Formulation and Antioxidant Activity Evaluation of Theaflavin Effervescent Tablet
Wenjing QI, Xueying WANG, Zhiling HU, Qi ZHENG, Qiyuan MAO, Shiming LI, Chang LIU
Abstract Theaflavins (TFs), as the major polyphenolic components of fermented tea, possess beneficial effects on human health. In this study, the effervescent tablets based on theaflavins were developed. The optimal formulation of TF effervescent tablets was obtained by response surface methodology with the Box-Behnken design. Then, the physiochemical properties were evaluated, including hardness, friability, effervescent time and pH of the solution. At last, the antioxidant ability of TF effervescent tablets was studied through DPPH and ABTS radical scavenging assay. According to the results, the optimal formulation of the tablets contained TF powder 9.09%, disintegrating agent 43.80% (the weight ratio of citric acid and sodium bicarbonate was 1:1), aspartame 1.86%, PEG-600 3%, and mannitol in balance. With the wet granulation method, the TF effervescent tablets displayed suitable hardness, fast disintegration time, good color, pleasant taste and high antioxidant activity. This study demonstrated that the TF effervescent tablets could be a valuable product for the supplement market and contribute to promoting practical application of TFs.
Key words Theaflavins; Effervescent tablet; Formulation; Physiochemical property; Antioxidant ability
Received: January 10, 2023 Accepted: March 11, 2023
Supported by Hubei Key Laboratory of Economic Forest Germplasm Improvement and Resources Comprehensive Utilization, Hubei Collaborative Innovation Center for the Characteristic Resources Exploitation of Dabie Mountains Fund (2021CX06).
Wenjing QI (2003-), female, P. R. China, major: food science and engineering.
*Corresponding author. E-mail: liuchang@hgnu.edu.cn.
Theaflavins (TFs), discovered by Roberts[1] (Roberts, 1958), are a group of compounds with benzotropolone skeleton which presented extensively in fermentated tea, such as black tea. During the fermentation process to form black and oolong tea, the released polyphenol oxidase initiates oxidation and polymerization of the catechins to theaflavins that contributes to the bright orange-red color of tea infusion. TFs are considered as the "golden molecules" because of their biological activity. Numerous studies show TFs possess beneficial effects on heath, including anti-oxidation, antibacterial, anticancer, anticancer, anti-obesity, and anti-atherosclerotic effects[2-4].
With the increasing research about the effectiveness of TFs, many TF products are developed as supplements and additives in nutritional medicines and functional food industry. TF products are mostly manufactured in the form of tablets and capsules, which are widely used solid oral dosage forms. However, it has been indicated that TFs possess poor systemic bioavailability by related research on the human absorption of TFs[5]. Mulder et al.[6] reported that less than 0.001% of consumed TFs was detected in plasma and urine after the intake of 700 mg of TF mixture by two volunteers, which was consistent with the findings of Pereira-Caro et al.[7]. Many studies confirmed that the formulation technologies play important roles in improving bioavailability by affecting the dissolution or release from the dosage form[8]. Hence, further research on the formulation of TF products is needed for promoting practical application of TFs.
Among the common forms, effervescent tablets are not only easy to use and store, but also better absorbed since it gets dissolved evenly. Effervescent tablets are manufactured by compressing the component ingredients containing soluble organic acid and alkali metal carbonate salt in the form of powder into a dense mass. Therefore, carbon dioxide is formed when effervescent tablets come into contact with water, which provides an interesting and unique feast for sense comparing to common tablets. The acid might be citric acid or tartaric acid, while the base might be sodium carbonate, sodium bicarbonate or potassium bicarbonate[9]. As for the formulation methodologies, the most widely applied approach for preparing effervescent tablet is wet granulation by making the material wetted by addition of binder solution and performing drying process to get dry granules[10]. Wet granulation process consists of wet massing of powder with binder solution, drying of wet mass to get dried granules, blending with lubricants and compression[11].
In this study, the TF effervescent tablets were fabricated by wet granulation method, and the effect of material composition on the sensory test was investigated. Based on the results, the optimal formulation of TF effervescent tablets was recommended and evaluated with pH, effervescent time and antioxidant activity.
Materials and methods
Materials
Theaflavin mixture powder (50%) from black tea extract was provided by Dehe Biotechnology Co., Ltd. (Wuxi, China). Citric acid, sodium bicarbonate, polyvinyl pyrrolidone (PVP), ethanol, aspartame, mannitol and PEG-600 were all purchased from Qilu Biotechnology Co., Ltd. (Shandong, China) and of food grade. 2,2-Diphenyl-1-picrylhydrazyl (DPPH), 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS), ascorbic acid, potassium persulfate were purchased from J&K Scientific Ltd. (Beijing, China). Ultrapure water was obtained from a Milli-Q system by Merck Millipore (Merck KGaA, Darmstadt, Germany).
Preparation of TF effervescent tablets
According to the wet granulation method, the effervescent tablets were prepared with TF powder, disintegrating agent (citric acid and sodium bicarbonate), PVP ethanol solution as binder solution, aspartame as sweetener, mannitol as excipient, and PEG-600 as lubricant. Firstly, citric acid and sodium bicarbonate were both mixed with TF powder, respectively. Subsequently, the acid and base mixtures were granulated separately with 6% PVP ethanol solution, which were then dried and mixed with equal proportion. Lastly, aspartame, mannitol and PEG-600 were added into the above mixture to form the uniform powder which were compressed by a tablet compressor at room temperature and lower than 50% humidity. Eventually, the TF effervescent tablets with 0.60 cm diameter and 0.30 cm thickness were obtained and stored for evaluation.
Sensory test
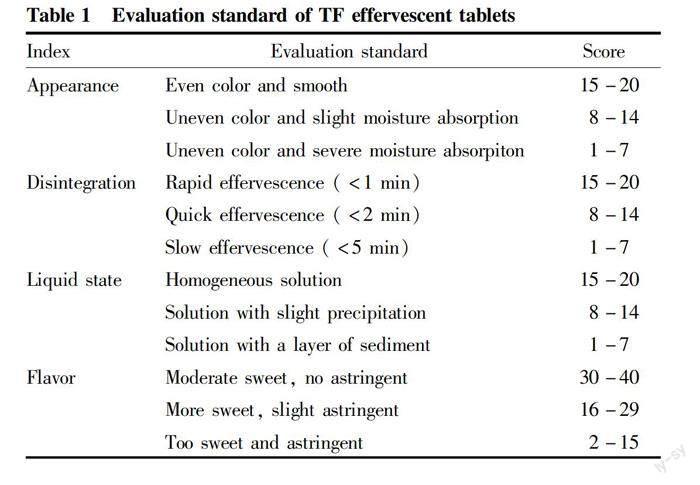
A sensory test for the prepared TF effervescent tablets was performed by evaluating the sensory properties, including appearance, effervescent time, flavor, and dissolution. Ten trained panelists aged from 18 to 25 with both genders were chosen from students of food science and technology. Every sample solution was prepared by adding four tablets into 200 ml of distilled water (solid-to-liquid ration was set as 1:50) at room temperature. By the end of disintegration, 20 ml of each sample was provided for panelists with random numbers. The standard for effervescent tablet evaluation is shown in Table 1. Total score of each group was 100 points, consisting of appearance (1-20 point), disintegration (1-20 point), liquid state (1-20 point) and flavor (1-40 point). The overall score of each tablet was calculated, and the average was taken as final score.
Optimization of experiment design with response surface method
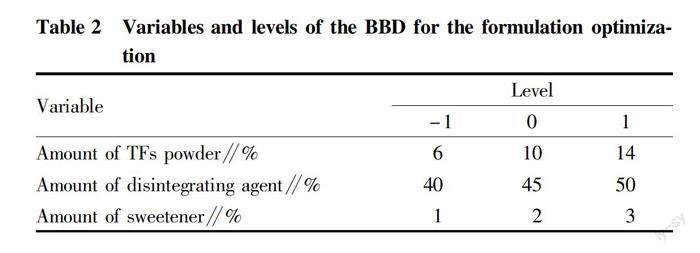
In order to optimize the formulation of TF effervescent tablets, the response surface method was used by conducting Box-Behnken Design (BBD). The amounts of active ingredient, disintegrating agent and sweetener in the effervescent tablets are usually main factors affecting their sensory properties[12-13]. Therefore, three factors with three levels in the BBD were the amounts of TF powder, disintegrating agent and sweetener, as shown in Table 2. According to the preliminary results, the proportion of lubricant (PEG-600) was fixed as 3%. The response and data analysis of BBD were performed using the Design-Expert 13.0.
Physicochemical evaluation of effervescent tablet evaluation
According to the quality control of effervescent tablets in China Pharmacopeia (2020 Edition), the physicochemical evaluation was conducted for TF effervescent tablets, including weight variation, thickness, hardness test, friability test, effervescent time and pH value. Twenty tablets were randomly collected and weighed individually, and the weight of each tablet was compared with the mean weight. The thicknesses of randomly selected 10 tablets were determined by a vernier caliper (Mitutoyo, Japan). Ten tablets were randomly selected and determined by the SY-6D Tablet four-use tester (Xinnuo, Shanghai, China) to perform the hardness and friability test of the formed TF effervescent tablets. In the hardness test, the force to break down the tablets in a compression is defined as the harness (N) of the tablets. In the friability test, tablets were weighed and shocked in the chamber revolving at 25 rpm for 4 min. Subsequently, these tablets were dedusted by rubber suction bulb and reweighed to calculate the friability. Tix tablets were placed individually into 200 ml of water at 20 ℃, and the effervescent time of each tablet was measured at the moment that clear solution was obtained. The pH value of the obtained solution was determined by a pH meter (Thermo, USA).
Antioxidant activity
The antioxidant activity of TFs effervescent tablets was evaluated by measuring the scavenging activities of DPPH and ABTS radicals. According to the method of Ruan et al.[14], the DPPH radical scavenging activity was studied as follows: 100 μl of different concentrations of TF effervescent tablet solutions (10, 50, 100, 150, 200, 250 mg/L) were added into the DPPH methanolic solution (5 ml, 100 μmol/L), and then incubated at dark for 30 min at room temperature. The absorbance of the solution was measured at 517 nm by the UV-Vis spectrophotometer (Mapada, Shanghai, China). The DPPH radical scavenging rate was calculated based on the following equation (1):
DPPH radical scavenging rate=[Ablank-(Asample-Acontrol)/Ablank]×100%(1)
in which Asample is the absorbance of the sample solution, Ablank is the absorbance of methanol, and Acontrol is the absorbance of sample solution without adding DPPH.
The ABTS radical scavenging activity of TF effervescent tablets was studied on the basis of the method of Xu et al.[15]. Briefly, ABTS radical could be generated by mixing 0.2 ml of ABTS solution (7.4 mmol/L) with 0.2 ml of potassium persulfate solution (2.6 mmol/L) at room temperature in the dark for 16 h. Then, the mixed solution was diluted with ethanol when the absorbance reached 0.70±0.02 at 734 nm (A0). Subsequently, 200 μl of different concentrations of TF effervescent tablet solutions (10, 50, 100, 150, 200, 250 mg/L) were added into the above prepared ABTS radical solution, and reacted for 5 min at room temperature. The absorbance value (Asample) was recorded at 734 nm. In addition, the absorbance value (Acontrol) of sample solution without ABTS radical was also measured at 734 nm. ABTS radical scavenging rate was calculated based on the following equation (2):
ABTS radical scavenging rate=[A0-(Asample-Acontrol)/A0]×100%(2)
Results and Discussion
Response surface experiment analysis
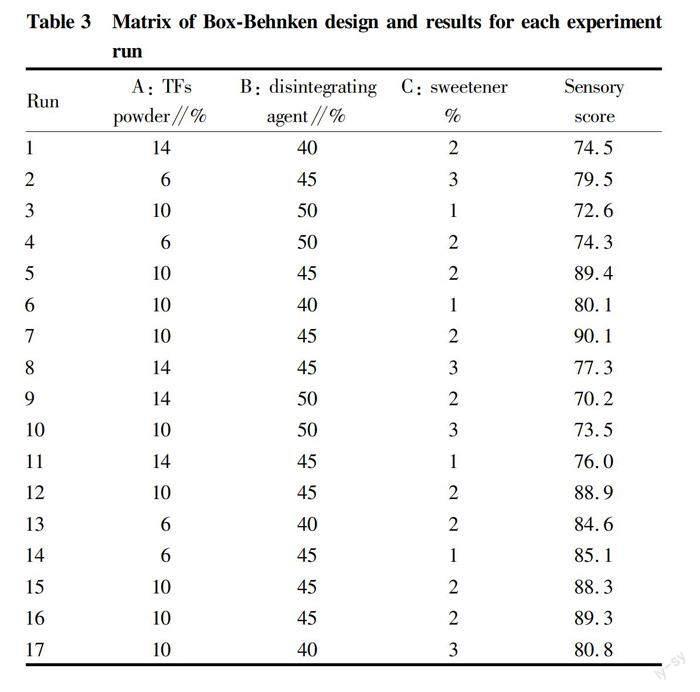
According to the preliminary experiment results, three factors were chosen to study their effects on the sensory evaluation (Y) of TF effervescent tablets, including TF powder (A), disintegrating agent (B) and sweetener (C). The matrix of Box-Behnken design and results for 17 experiment runs are shown in Table 3.
The experimental data were fitted by a quadratic equation to reveal the relationship between the response variable and intendent variables, which was demonstrated as follows:
Y=89.20-3.19A-3.67B-0.337 5C+1.50AB+1.72AC+0.05BC-5.29A2-8.01B2-4.44C2
where Y is the sensory score. The correlation coefficient (R2) was 99.03% that indicating the model fitted the variation well. And the adequate precision measures the signal to noise, which was calculated as 24.05 greater than 4, indicating an adequate signal. The analysis of variance (ANOVA) for the model is shown in Table 4. Based on Table 4, the F-value of the model was 79.50 and P-value was less than 0.000 1, which implied the model is significant. In addition, the lack of fit F-value and P-value were 4.10 and 0.103 3, respectively, confirming the good fit and suitability of the model for the experiment results. The above results proved that the model could be used for the optimization the formulation.
In order to obtain more clear expression about the effect of the interaction of factors on the sensory evaluation, the 3D surface and contour plot are demonstrated in Fig. 1-Fig. 3. It is very useful to find out different surface shapes for different variables and effectiveness on the sensory score. Similar to 3D surface plot, contour plot also unveil the interaction among variables which is more straightforward than the former[16]. The response surface attained by plotting the variables TF powder versus disintegrating agent, represented in Fig. 1, shows a clear surface, suggesting that the optimum sensory score was well inside the design boundary. The sensory score demonstrated obvious first increasing and then decreasing with the increasing of both TF powder and disintegrating agent. The reason behind this was TF powder and disintegrating agent had comprehensive influence on the sensory evaluation of tablets. Since theaflavins contribute to the astringency (briskness) and brightness of black tea[17], the excessive amount of TF powder would result in dark color, uneven solution and astringent flavor. Similarly, the disintegrating agent would contribute to the rapid effervescent time, while leading to the sour flavor. Hence, disintegrating agent and TF powder had significant effect on the sensory score of the tablets, which was confirmed by the ANOVA results. From the contour plot, the effect of variables could be more clearly observed, and the sensory score reached to the maximum when the amount of TF powder and disintegrating agent were about 8%-10% and 43%-45%, respectively. Compared with the TF powder and disintegrating agent, sweetener had little influence on the sensory score which could be seen in the contour plot in Fig. 2 and Fig. 3. Since the maximum usage of aspartame in China National Food Safety Standard (GB 2760-2014) is 3.0% in effervescent tablets, the amount of sweetener in the range of 1%-3% might have low impact on the flavor of tablets as well as the sensory score.
According to the model, the optimal formulation composition was TF powder 9.09%, disintegrating agent 43.80%, and sweetener 1.86% with a sensory score of 90.2 point. As the most dominant factor affecting consumers preference for foods, the sensory evaluation plays the key role in consumer appeal, purchasing decisions and ultimate consumption. Hence, the optimal formulation possessed the maximum sensory score was obtained and subsequently studied by physicochemical properties as well as antioxidant ability.
Effervescent tablet evaluation
With the optimal formulation, the TF effervescent tablets with 0.60 cm diameter, 0.30 cm thickness and 1.003 g were prepared. The physiochemical properties of the tablets were obtained in Table 5. Hardness is one of the most important parameters to reflect the strength of the tablet and the density of the tablet ingredients[10,18]. The inappropriate hardness of tablet would lead to the damage and crumble in the storage or insolubility in water for tablets. The hardness of TF effervescent tablets was measured as 67.80 N, meeting the tablets requirement for hardness, which could also be proved by the friability of the tablets. The friability of TFs effervescent tablets was 0.65% (less than 1%), which was in compliance with the requirement in China Pharmacopeia. The effervescent time refers to the time required for the disintegration and dissolution of the tablets. The effervescent time within 5 min for oral effervescent tablets was acceptable for several countries[19]. The TF effervescent tablets possessed rapid effervescent time, less than 1 min, which might due to the proper formulation and granulation method. At last, the pH of the solution was 6.36, showing the complete effervescent reaction.
Antioxidant activity
It is well-known that theaflavins have excellent antioxidant properties. Evaluation of antioxidants activity of TF effervescent tablets, specifically the free radical scavenging ability, could be performed to confirm the function of TF products. DPPH and ABTS assays are the most widely used tools for antioxidant activity evaluation in food industries due to their great reproducibility. Thus, the DPPH and ABTS radical scavenging effect of the TF effervescent tablets was studied and shown in Fig. 4. With the increasing concentration of effervescent tablets from 10 to 250 mg/L, the scavenging rates for both DPPH and ABTS radical were rising and close to 100%. The ascorbic acid was used as positive control, and it turns out that the antioxidant ability of TF effervescent tablets was slightly less than that of Vc.
Conclusion
This study developed an optimal formulation of TF effervescent tablets by response surface methodology with the Box-Behnken design. Then, the physiochemical properties were evaluated, including hardness, friability, effervescent time and pH of the solution. At last, the antioxidant ability of TF effervescent tablets was studied through DPPH and ABTS radical scavenging assay. The optimal formulation of tablets contained TF powder 9.09%, disintegrating agent 43.80% (the weight ratio of citric acid and sodium bicarbonate was 1:1), aspartame 1.86%, and PEG-600 3%, and the remaining amount was mannitol. With the wet granulation method, the obtained TF effervescent tablets displayed appropriate hardness, fast disintegration time, good color, pleasant taste and high antioxidant activity. The TF effervescent tablets could be a valuable product for the supplement market and contribute to promoting practical application of TFs.
References
[1] ROBERTS E. The phenolic substances of manufactured tea[J]. Journal of the Science of Food and Agriculture, 1958, 9(4): 212-216.
[2] MOHAMED IMA, OGAWA H, TAKEDA Y. In vitro virucidal activity of the theaflavin-concentrated tea extract TY-1 against influenza A virus[J]. Journal of Natural Medicines, 2022, 76(1): 152-160.
[3] ONEILL EJ, TERMINI D, ALBANO A, et al. Anti-cancer properties of theaflavins[J]. Molecules, 2021, 26(4): 987.
[4] TAKEMOTO M, TAKEMOTO H. Synthesis of theaflavins and their functions[J]. Molecules, 2018, 23(4): 918.
[5] CLIFFORD MN, VAN DER HOOFT JJ, CROZIER A. Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols[J]. The American Journal of Clinical Nutrition, 2013, 98(6): 1619S-1630S.
[6] MULDER TPJ, VAN PLATERINK CJ, WIJNAND SCHUYL PJ, et al. Analysis of theaflavins in biological fluids using liquid chromatography–electrospray mass spectrometry[J]. Journal of Chromatography B: Biomedical Sciences and Applications, 2001, 760(2): 271-279.
[7] PEREIRA-CARO G, MORENO-ROJAS JM, BRINDANI N, et al. Bioavailability of black tea theaflavins: Absorption, metabolism, and colonic catabolism[J]. Journal of Agricultural and Food Chemistry, 2017, 65(26): 5365-5374.
[8] HOAG SW, HUSSAIN AS. The impact of formulation on bioavailability: Summary of workshop discussion[J]. The Journal of Nutrition, 2001, 131(4): 1389S-1391S.
[9] NGUYEN VT, PHAM QT. Effect of raw material and processing factors on the production of effervescent artichoke (Cynara scolymus L.) tea tablets[J]. International Journal of Food Engineering, 2011, 7(1): 5.
[10] PATEL SG, SIDDAIAH M. Formulation and evaluation of effervescent tablets: A review[J]. Journal of Drug Delivery and Therapeutics, 2018, 8(6): 296-303.
[11] PRAJAPATI B, MISHRA O. Concept, manufacturing and characterization of effervescent tablets: A review[J]. SunText Review of Pharmaceutical Sciences. 2021, 2(1): 110.
[12] NAJI-TABASI S, EMADZADEH B, SHAHIDI-NOGHABI M, et al. Physico-chemical and antioxidant properties of barberry juice powder and its effervescent tablets[J]. Chemical and Biological Technologies in Agriculture, 2021, 8(1): 23.
[13] WU S, YUAN Y, YIN J, et al. Characteristics of effervescent tablets of Aronia melanocarpa: Response surface design and antioxidant activity evaluation[J]. Journal of Food Measurement and Characterization, 2022, 16(4): 2969-2977.
[14] RUAN J, PEI H, LI T, et al. Preparation and antioxidant activity evaluation of tea polyphenol-collagen-alginate microspheres[J]. Journal of Food Processing and Preservation, 2021, 45(2): e15187.
[15] XU X, HU W, ZHOU S, et al. Increased phenolic content and enhanced antioxidant activity in fermented glutinous rice supplemented with Fu brick tea[J]. Molecules, 2019, 24(4): 671.
[16] KUMAR M, MISHRA PK, UPADHYAY SN. Pyrolysis of Saccharum munja: Optimization of process parameters using response surface methodology (RSM) and evaluation of kinetic parameters[J]. Bioresource Technology Reports, 2019(8): 100332.
[17] OWUOR PO, MCDOWELL I. Changes in theaflavin composition and astringency during black tea fermentation[J]. Food Chemistry, 1994, 51(3): 251-254.
[18] YU D, SEELAM RR, ZHANG F, et al. Evaluation of tableting performance of poly (ethylene oxide) in abuse-deterrent formulations using compaction simulation studies[J]. Journal of Pharmaceutical Sciences, 2021, 110(7): 2789-2799.
[19] ZHAO F, LI M, MENG L, et al. Characteristics of effervescent tablets of Lactobacilli supplemented with Chinese ginseng (Panax ginseng C.A. Meyer) and Polygonatum sibiricum[J]. Applied Sciences, 2020, 10(9): 3194.
- 农业生物技术(英文版)的其它文章
- Improving the Heat Resistance of β-1,4 Glucanase by Introducing Disulfide Bonds
- Comparative Study on Biological and Commercial Characteristics of Mul-tiple Varieties of Broccoli (Brassica oleracea L. var. italica Plenck) and Vegetable Soybean (Glycine max (L.) Merr.)
- Effects of Bamboo Charcoal-based Biochar on Soil Enzyme Activity and Microbial Community Structure
- Summary of Short-vine Watermelon Breeding Practice
- Effects of Combined Application of Biochar-based Organic Fertilizer and Reduced Nitrogen Fertilizer on Soil Enzyme Activity and Yield of Purple Cabbage (Brassica oleracea var. capita rubra)
- Effects of Microelement Fertilizers on Main Economic Characters and Yield of Peanut

