].Our study also showed that SMA type II and III patients,including those on mechanical ventilation,presented with better motor outcomes after administration of nusinersen.However,compared to SMA patients who did not require mechanical ventilation,those on invasive/noninvasive ventilation showed poorer motor outcomes at six months.
In addition to patients on respiratory support via mechanical ventilation,this study showed that the majority of SMA patients,including those with a history of scoliosis surgery,were safely treated with CT-guided intrathecal injection of nusinersen.There were no significant side effects,except postlumbar-puncture headache,back pain,or temporary sensory abnormalities.Therefore,some studies have suggested the facilitation of administration methods,such as additional orthopedic surgery.Machida et al.reviewed the data of four SMA patients who underwent posterior spinal corrective fusion surgery before successful intrathecal injection of nusinersen [20].Alternative methods of intrathecal injection include insertion of a device,such as an Ommaya reservoir or intrathecal port,for effective administration of nusinersen in SMA patients with severe scoliosis [20–23].Flotats-Bastardas et al.described a device that could be applied via a subcutaneous port connected to a permanent intrathecal catheter.These invasive procedures have increased risk factors,such as drug leakage,catheter-related infection,or complications related to surgery,such as bleeding [21].
Recent studies have shown that nusinersen can be effectively administered without invasive procedures if guided by imaging modalities,such as CT,ultrasonography,or fluoroscopy [24–28].Stolte et al.reviewed the data of 28 adult SMA patients who were administered intrathecal nusinersen [24].They recommended fluoroscopy-assisted lumbar puncture in adults with SMA type III and advanced scoliosis and CT-guided lumbar puncture in patients with SMA type III or spondylodesis [24].When proceeding with CT-guided lumbar puncture,the interlaminar approach is the most accessible and safest method,but transforaminal and posterior laminar approaches must also be considered in patients with complex anatomy of the spine [27,29,30].Because recurrent CT-guided lumbar punctures may lead to accumulated radiation exposure,Rosiak et al.even proposed a protocol for CT-guided injection of nusinersen that could significantly decrease the radiation dose,which may allow nusinersen administration to be more accessible to a broader spectrum of SMA patients [31].In this study,most of the patients were treated with nusinersen via the transforaminal approach,without complications,which is consistent with previous findings.
Apart from nusinersen,the approved therapeutic agents for SMA patients include onasemnogene abeparvovec-xioi,and risdiplam.Data comparing the efficacy of the onasemnogene abeparvovec-xioi or risdiplam to that of nusinersen are limited.To date,no prospective study has compared these drugs.Mirea et al.reported seven patients who received both the onasemnogene abeparvovec-xioi and nusinersen.After combination treatment,all patients showed motor function improvement,especially in the six patients who were treated first with nusinersen.In addition,there was a subtle improvement in respiratory function after six months of treatment [32].Harada et al.reported five patients on combination therapy of the onasemnogene abeparvovec-xioi and nusinersen,and this method was also found to be safe [33].In this trial,patients showed better gain in motor function when administered nusinersen compared with those who received the onasemnogene abeparvovec-xioi [33].Currently,there is an ongoing trial of patients being treated with risdiplam after nusinersen,and the results seem to be prognostic [34].To date,there is no literature regarding the administration of intravenous onasemnogenes to patients with severe scoliosis.D'Silva et al.reviewed 21 patients who were treated with onasemnogenes after nusinersen,and seven infants required mechanical ventilation at baseline [35].After treatment,five patients (71.4%) continued to require mechanical ventilation,and 16 patients (75%) had at least one World Health Organization motor milestone [35].Regarding risdiplam,Mercuri et al.reported improved motor function in patients with SMA type II or III,even those on mechanical ventilation or with a history of scoliosis surgery or severe scoliosis [36].
To have a comprehensive view of the efficacy of nusinersen,it is important to know the effect it may have in a variety of races and ethnicities.Accordingly,this study adds more value to the efficacy of nusinersen.Furthermore,this study included a wide variety of patients,including those with severe scoliosis or requiring mechanical ventilation,which further expands the spectrum of patients.The extant literature provides limited data regarding the treatment of SMA patients with high-risk factors.In this study,patients with high-risk factors were treated safely with nusinersen via CT-guided intrathecal injection.
This retrospective study has several limitations.First,this was a single-center study,and the number of patients was small.In addition,a longer follow-up duration may have provided further validation.Assessment of motor function was evaluated through HFMSE,which did not include upper limb activities,such as the Revised Upper Limb module,which may reflect the improvement in motor function more accurately in non-ambulatory SMA patients.
Further research is needed regarding an accurate method of motor function assessment for all types of SMA.Recently,Weststrate et al.described the validation of a scale to assess bulbar function [37].De Wel et al.reported a method for assessing hand motor function [38].Moreover,the efficacy of nusinersen should be evaluated not only through motor function but also by pulmonary function and improvement in daily life activities [39].Whether nusinersen improves pulmonary function in SMA patients remains controversial,and previous studies have mainly focused on SMA type I patients [40–44].Serum SMN protein levels or other biomarkers should be investigated,and the dose of medication should be adjusted if needed [45,46].More studies should be performed regarding the efficacy of nusinersen in patients with high-risk factors,such as tracheostomy status,gastrostomy status,history of scoliosis surgery,and permanent ventilatory support [18,47].
In conclusion,nusinersen could be effective and safe in a broad spectrum of patients with SMA type II or III,including both children and adults,regardless of severe scoliosis or respiratory support via mechanical ventilation.Although treatment outcomes may seem subtle in older patients,significant progress was noted in motor outcomes.Therefore,therapeutic agents,such as nusinersen,should be considered in all patients with SMA.Patients with difficult lumbar anatomy may be treated with CT-guided intrathecal injection of nusinersen,which is safe and effective.Standardized protocols are expected to ensure safety in the near future.Furthermore,although patients requiring the use of noninvasive ventilation had poorer motor function,a longer treatment duration led to a higher number of patients with improved motor function.Long-term treatment with nusinersen should be analyzed to further validate the efficacy of this drug.
AcknowledgementsWe would like to thank Editage ( www.edita ge.co.kr) for the English language editing.We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Author contributionsSHJ:data curation,formal analysis,investigation,methodology,validation,visualization,writing–original draft,and review &editing;NJH:data curation,supervision,and writing–review &editing;LH:data curation;LYM:conceptualization,supervision,writing-review &editing,project administration,and final approval.
FundingThe authors received no financial support for the research,authorship,and/or publication of this article.
Data availability statementThe authors confirm that the data supporting the findings of this study are available within the article.Raw data that support the findings of this study are available from the corresponding author,upon request.
Declarations
Conflict of interestNo financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.Ethical approval We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.This study was approved by the Institutional Review Board of Gangnam Severance Hospital,Yonsei University College of Medicine (3-2022-0111).The board waived the requirement for informed consent.
 World Journal of Pediatrics2023年5期
World Journal of Pediatrics2023年5期
- World Journal of Pediatrics的其它文章
- Multigenerational birth cohort study in China:importance,necessity and beyond
- Biliatresone:progress in biliary atresia study
- Sphingosine phosphate lyase insufficiency syndrome:a systematic review
- Early recombinant human growth hormone treatment improves mental development and alleviates deterioration of motor function in infants and young children with Prader–Willi syndrome
- Relationship between early nutrition and deep gray matter and lateral ventricular volumes of preterm infants at term-equivalent age
- Impact of the COVID-19 kindergarten closure on overweight and obesity among 3-to 7-year-old children
