Effects of Bamboo Charcoal-based Biochar on Soil Enzyme Activity and Microbial Community Structure
Yizu PAN, Sihai ZHANG
Abstract [Objectives] This study was conducted to reveal the effects of bamboo charcoal-based biochar (or bamboo charcoal for short) on soil enzyme activity and microbial community structure.
[Methods] The field experiment was carried out at the Modern Agriculture Demonstration Base of Gaoping Village, Gaoping Town, Suichang County, Zhejiang Province. Bamboo charcoal was applied at four different levels: T0 (no bamboo charcoal), T1 (1 125 kg/hm2 bamboo charcoal), T2 (2 250 kg/hm2 bamboo charcoal) and T3 (3 375 kg/hm2 bamboo charcoal). Soil physicochemical properties and enzyme activities in different treatments were measured.
[Results] The soil fungal, bacterial and actinomycete populations increased significantly in the soils surrounding capsicum roots. The bacterial population, fungal population and fungus/bacterium ratio peaked in Treatment T2, up to 7.32×106 cfu/g, 2.65×104 cfu/g and 0.36×10-2, respectively. The effect of bamboo charcoal in promoting β-glucoside, catalase, acid phosphatase and sucrase activities was T2>T3>T1>T0. With bamboo charcoal increasing, the bacterium population, fungus population, fungus/bacterium ratio, β-glucoside, catalase, acid phosphatase and sucrase activities all increased at first and then decreased. T2 treatment showed the best effects in improving soil physicochemical properties and microbial community structure.
[Conclusions] Bamboo charcoal significantly improves soil enzyme activity and increases soil microbial population, and thus has important positive effects on the soil ecosystem.
Key words Bamboo charcoal-based biochar; Soil enzyme activity; Microbial community structure
Received: January 5, 2023 Accepted: March 6, 2023
Supported by Special Fund of Lishui City for Public Interest (2021GYX11); Special Fund of Zhejiang Provincial Department of Finance for Basic Research and Development of Bamboo Charcoal-based Soil Conditioner (20180021); Key Research and Development Project of Zhejiang Province (2018C02031).
Yizu PAN (1999-), male, P. R. China, master, devoted to the research in soil ecology and environmental restoration.
*Corresponding author. E-mail: zhangsihai2352@163.com.
Biochar is a stable solid made from biomass (primarily carbon, hydrogen and oxygen) via pyrolysis of incomplete combustion in the absence of oxygen, and rich in carbon, with large pore volume, large specific surface area, strong adsorption and high degree of aromatization[1-2]. According to the source of materials, biochar can be classified into wood charcoal, bamboo charcoal, straw charcoal, rice husk charcoal, animal manure charcoal, etc.[2]. Bamboo charcoal-based biochar (or bamboo charcoal for short) is made up of bamboo, better than other types of biochar in specific surface area, specific pore volume and average pore size. Studies have shown that the specific surface area and total pore volume of bamboo charcoal are twice that of rice straw charcoal, and it has significantly higher cation exchange capacity (CEC) in soil than rice straw char[3]. A series of biological reactions occur after bamboo charcoal is applied to soils, and many organisms are involved in these reactions, including bacteria, algae, fungi, actinomycetes, protozoa, nematodes, mites and isopods, among which bacteria are most numerous and essential[4], while protozoa and nematodes also play an important role[5]. Protozoa regulate bacterial communities by feeding on bacteria; nematodes regulate both bacterial and fungal communities. Bacteria, protozoa (including three functional populations: flagellates, ciliates and amoebae), and bacterial-feeding nematodes form a pathway to degrade organic matters (sugars, fats, and starch) which have low carbon to nitrogen ratio (C/N) and are easy to decompose, and the degradation is faster. Fungi, fungal-feeding nematodes, omnivorous nematodes and predatory nematodes form another pathway to degrade cellulose and lignin, etc., which have high C/N ratio and are difficult to decompose, and the degradation is relatively slow[6-9]. The number and diversity of soil microorganisms are important biological indicators for characterizing the changes in effective soil nutrients and monitoring the dynamics of soil ecology[10-12]. Soil microorganisms play an important role in soil food webs, and the changes in microbial community can directly or indirectly affect the distribution, quantity, abundance, community structure and diversity of other ecological niches[13].
Materials and Methods
Materials
The field experiment was carried out at the Modern Agriculture Demonstration Base in Gaoping Village, Gaoping Town, Suichang County, Zhejiang Province. After years of continuous cropping and serious diseases, the crop yield decreased remarkably at this base. The background values of the soil samples were 20.89 g/kg organic matter, 145.65 mg/kg available nitrogen, 20.82 mg/kg available phosphorus, 1 116.74 mg/kg available potassium, pH 6.00.
Experimental design
A plastic greenhouse which was south-facing, 8 m wide and 102 m long was selected, divided into 16 plots, each with a width of 8 m, a length of 6 m and an area of 48 m2. Bamboo charcoal were applied at four different levels: T0 (no bamboo charcoal), T1 (1 125 kg/hm2 bamboo charcoal powder), T2 (2 250 kg/hm2 bamboo charcoal powder) and T3 (3 375 kg/hm2 bamboo charcoal powder) were set. The bamboo charcoal powder used in the experiment was made up of moso bamboo straw via being charred at 800 °C and then passed through an 80-mesh sieve. On April 9, 2021, bamboo charcoal powder was evenly spread along with base fertilizer (organic fertilizer) to the experimental plots, which were then plowed and harrowed before beds were prepared. Pepper seedlings were transplanted on May 2. Rhizosphere soils (0-20 cm deep) were sampled along an S-shaped line in each plot on November 10, brought back to our laboratory, and processed for different tests.
Measurement items and methods
Soil nutrient indexes were determined by referring to "Soil Agro-chemical Analysis (3rd edition)"[13] by Shidan Bao. Organic matter contents were measured via oxidation with potassium dichromate. Available nitrogen was measured using a micro-diffusion method, in which ammonia was released from the soil sample by sodium hydroxide and then absorbed by boric acid. Available potassium was measured via ammonium acetate extraction and flame photometry, available phosphorus by Olsen-P method. Available zinc was determined by being boiled in aqua regia and measured by ICE3000 atomic absorption spectrometry. Soil peroxidase activity was assayed via UV spectrophotometry, acid phosphatase activity via colorimetry using disodium p-nitrophenyl phosphate as the substrate. Urease activity was assayed by means of phenol-hypochlorite reaction. Sucrase activity was assayed by using the 3,5-dinitrosalicylic acid method. Soil bacterial population was determined using beef paste peptone medium, actinomycete population using Gauzes Synthetic Medium No.1, and fungal population using Martins medium.
Data processing
The data were processed using Excel 2003, DPS and SPSS 16.0 software, and the significant differences of soil microbial populations between treatments were determined by means of canonical correspondence analysis (CCA) and analysis of variance (ANOVA).
Results and Analysis
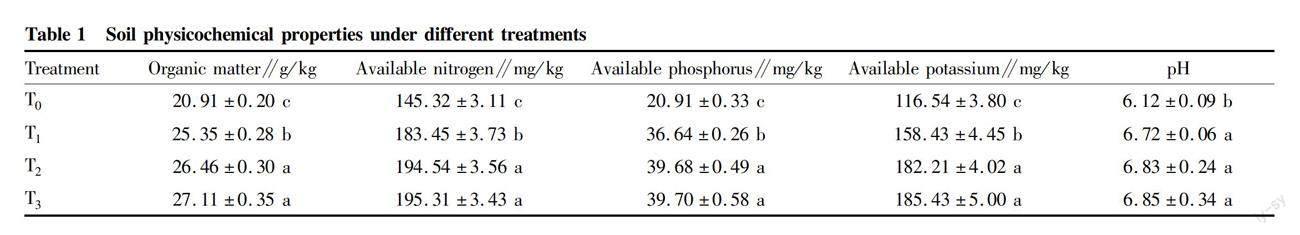
Effect of bamboo charcoal on soil physicochemical properties
As shown in Table 1, bamboo charcoal significantly increased soil organic matter content in a concentration-dependent manner. Treatment T3 had the highest content of organic matter, up to 27.11 g/kg, while treatment T0 had lowest, which was only 20.91 g/kg. Compared with Treatment T0, bamboo charcoal increased soil organic matter by 21.23% to 29.65% in treatments T1, T2 and T3. In addition, bamboo charcoal also increased the contents of soil available nitrogen, phosphorus and potassium in a concentration-dependent manner, so all the three indices peaked in T3 treatments, up to 195.31, 185.43 and 39.70 mg/kg, respectively. Compared with Treatment T0, soil available nitrogen, phosphorus and potassium in Treatment T3 were raised by 34.41%, 59.11 % and 89.86%, respectively. The contents of available nitrogen, phosphorus and potassium in treatments T2 and T3 were significantly higher than those in Treatment T1, while no significant difference was observed between treatments T2 and T3. Soil pH changed in a similar trend with organic matter content when bamboo charcoal was applied, which also reached the highest in treatment T3 and the lowest in treatment T0, showing significant difference between bamboo charcoal treatments and Treatment T0 (P<0.05).
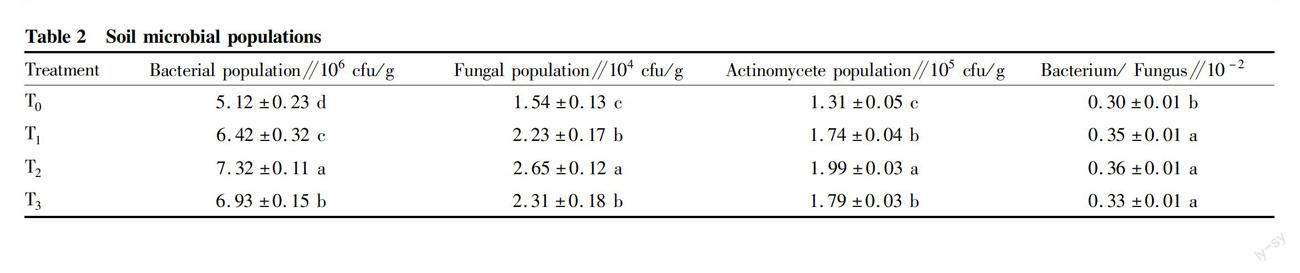
Effect of bamboo charcoal on soil microbial community structure
As shown in Table 2, the microbial populations in all bamboo charcoal treatments were significantly different from those in Treatment T0 (P<0.05). Among them, Treatment T2 had the biggest bacterial, fungal, actinomycete populations, up to 7.32×106 cfu/g, 2.66×104 cfu/g and 1.89×105 cfu/g, respectively, followed by Treatment T3, while Treatment T0 had the smallest microbial populations. Compared with Treatment T0, the bacterial, fungal and actinomycete populations and bacterium/fungus ratio in bamboo charcoal treatments were increased by 25.31%-42.90%, 44.70%-72.45%, 32.77%-43.67%, and 11.01%-20.68%, respectively.
Effect of bamboo charcoal on soil enzyme activity
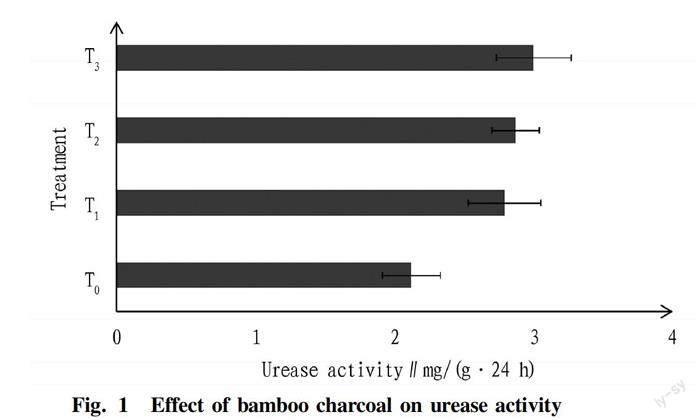
Effect of bamboo charcoal on urease and acid phosphatase activity
From Fig. 1 and Fig. 2, it could be seen that the soil urease and acid phosphatase activities were both increased by bamboo charcoal. Soil urease activity showed significant difference between the control and bamboo charcoal treatments (P<0.05), reached the highest value 3.00 mg/(g·24 h) in Treatment T3 and the lowest value 2.12 mg/(g·24 h) in treatment T0, and showed no significant difference between these bamboo charcoal treatments. Acid phosphatase activity showed no significant difference between the control and bamboo charcoal treatments. This index reached the highest 2.05 mg/(g·24 h) in treatments T1 and T2, the lowest 2.03 mg/(g·24 h) in Treatment T0. And the difference in acid phosphatase activity between these bamboo charcoal treatments was insignificant.
Effect of bamboo charcoal on catalase, sucrase and β-glucosidase activities
As can be seen from Fig. 3, 4 and 5, soil catalase, sucrase and β-glucosidase increased at first and then decreased with bamboo charcoal increasing. Among them, catalase activity showed significant difference between the control and bamboo charcoal treatments(P<0.05), and peaked in T2 treatment, up to1.37 mg/(g·20 min). Sucrase activity showed significant difference between the control and Treatment T1, Treatment T2 (P<0.05), and reached the highest value 12.05 mg/(g·24 h) in Treatment T2, and the lowest value 7.82 mg/(g·24 h) in the control. Soil β-glucosidase activity, was the highest up to 66.67 μg/(g·h) in Treatment T2, followed by that in Treatment T1, 47.47 μg/(g·h), and the lowest was 39.59 μg/(g·h) in the control. And β-glucosidase activity in Treatment T3 showed no significant difference from that of treatments T2 and T1.
Conclusion and Discussion
The present study suggests that bamboo charcoal significantly increase soil microbial population. With bamboo charcoal increasing, bacterial, fungal, actinomycete populations and the fungus/bacterium ratio all increased at first, peaked in Treatment T2 and then decreased (T2>T3>T1>T0). To sum up, bamboo charcoal-based biochar remarkably increases soil bacteria, fungi and actinomycetes.
Bamboo charcoal also promoted the activities of sucrase, urease, catalase and β-glucosidase, but the difference in acid phosphatase between these treatments was not significant. The effect of bamboo charcoal in increasing β-glucosidase, catalase, acid phosphatase and sucrase in all the treatments was T2>T3>T1>T0. With bamboo charcoal-based biochar increasing, β-glucosidase, catalase, acid phosphatase and sucrase activities all increased at first and then decreased.
References
[1] YANG QJ, WANG HC, REN N, et al. Study on the preparation and application of biochar[J]. Applied Chemical Industry, 2020, 49(7): 1835-1838.
[2] ZHANG ZR, LIU QH, WANG WG, et al. Effect of pyrolysis temperature on the physical and chemical characteristics of bamboo-based biochar[J]. Environmental Engineering, 2021, 39(11): 96-102.
[3] LIU YX, WANG YF, LYU HH, et al. Effects of different application rates of rice straw biochar and bamboo biochar on yield and quality of greengrocery (Brassica chinensis) and soil properties[J]. Journal of Plant Nutrition and Fertilizer Science, 2013, 19(6): 1438-1444.
[4] STEEL H, PENA EDL, FONDERIE P, et al. Nematode succession during composting and the potential of the nematode community as an indicator of compost maturity[J]. Pedobiologia, 2010, 53(3): 181-190.
[5] Ingham ER. The Compost Tea Brewing Manual (5th edition)[M]. Oregon: Soil Foodweb Incorporated, 2005.
[6] FERRIS H. The structure and functions of the soil food web[C]. Santa Paula: Proceedings of the Landscape Disease Symposium, 2005.
[7] CHEN YF, HU C, LI SL, et al. Managing farmland soil food web: principles and methods[J]. Acta Ecologica Sinica, 2011, 31(1): 286-292.
[8] BERNAL MP, ALBURQUERQUE JA, MORAL R. Composting of animal manures and chemical criteria for compost maturity assessment. A review[J]. Bioresource Technology, 2009, 100(22): 5444-5453.
[9] MEHTA CM, PALNI U, FRANKE-WHITTLE IH, et al. Compost: Its role, mechanism and impact on reducing soil-borne plant diseases[J]. Waste Manage, 2014, 34(3): 607-622.
[10] CHEN WF, ZHANG WM, MENG J. Research progress and prospects of agricultural biochar[J]. Scientia Agricultura Sinica, 2013, 46(16): 3324-3333.
[11] HUANG YD, DU YQ, CHEN YJ, et al. Physicochemical properties of biochars originated from different agricultural wastes and their impact on the yield of Brassica campestris L.[J]. Ecology and Environment Sciences, 2018, 027(2): 356-363.
[12] LI LL, WU ZC, CHEN B, et al. The effect of biochar on soil enzyme activity and microbial community structure in the soil under autotoxicity stress[J]. Acta Agriculturae Boreali-Sinica, 2015, 30(4): 219-225.
[13] BAO SD. Soil Agro-chemical analysis[M]. Beijing: China Agriculture Press Co., Ltd., 2013.
- 农业生物技术(英文版)的其它文章
- Improving the Heat Resistance of β-1,4 Glucanase by Introducing Disulfide Bonds
- Comparative Study on Biological and Commercial Characteristics of Mul-tiple Varieties of Broccoli (Brassica oleracea L. var. italica Plenck) and Vegetable Soybean (Glycine max (L.) Merr.)
- Summary of Short-vine Watermelon Breeding Practice
- Effects of Combined Application of Biochar-based Organic Fertilizer and Reduced Nitrogen Fertilizer on Soil Enzyme Activity and Yield of Purple Cabbage (Brassica oleracea var. capita rubra)
- Effects of Microelement Fertilizers on Main Economic Characters and Yield of Peanut
- Effects of Microbial Fertilizers in Improving Acidic Tobacco-planting Soil and Tobacco Leaf Quality

