Improving the Heat Resistance of β-1,4 Glucanase by Introducing Disulfide Bonds
Guodong WANG, Junqing WANG
Abstract Each possible pair of residues in β-1,4 glucanase for disulfide formation was assessed using online websites, and four pairs L28C-S256C, Q41C-P278C, S122C-N163C and A184C-A215C were selected. Accordingly, four recombinant plasmids pET28a (+) EccslH28, pET28a (+) EccslH41, pET28a (+) EccslH122 and pET28a (+) EccslH184 were prepared and transformed into E. coli to express the recombinant enzymes. Then analysis on enzymatic properties showed that T50 of the recombinant enzymes was increased from 10 min for EccslHt2 to 90 min for EccslH28 and 40 min for EccslH41 at 70 ℃, while their optimum pH value and pH stability were not affected, which proved that the introduction of disulfide bond improved the thermal stability of β-1,4 glucanase.
Key words β-1,4-Glucanase; Disulfide bond; Thermal stability; Plasmid construction
Received: January 1, 2023 Accepted: March 3, 2023
Supported by the National Key Research and Development Plan of China (2019YFC1905902, 2019YFC1905900); Key Research and Development Plan in Shandong Province (2020CXGC010603, 2021ZDSYS10, 2022CXGC020206); "Open Competition Mechanism" Project of Qilu University of Technology (Shandong Academy of Sciences) (2022JBZ01-06); Taishan Industry Leading Talent Program (tscy20180103); National Natural Science Foundation of China (31801527).
Guodong WANG(1997-), male, P. R. China, master, devoted to the research about biology and medicine.
*Corresponding author. E-mail: wjqtt.6082@163.com.
Beta-1,4-glucanase (EC3.2.1.4) is a conventional cellulase capable of randomly hydrolyzing β-1,4 glycosidic bond-linked glucan, commonly used to cut fibers into a large number of short cellulose chains with reducing ends[1]. An endoglucanase is essential for the initiation and maintenance of cellulose hydrolysis, and can randomly cleave cellulose polymers into oligosaccharides and oligo-polysaccharides. Cellulose, the cheapest and most widely distributed biomass, can be hydrolyzed by bioenzymes such as glucanase into monosaccharides, which are then converted to high-value-added products such as ethanol[2]. So, it has been studied a lot all over the world. For instance, cellulase isolated from Trichoderma reesei was used to synthesize new oligomeric β-glycosides[3]. The majority of cellulases have an optimum pH of 4-5 and an optimum temperature near 55 ℃[4], which limit their application in laundry, pulping and paper making, etc. Higher heat resistance can prolong the duration and thus reduce the cost of β-1,4 glucanase[5]. Given this, there is to develop highly heat-resistant β-1,4 glucanase.
Disulfide bond, formed by a pair of sulfhydryl (-SH) groups on the side chain of cysteine inside protein molecules, is important for stabilizing the protein structure and improving protein thermal stability. For example, in the study of Du et al.[6], the thermal stability of zearalenone-degrading enzyme ZLHY- at 45 ℃ was increased by 10 folds by introducing disulfide bonds. Zhu et al.[7] improved the heat resistance of keratinase at 70 ℃ and Niu et al.[8] improved the half-life value of glucanase at 60 ℃ by 38% via the introduction of disulfide bonds.
In the present study, through an extensive literature search, β-1,4-glucanases (EC3.2.1.4) of the GH5 family, from an alkaliphilic Bacillus isolate[9] was selected and modified to be a mutant enzyme (EccslHt2) in our laboratory. Among the families of cellulases whose structure have been analyzed and determined, the GH5 family is the only one that contains three types of cellulase, including 222 333 enzymes in total, showing a structure of eight β-sheets wrapped with eight α-helices[10]. The enzyme has an optimum temperature of 65 ℃, an optimum pH of 10, a molecular mass of 50 kDa, and a half-life value T50 of 10 min measured at 70 ℃. It consists of 1 476 bases encoding 492 amino acids, with a distinct structure, repeated carbohydrate-binding module (CBM) of the GH12 family at its C-terminal end. By introducing a disulfide bond into EccslHt2 at four different sites, four new mutant enzymes EccslH28, EccslH41, EccslH122 and EccslH184 were obtained in the present study. Among them, EccslH122 and EccslH184 showed improved temperature stability at 70 ℃.
Materials and Methods
Materials
Plasmids, strains and primers
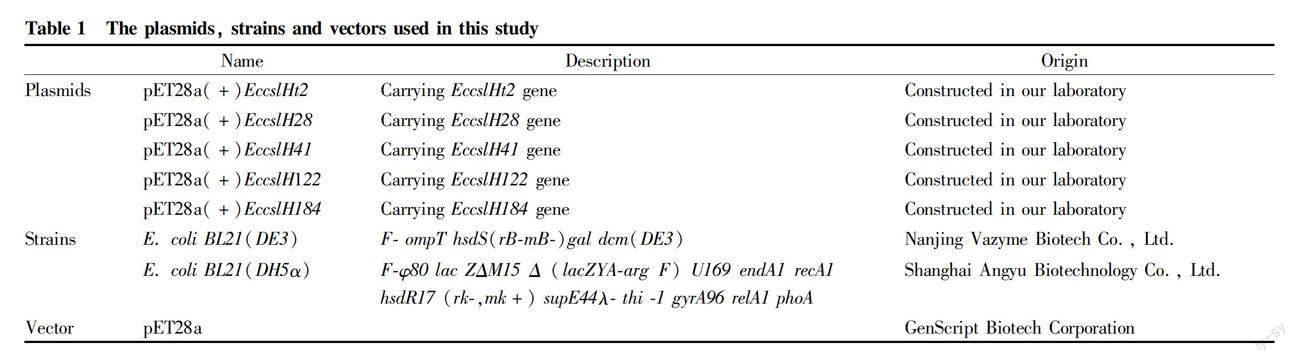
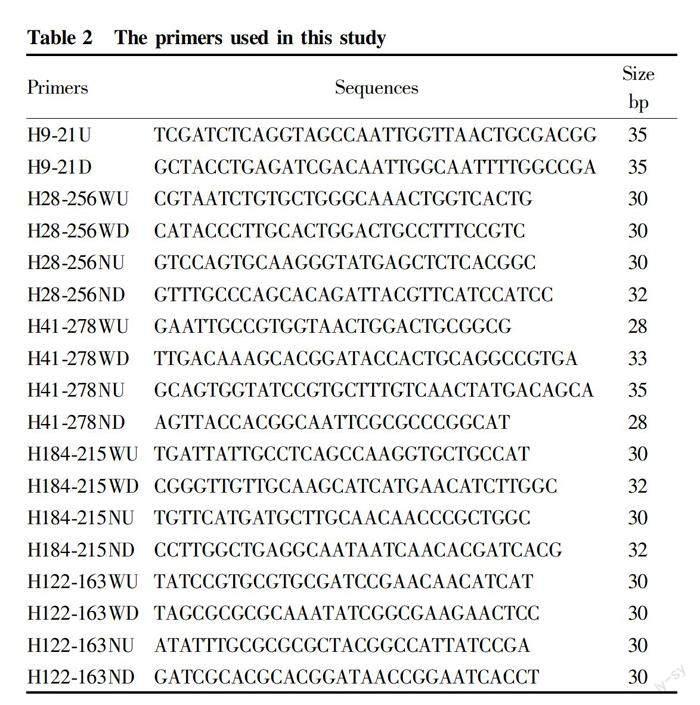
The plasmids, strains and vectors used in this study are shown in Table 1, and the primers in Table 2.
Reagents
Sodium carboxymethyl cellulose and microcrystalline cellulose were purchased from Shanghai Aladdin Biochemical Technology Co. Ltd. Anhydrous glucose and sodium acetate tetrahydrate were purchased from Beijing Solarbio Science & Technology Co., Ltd. PBS and 3,5-dinitrosalicylic acid (DNS) were purchased from Beijing Coolaber Science & Technology Co., Ltd. Precast gels were bought from Changzhou Smart-Lifesciences Biotechnology Co., Ltd. Protein marker (10-180 kDa) and Nucleic acid 2 000 marker, 5 000 marker and 12 000 marker were bought from Qingdao Lige Biotechnology Co., Ltd. Cobalt chloride and Copper sulfate were bought from Shanghai Hushi. Plasmid extraction kits were purchased from Tiangen Biotech (Beijing) Co., Ltd., Gel Recovery Kit from Nanjing Vazyme Biotech Co., Ltd., and zinc sulfate from Tianjin Kermel Chemical Reagent Co., Ltd.
Main solutions and media
Sodium carboxymethyl cellulose solution was prepared following the steps: 1.6 g of sodium carboxymethyl cellulose was weighed, added to 80 ml PBS buffer solution, heated with a magnetic stirrer until the substrate was completely dissolved, and pH was adjusted to 7. Finally, PBS buffer was added to bring the volume up to 100 ml.
Kanamycin solution (50 mg/ml) was diluted in 10 ml of double-distilled water to make the final concentration of 50 μg/ml, and passed through 0.22-μm pore size membrane filter, and stored at -20 ℃.
Isopropyl-β-D-1-thiogalactopyranoside (IPTG) was diluted to a final concentration of 20 μg/ml: 2.383 g of IPTG was dissolved in sufficient water, and the volume was made up to 10 ml, and finally the solution was passed through a 0.22-μm pore size syringe filter, stored at -20 ℃.
LB liquid medium contained 0.5% yeast extract powder, 1% peptone, and 1% sodium chloride. And LB plates were prepared by adding 2% agar powder to liquid medium and adjusting pH to 7.0.
To prepare DNS reagent, 6.3 g of 3,5-dinitrosalicylic acid was added to 1 000 ml of water, incubated in water bath at 45 ℃, when 200 ml of sodium hydroxide solution (200 g/L) was slowly added, before 182 g of potassium sodium tartrate tetrahydrate, 5.0 g of phenol and 5.0 g of sodium sulfite were successively added. After they were completely dissolved, the solution was cooled to room temperature, and the volume was made up to 2 L.
Instruments and software
Carbohydrate-Active Enzyme Database (http:∥www.cazy.org/) was used for analyzing the structural features of different families of glucanases, SignalP5.0 (http:∥www.cbs.dtu.dk/services/Signa lP/) for predicting signal peptides, Snapgene and Geneious for sequence comparison, SWISS-MODEL website (https:∥swissmodel.expasy.org/) for homology modeling of 3D protein structure, PIC (http:∥pic.mbu.iisc.ernet.in/) for protein structure analysis, and Disulfide by Design website (http:∥cptweb.cpt.wayne.edu/DbD2/) for predicting the potential sites for disulfide engineering.
Methods
Construction of β-1,4 glucanase recombinant plasmids
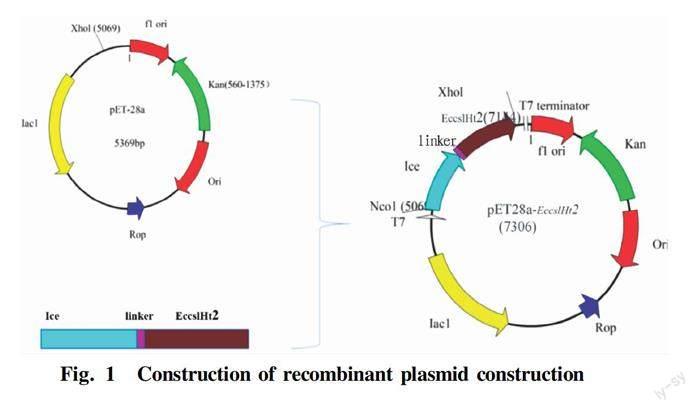
The β-1,4-glucanase gene of GH5 family was selected and engineered to produce EccslHt2. The signal peptide of the introduced gene fragment was predicted by SignalP5.0 (http:∥www.cbs.dtu.dk/services/Signa lP/). A gene fragment expressing ice nucleation protein and the linker were introduced at the N-terminal end, while His tag was introduced to the C-terminal end. After codon optimization, the target gene sequence was ligated to the expression vector pET28a that had been digested using XhoI and NocI. The ligated product was then transformed into E. coli BL21(DE3) competent cells.
Induction, expression and purification of β-1,4-glucanase
The plasmid pET28a(+)EccslHt2 constructed above was incubated on ice in advance, then 5 μl was added to BL21(DE3) competent cells that had been thawed by incubation at 37 ℃ for 5 min. The reaction solution was incubated on ice for 30 min, in a water bath at 45 ℃ for 90 s, on ice for 2 min, and finally transferred into 900 ml of liquid LB medium supplemented with kanamycin, incubated for 1 h at 37 ℃. Then, the medium was centrifuged at 5 000 r/min for 3 min before 100 μl of the supernatant was resuspend and spread on a LB plate containing kanamycin.
After overnight incubation, single colonies were picked and transferred into glycerol tubes containing 1 ml LB liquid medium and 50 μg/ml kanamycin, incubated for 16 h. Then, the broth was transferred to LB liquid medium in 250 ml conical flasks containing kanamycin, and incubated at 37 ℃ and 200 r/min, until the OD value was between 0.6 and 1.0. The expression of target protein was induced by adding isopropyl-β-D-1-thiogalactopyranoside (IPTG), followed by incubation at 28 ℃ for 8 h.
50 ml of the fermentation broth was centrifuged at 12 000 r/min and 4 ℃ for 20 min. The supernatant was collected, and the bacterial precipitate was resuspended with 10-20 ml PBS buffer, sonicated on ice at output power of 300 W for 20 min, in a pulsed mode 4 s on/6 s off, centrifuged again at 12 000 r/min, 4 ℃ for 20min to collect the supernatant (crude enzyme solution). After PBS was added to the crude enzyme solution to bring the volume up to 50 ml, the target protein was purified by using Ni-NTA column at 4 ℃, and eluted with 50 mmol/L imidazole. The eluted protein was mixed with protein loading buffer at a ratio of 1:5, incubated in a boiling water bath for 10 min and allowed to cool before it was loaded for SDS-PAGE. The protein marker 10-180 KD was selected as the standard. The gel was stained with Coomassie Brilliant Blue for 1 h after electrophoresis, and then washed with deionized water.
Design of disulfide bonds
EccslHt2 was used as a template to design the introduction of disulfide bonds. The amino acid sequence of Paenibacillu β-1,4-glucanase (EccslHt2) obtained in Genbank was submitted to SWISS-MODEL online server for homology modeling. The potential residues sites where disulfide bonds can form were predicted using Disulfide by Design V 1.20. First, the residue sites were selected based on χ3 torsion angle about -87° or + 97° ± 30, energy values between 2.5 and 2.6 kcal/mol, and B- factor ranging from 1.5 to 1.9. Then, the sites lying within 10from the substrate binding site were excluded to ensure the integrity of the catalytic center. Finally, the mutant residue pairs that negatively affect the structure of the protein itself were also excluded.
Analysis of β-1,4-glucanase enzymatic properties
Calculation of enzyme activity
By referring to NY/T 912-2020, 2 ml of β-1,4 glucanase solution that had been properly diluted (usually 100 folds) with PBS buffer was mixed with 2 ml of 1.6 g/L carboxymethyl cellulose sodium that had been preheated at 37 ℃ for 10 min. The reaction was carried out at 55 ℃ for 15 min before it was terminated by adding 5 ml of DNS. Then, the mixture was incubated in a boiling water bath for 10 min, and the volume was made up with water to 25 ml. To prepare the negative control, 2 ml of the diluted enzyme solution was mixed with 5 ml of DNS reagent at first to deactivate the enzyme, and then with 2 ml of sodium carboxymethyl cellulose substrate, followed by incubation at 55 ℃ for 15 min. Their absorbance was measured at 540 nm. The standard curve of absorbance and reducing sugar concentration was made by mixing an equal volume of glucose solution with buffer. Three repetitions were set for each sample. One unit (1 U) of enzyme activity was defined as the amount of enzyme required to decompose sodium carboxymethyl cellulose into 1 μmol of reducing sugar per minute at 55 ℃ and pH7.0. The enzyme activity x was calculated using the formula as follows:
x=(c-c0)×100×n2×15×180.2×1 000
Where, c and c0 are obtained from the standard curve, 100 refers to the dilution ratio of enzyme solution, n is the dilution ratio of above 100-fold diluted enzyme solution, 1 000 is conversion coefficient, 2 is sampling volume, 15 is reaction time, and 180.2 is the relative molecular mass of glucose.
Optimal temperature and temperature stability
Appropriate amount of enzyme solution was taken and incubated at 40, 45, 50, 55, 60, 65, 70 and 75 ℃ for 15 min, and the temperature at which the highest enzyme activity was measured was considered as its optimal temperature.
To determine temperature stability, enzyme reaction was carried out at the optimal temperature determined above for 10, 20, 30, 50, 70, 90, 120 and 140 min respectively before enzyme activity was measured.
Optimal pH and pH stability
The buffers with different pH were prepared as: pH 4-5: acetic acid-sodium acetate, pH 6-8: sodium dihydrogen phosphate-disodium hydrogen phosphate, pH 8-9: Tris-hydrochloric acid, pH 10-11: glycine-sodium hydroxide. The substrate was also prepared with above buffers. The reactions between each mutant enzyme and the substrate were carried out at 55 ℃ for 15 min. The pH at which the highest enzyme activity was measured was considered as the optimal pH.
To determine pH stability, the enzyme solution was diluted with above buffers of different pH, and the remaining enzyme activity was measured after incubation at 37 ℃ for 1 h. A curve was made with pH on the horizontal coordinate and enzyme activity on the vertical coordinate.
Results and Analysis
Recombinant plasmid pET28a(+)EccslHt2
The scheme for the construction of recombinant plasmid pET28a(+)EccslHt2 is shown in Fig. 1. The N-terminal signal peptide predicted by SignalP5.0 (http:∥www.cbs.dtu.dk/services/Signa lP/) consists of 26 amino acids: MKQKKIVTIGLVLVLSTMLWASSALG. The gene fragment encoding EccslHt2 was 1 389 bp (excluding the signal peptide), encoding a protein of 51.175 kDa, with an isoelectric point of 4.01. After ice nucleoprotein and the linker were inserted, the new protein was 74.333 kDa, with an isoelectric point of 4.28.
Construction of engineered plasmids with introduced disulfide bond
Extraction of pET28a(+)EccslHt2 plasmid
Recombinant pET28a(+)EccslHt2 plasmid was extracted following the kit instruction, and the genome was recovered with double-distilled water that had been preheated at 55 ℃.
Construction of engineered plasmid pET28a(+)EccslH28
Primers H28-256NU, H28-256ND, H28-256WU and H28-256WD were designed using plasmid pET28a(+)EccslHt2 which harbors the nucleotide sequence of heterozyme β-1,4-glucanase (EccslHt2) as the template. The target gene EccslH28T was synthesized via PCR in the presence of primers 256NU and H28-256ND, and plasmid pET28a(+)EccslHt2 as the template. And the target gene EccslH28H was synthesized via inverse PCR in the presence of primers H28-256WU and H28-256WD and plasmid pET28a(+)EccslHt2 as the template. Then, the two genes EccslH28H and EccslH28T were ligated together to produce a mutant plasmid, which was then transformed into E. coli BL21, and induced to express a heat-resistant β-1,4-glucanase mutant EccslH28.
Construction of engineered plasmid pET28a(+)EccslH41
Primers H41-278NU, H41-278ND, H41-278WU and H41-278WD were also designed using plasmid pET28a(+)EccslHt2 as the template. The target gene EccslH41T was synthesized via PCR in the presence of primers H41-278NU and H41-278ND, and plasmid pET28a(+)EccslHt2 as the template. The target gene EccslH41H was synthesized via inverse PCR in the presence of primers H41-278WU and H41-278WD and plasmid pET28a(+)EccslHt2 as the template. Then, the two target genes were ligated together to produce a mutant plasmid, which was then transformed into E. coli BL21, and induced to express a heat-resistant β-1,4-glucanase mutant EccslH41.
Construction of engineered plasmid pET28a(+)EccslH122
Primers H122-163NU, H122-163ND, H122-163WU and H122-163WD were also designed using plasmid pET28a(+)EccslHt2 as the template. The target gene EccslH122T was synthesized via PCR in the presence of primers H122-163NU and H122-163ND, and plasmid pET28a(+)EccslHt2 as the template. The target gene EccslH122H was synthesized via inverse PCR in the presence of primers H122-163WU and H122-163WD, and plasmid pET28a(+)EccslHt2 as the template. Then, the two target genes EccslH122H and EccslH122T were ligated together to produce a mutant plasmid, which was then transformed into E. coli BL21, and induced to express a heat-resistant β-1,4-glucanase mutant EccslH122.
Construction of engineered plasmid pET28a(+)EccslH184
Primers H184-215NU, H184-215ND, H184-215WU and H184-215WD were also designed using plasmid pET28a(+)EccslHt2 as the template. The target gene EccslH184T was synthesized via PCR in the presence of primers H184-215NU and H184-215ND, and plasmid pET28a(+)EccslHt2 as the template. The target gene EccslH184H was synthesized via inverse PCR in the presence of primers H184-215WU and H184-215WD, and plasmid pET28a(+)EccslHt2 as the template. Subsequently, the two target genes EccslH184H and EccslH184T were ligated together to produce a mutant plasmid, which was then transformed into E. coli BL21, and induced to express a heat-resistant β-1,4-glucanase mutant EccslH184.
Construction of mutant enzymes
As shown in Fig. 2a, lanes 1 and 2 are extracted pET28a(+)EccslHt2 plasmid, and lanes 5 and 6 are the target gene EccslH28H, which is 6 691 bp in size. As shown in Fig. 2b, lanes 1 and 2 are the target gene EccslH41H, which is 6 592 bp in size, lanes 3 and 4 are the target gene EccslH122H, which is 6 691 in size, lanes 5 and 6 are the target gene EccslH184H, which has a size of 7 213 bp.
As shown in Fig. 2c, the target gene EccslH28T, which has a size of 687 bp, is displayed in lanes 1 and 2; the target gene EccslH41T, which has a size of 714 bp, is shown in lanes 3 and 4; the target gene EccslH122T, which has a size of 123 bp, is displayed in lanes 5 and 6. Lanes 1 and 2 in Fig. 2d show the target gene EccslH184T, which has a size of 93 bp.
The nucleic acid concentration of amplified EccslH28T, EccslH41T, EccslH122T, EccslH184T, EccslH282H, EccslH41H, EccslH122H and EccslH184H was measured using MD2000 microscale spectrophotometer. Ligation reaction was performed using C112 One Step Cloning Kit. Then the four plasmids pET28a(+)EccslH28, pET28a(+)EccslH41, pET28a(+)EccslH122 and pET28a(+)EccslH184 introduced with disulfide bond were chemically transformed into E. coli BL21(DE3), and spread on LB solid plates, which were then incubated overnight. Single colonies were inoculated into flasks, incubated in a shaking incubator at 37 ℃. The culture broths were shipped in glycerol tubes to Sangon Biotech (Shanghai) Co., Ltd. for sequence analysis with T7-T7er universal primers. Analysis of mutant enzymes showed that disulfide bonds were indeed introduced. Subsequently, target enzymes were induced at 8 ℃. EccslH28 enzyme is displayed in lanes 1, 2 and 3 in Fig. 3a, EccslH41 in lanes 4, 5 and 6 in this figure, EccslH122 in lanes 1, 2 and 3 in Fig. 3b, EccslH184 in lanes 4, 5 and 6 in this figure. As the extracellular level of the recombinant enzymes was extremely low, intracellular enzymes were isolated and purified after cell ultrasonic treatment. And our results showed that these recombinant enzymes had a size of 75.6 kDa.
Changes in enzymatic properties after disulfide bond was introduced
Resistances to high temperature, acids and alkalis are important parameters for measuring enzymes. In order to determine the optimal temperature of the four recombinant enzymes with disulfide bond, their activity was measured after reaction at 50 to 70 ℃ for 15 min. The enzyme activity of each enzyme at its optimal temperature was considered as 100%. The optimum temperature for EccslH28 and EccslH41 was 65 ℃, and the optimum temperature for EccslH122 and EccslH184 was 60 ℃, as shown in Fig. 4a. Compared with the optimum temperature of EccslHt2 which was 65℃ when disulfide bond was not introduced, the optimum temperature of the mutant enzymes was not improved significantly. However, the enzyme activity of EccslH122 and EccslH184 at 70 ℃ was increased. So, we investigated the temperature stability of the four recombinant enzymes at 70 ℃, and the results are shown in Fig.4b. Among the four enzymes, EccslH28 was most stable, and still retained over 55% enzyme activity after incubation at 70℃ for 60 min, and its half-life time prolonged to 90 min. And the half-life time was 40 min for EccslH41, 20 min for EccslH122 and EccslH184. Given that enzyme EccslHt2 can completely lost its activity after 10 min of incubation at 70 ℃ when disulfide bond is not introduced, the introduction of disulfide bond indeed improves its heat resistance at 70 ℃, and the effect is most pronounced in EccslH28.
By evaluating the enzymatic activity of the four recombinant enzymes, EccslH28, EccslH41, EccslH122 and EccslH184 at pH ranging from 5 to 11, we found that the optimum pH for EccslH28, EccslH41 and EccslH184 was 8, while that for EccslH122 was 7 (Fig. 5a), proving that the introduction of disulfide bond has little effect on the optimum pH of the recombinant enzymes. From Fig. 5b, we could see that the enzyme activity of the four recombinant enzymes remained stable at above 70% between pH 4 and 10, which proves that the introduction of disulfide bonds has little effect on their pH stability.
Conclusion and Discussion
In the present study, the possible residue sites for disulfide formation were assessed using online websites based on the sequence of EccslHt2, and four sites L28C-S256C, Q41C-P278C, S122C-N163C and A184C-A215C were selected. Accordingly, four recombinant plasmids pET28a(+) EccslH28, pET28a(+)EccslH41, pET28a(+)EccslH122 and pET28a(+)EccslH184 were prepared and transformed into E. coli to express the recombinant enzymes. Then analysis on enzymatic properties showed that T50 of the recombinant enzymes was increased from 10 min for EccslHt2 to 90 min for EccslH28 and 40 min for EccslH41 at 70 ℃, proving that the introduction of disulfide bond improves their heat resistance. In addition, their optimum pH still remains at 8 to 9. To sum up, the introduction of disulfide bond broadens the scope of β-1,4-glucanase application, especially at high temperature and in alkaline environment. For example, it can be used as laundry detergent additives[11], or for straw pulp pretreatment[12] in the pulping and papermaking field, and for sugar and wine fermentation[13].
References
[1] SAN T, TEERAVIVATTANAKIT T, RATANAKHANOKCHAI K, et al. Cloning, expression, and preliminary characterization of a putative endo-1,4-β- glucanases from Clostridium sp. Z-7206. 2021[C].∥The 30th Annual Meeting of the Thai Society for Biotechnology and International Conference. Thailand, 2021: 1-10.
[2] DIONISI D, ANDERSON JA, AULENTA F, et al. The potential of microbial processes for lignocellulosic biomass conversion to ethanol: a review[J]. Journal of Chemical Technology and Biotechnology, 2015, 90(3): 366-383.
[3] YORK WS, HAWKINS R. Preparation of oligomeric β-glycosides from cellulose and hemicellulosic polysaccharides via the glycosyl transferase activity of a Trichoderma reesei cellulase[J]. Glycobiology, 2000, 10(2): 193-201.
[4] SARANGTHEM I, RAJKUMARI L, NGASHANGVA N, et al. Isolation and characterization of bacteria from natural hot spring and insights into the thermophilic cellulase production[J]. Current Microbiology, 2023, 80(2): 64.
[5] DOTSENKO A, DENISENKO J, OSIPOV D, et al. Testing and improving the performance of protein thermostability predictors for the engineering of cellulases[J]. Journal of Bioinformatics and Computational Biology, 2023.
[6] DU HL, LUO SJ, ADEGOKE TV, et al. Thermal stability modification of zearalenone detoxification enzyme ZLHY-6 based on thermolabile segment analysis and disulfide bond prediction[J]. Journal of Food Safety & Quality, 2022, 13(22): 8. (in Chinese)
[7] ZHU FJ, Rational design of disulfide bonds to improve the thermal stability of Thermobifida fusca keratinase[D]. Wuxi: Jiangnan University, 2020. (in Chinese)
[8] NIU CH, ZHU LJ, XU X, et al. Rational design of disulfide bonds increases thermostability of a mesophilic 1,3-1,4-β-glucanase from Bacillus terquilensis[J]. Plos One, 2016. 11(4): e0154036.
[9] ENDO K, HAKAMADA Y, TAKIZAWA S, et al. A novel alkaline endoglucanase from an alkaliphilic Bacillus isolate: enzymatic properties, and nucleotide and deduced amino acid sequences[J]. Applied Microbiology and Biotechnology, 2001, 57(1-2): 109-116.
[10] Dos Santos CR, Cordeiro RL, Wong DWS, et al. Structural basis for xyloglucan specificity and α-d-Xylp(1 → 6)-d-Glcp recognition at the 1 subsite within the GH5 family[J]. Biochemistry, 2015, 54(10): 1930-1942.
[11] SHACK N. Detergent composition and substitution of optical brighteners in detergent composition: United States, US20150064773A1[P]. 2015-03-05.
[12] WANG MM, YE YX, LI XZ, et al. Utility of co-expressed alkali-tolerant endoglucanase and xylanase in ameliorating wheat straw chemical pulp properties[J]. Cellulose, 2017. 24(5): 2299-2311.
[13] SHARMA R, NEHRA S, KUMAR D. Microbial cellulases: An approach toward recent advances in research, their application, and future perspectives[A]. TULI DK and KUILA A, Current Status and Future Scope of Microbial Cellulases[C]. United States: Elsevier, 2021: 295-311.
- 农业生物技术(英文版)的其它文章
- Comparative Study on Biological and Commercial Characteristics of Mul-tiple Varieties of Broccoli (Brassica oleracea L. var. italica Plenck) and Vegetable Soybean (Glycine max (L.) Merr.)
- Effects of Bamboo Charcoal-based Biochar on Soil Enzyme Activity and Microbial Community Structure
- Summary of Short-vine Watermelon Breeding Practice
- Effects of Combined Application of Biochar-based Organic Fertilizer and Reduced Nitrogen Fertilizer on Soil Enzyme Activity and Yield of Purple Cabbage (Brassica oleracea var. capita rubra)
- Effects of Microelement Fertilizers on Main Economic Characters and Yield of Peanut
- Effects of Microbial Fertilizers in Improving Acidic Tobacco-planting Soil and Tobacco Leaf Quality

