Simultaneous Determination of Six Components in Cortex Phellodendri by HPLC
Lian LIAN, Guosheng WAN, Weili JIA, Huiyuan GAO

Abstract [Objectives] This study was conducted to establish the method for simultaneous determination of six active components.
[Methods] Simultaneous determination of chlorogenic acid, phellodendrine, magnoflorine, jatrorrhizine, palmatine and berberine in Cortex Phellodendri was carried out by HPLC with a Diamonsil C18 (4. 6 mm×250 mm, 5 μm) column was used. The mobile phase was acetonitrile-water (1‰ acetic acid, 2 mmol ammonium acetate) solution in gradient elution. The detection wavelength was set at 280 nm, and the column temperature was kept at 25 ℃ and the flow rate was 1 ml/min.
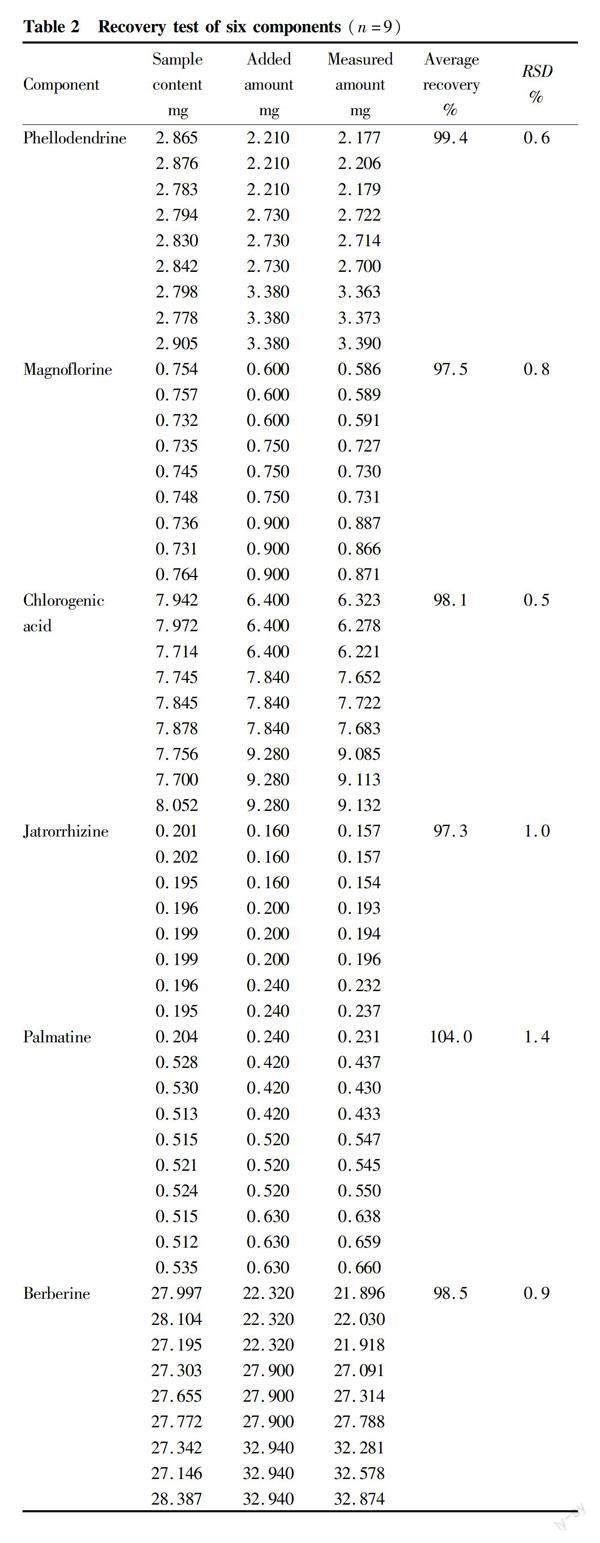
[Results] The linear ranges of chlorogenic acid, phellodendrine, magnoflorine, jatrorrhizine, palmatine and berberine were 20.00-320.00, 18.75-130.00, 25.00-200.00, 5.00-100.00, 20.00-200.00, and 0.09-1.80 mg/L, respectively. The average recovery was 98.1%, 99.4%, 97.5%, 97.3%, 104.0%, and 98.5%, respectively; and the RSDs were 0.5%, 0.6%, 0.8%, 1.0%, 1.4%, and 0.9%, respectively.
[Conclusions] The method is convenient, stable, reliable and suitable for quality control of Cortex Phellodendri.
Key words Cortex Phellodendri; Chlorogenic acid; Phellodendrine; Magnoflorine; Jatrorrhizine; Palmatine; Berberine; Determination
Received: January 3, 2023 Accepted: March 4, 2023
Supported by Liaoning Provincial Doctoral Research Start-up Fund (20101049); Outstanding Young Scholar Development Plan for Higher Education Institutions of Liaoning Province (LJQ2011129).
Lian LIAN (1972-), female, P. R. China, associate professor, PhD, devoted to research about active ingredients and processing principles of traditional Chinese medicine.
*Corresponding author. E-mail: lotus0216@126.com.
Cortex Phellodendri refers to the dry bark of Phellodendron plants of Rutaceae, commonly known as Phellodendron amurense Rupr. (PA) and Phellodendron chinense (PC)[1]. P. chinense is mainly produced in Hubei, northwestern Hunan, and eastern Sichuan. P. amurense is mainly produced in various provinces of Northeast and North China, and also distributed in Henan, northern Anhui, and Ningxia, and a small amount is cultivated in Inner Mongolia[2].
Phellodendron was first recorded in Sheng Nongs herbal classic, originally named "Bomu", and listed as the top grade[3]. The 1995 edition of Chinese Pharmacopoeia (Part 1) listed the sources of Cortex Phellodendri as P. chinense and P. amurense under the category of Phellodendron medicinal herbs, and provided a description of their characteristics. With the deepening of research, since the 2005 edition of Chinese Pharmacopoeia, "P. chinense" and "P. amurense" have been classified as two medicinal herbs, but their functional indications have been described in the same way. Both are bitter and cold in nature and flavor, and have functions such as clearing away heat and drying dampness, purging the pathogenic fire, relieving internal heat or fever and treating sores, and reducing deficiency heat. They are commonly used in clinical practice to treat diseases such as damp-heat dysentery, diarrhoea, jaundice, and skin eczema[4].
Although the pharmacopoeia mainly states that P. chinense and P. amurense have the same efficacy and nature and flavor, with the advancement of modern analytical technology, there are still differences in their composition and content, leading to different emphasis on pharmacology and biological activity. In order to gain a deeper understanding of the main active components such as alkaloids and phenolic acids and to better control the quality of the two Phellodendron herbs, referring to relevant literature[5-10], a method for simultaneous determination of six common components in the two types of Phellodendron herbs was established using high-performance liquid chromatography (HPLC), and the contents were determined.
Instruments and Agents
LC-20AB high-performance liquid chromatograph (Shimadzu Corporation, Japan); SPD-M20A PDA detector (Shimadzu Corporation, Japan); Diamonsil C18 (46 mm × 250 mm, 5 μm) chromatographic column (Dikama technologies); AB135-S electronic balance (Shanghai Mettler Toledo Instrument Co., Ltd.); KQ-250DB CNC ultrasonic cleaner (Kunshan Ultrasonic Instrument Co., Ltd.).
P. chinense (the first branch of Sichuan Dayi Traditional Chinese Medicine Co., Ltd.) and P. amurense (purchased from Benxi, Liaoning) materials were identified by Professor Lu Jincai of Shenyang Pharmaceutical University as the dried bark of P. chinense Schneid. in Rutaceae and the dried bark of P. amurense Rupr. in Rutaceae, respectively. Chlorogenic acid reference substance, phellodendrine reference substance, magnoflorine reference substance, jatrorrhizine reference substance, palmatine reference substance and berberine reference substance were all self-made by our laboratory (the purity determined by HPLC was greater than 98%). Methanol and acetonitrile were chromatographically pure. Water used was ultrapure water, and all other reagents are analytically pure.
Methods and Results
Chromatographic conditions
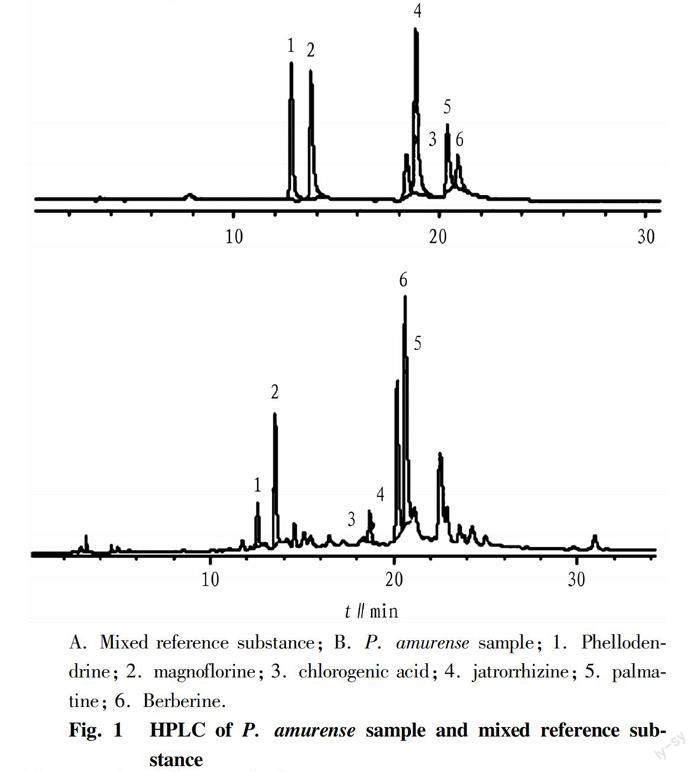
The chromatographic conditions were as follows: Dikama Diamonsil C18 chromatographic column (46 mm×250 mm, 5 μm), column temperature 25 ℃, flow rate 1 ml/min, injection volume 20 μl, detection wavelength 280 nm, and gradient elution with acetonitrile and water (1‰ acetic acid, 2 mmol/L ammonium acetate) as mobile phases, of which acetonitrile served as mobile phase A, and water served as mobile phase B. The gradient elution was programmed as 0 min, 5%; 20 min, 35%; 35min, 50%; 45 min, 85%. Under these chromatographic conditions, the resolution between the target components and other components in samples was good, as shown in Fig. 1.
Preparation of reference substance stock solutions
The reference substances, phellodendrine 13.0 mg, magnoflorine 10.0 mg, chlorogenic acid 16.0 mg, jatrorrhizine 10.0 mg, palmatine 10.0 mg and berberine 18.0 mg, were accurately weighed in 10 ml volumetric flasks, respectively. Each reference substance was dissolved with methanol and diluted to constant weight. After shaking well, the prepared stock solutions, 1.3 g/L phellodendrine, 1.0 g/L magnoflorine, 1.6 g/L chlorogenic acid, 1.0 g/L palmatine, and 1.8 g/L berberine, were filtered with 0.45 μm microporous membranes to filtrates for later use.
Preparation of test solutions
A 1.0 g of Cortex Phellodendri sample was accurately weighed and added into a 50 ml triangular flask. Then, it was ultrasonically extracted with 10 ml of 75% methanol (by volume) for 3 times, 1 h each time. The filtrates were merged, and 25 ml was transferred to a 50 ml measuring flask and diluted with 75% methanol to constant volume. The obtained solution was shaken well and filtered with a 0.45 μm microporous membrane to obtain a test solution for later use.
Investigation of linear relationships
Appropriate amounts of phellodendrine, magnoflorine, chlorogenic acid, jatrorrhizine, palmatine and berberine stock solutions were precisely measured and added into 10 ml volumetric flasks, respectively. The solutions were diluted with methanol to constant volume to obtain 0.13, 0.2, 0.32, 0.1, 0.2 and 0.9 g/L reference solutions, respectively. Next, each solution was diluted step by step to prepare a series of reference solutions with five mass concentrations. Next, 20 μl of reference solutions of the above series of concentrations were precisely injected into liquid chromatograph, and determined according to the chromatographic conditions under item "Chromatographic conditions" to record the peak areas. A standard curve was drawn with peak area (Y) as the ordinate and mass concentration (X) as the abscissa. The linear regression equation, correlation coefficient, and linear range of each reference substance are shown in Table 1.
Precision test
First, 1 ml of 0.13 g/L phellodendrine, 0.2 g/L magnoflorine, 0.32 g/L chlorogenic acid, 0.1 g/L jatrorrhizine, 0.2 g/L palmatine and 0.9 g/L berberine reference solutions were added into the same 10 ml volumetric flask and diluted to constant volume to obtain a mixed reference solution, which was shaken well. The obtained solution was injected and determined according to the chromatographic conditions under item "Chromatographic conditions" for 6 times for pear areas. The RSDs of peak areas of phellodendrine, magnoflorine, chlorogenic acid, jatrorrhizine, palmatine and berberine were calculated to be 0.9%, 0.6%, 0.5%, 0.7%, 1.3% and 1.2%, respectively, indicating good instrument precision.
Stability test
The test solution of P. chinense was injected at 0, 2, 4, 6, 8, 12 and 24 h for determination. The RSDs of peak areas of phellodendrine, magnoflorine, chlorogenic acid, jatrorrhizine, palmatine and berberine at 0-24 h were 1.3%, 0.6%, 0.8%, 1.7%, 1.5%, and 2.0%, respectively, indicating that the test solution was stable within 24 h.
Repeatability test
Six portions of P. chinense powder, about 1.0 g each, were accurately weighed in parallel. Test solutions were prepared according to the method under item "Preparation of test solutions",
and determined according to the chromatographic conditions under item "Chromatographic conditions" for peak areas, and the contents were calculated. The average contents of phellodendrine, magnoflorine, chlorogenic acid, jatrorrhizine, palmatine and berberine were 115, 29, 361.7, 8.2, 20.5, and 1 150 mg/L, respectively. The RSDs were 2.3%, 1.2%, 1.3%, 1.7%, 1.4%, and 1.5%, respectively, indicating good repeatability of the method.
Recovery test
Nine portions of P. chinense medicinal powder with known contents, approximately 0.5 g each, were weighed in parallel, and divided into three groups, which were, respectively, added with appropriate amounts of 1.3 g/L phellodendrine, 1.0 g/L magnoflorine, 1.6 g/L chlorogenic acid, 1.0 g/L jatrorrhizine, 1.0 g/L palmatine, and 1.8 g/L berberine (each equivalent to 80%, 100% and 120% of the original content of the tested medicinal material, respectively). Test solutions were prepared according to the method under item "Preparation of test solutions", and determined according to the chromatographic conditions under item "Chromatographic conditions", and the recovery was calculated, as shown in Table 2.
Determination of contents in test samples
P. amurense and P. chinense medicinal herbs were weighed and prepared into test solutions according to the method under item "Preparation of test solutions", and determined by HPLC according to the chromatographic conditions under item "Chromatographic conditions". Each batch of sample was measured three times in parallel, and the contents of phellodendrine, magnoflorine, chlorogenic acid, jatrorrhizine, palmatine and berberine in the two kinds of Phellodendron materials were calculated by the one-point external standard method. The results are shown in Table 3.
Discussion
It was found by using a PDA detector and comparing the absorption of different wavelengths that the absorption peaks of various compounds at the wavelength of 280 nm were good, while the absorption of impurities was low. Therefore, this wavelength was chosen as the detection wavelength. According to the chromatographic conditions of berberine and palmatine in the current pharmacopoeia[11], it was found that multiple components could not achieve good separation results. Therefore, after improvement, the gradient elution method using acetonitrile-water (0.1% acetic acid, 2 mmol ammonium acetate) as the mobile phase achieved good separation results for various components.
From the content data, the contents of berberine and phellodendrine in P. chinense and the contents of berberine and palmatine in P. amurense measured in this study were in line with the provisions in the 2010 edition of Chinese Pharmacopoeia. After analysis, it was found that there were significant differences in the contents of the six common components between the two types of Phellodendron herbs, with the main pharmacological component berberine being the most obvious. The berberine content in P. chinense was almost 10 times higher than that in P. amurense, which is consistent with the trend of content differences specified in the pharmacopoeia. In addition, there was a significant difference in the content of chlorogenic acid between the two Phellodendron herbs, which should also be taken seriously when further studying the pharmacological mechanism of Cortex Phellodendri. Therefore, it is believed that when both are used as genuine Cortex Phellodendri, it is not possible to ensure the uniformity and stability of the chemical components and their contents in the finished product, and it is not easy to control the quality of the drug. It is not recommended to use both as genuine Cortex Phellodendri, and they should be distinguished and noted in clinical medication. Further research should be conducted on the chemical composition of the two, clarifying the differences in their chemical composition, in order to serve the rational use of drugs in clinical practice.
References
[1] ZHANG GY, DONG RJ, LIAN L. Advances in study on chemical constituents and pharmacological activities of Phellodendron chinense Schineid. and Phellodendron amurese Rupr.[J]. Journal of Shenyang Pharmaceutical University, 2012, 29(10): 812. (in Chinese).
[2] YONG MH, GUANG HAI SUB, STEPHEN CHO-WING SZEA, etc. Quality assessment of Cortex Phellodendri by high-performance liquid chromatography coupled with electrospray ionization mass spectrometry[J]. Biomed. Chromatogr., 2010(24): 438-453
[3] National Administration of Traditional Chinese Medicine. Chinese materia medica[M]. Shanghai: Shanghai Scientific & Technical Publishers, 1999. (in Chinese).
[4] ZHU SL, DOU SS, LIU XR. Qualitative and quantitative analysis of alkaloids in Cortex Phellodendri by HPLC-ESI-MS/MS and HPLC-DAD[J]. Chem. Res. Chinese Universities, 2011, 27(1): 38-44.
[5] Chinese Pharmacopoeia Commission. Chinese pharmacopoeia (First part)[S]. Beijing: Chemical Industry Press, 2005. (in Chinese).
[6] LIN JZ, FU CM, MAO X, et al. Comparison of changes of content of berberine hydrochloride and yield of dry extract from pieces and powder of Phellodendri chinense in different boiling time[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2012, 18(12): 41-43. (in Chinese).
[7] SHEN J, YIN L, DUAN JA. Determination and comparison of alkaloids content between Cortex Phellodendri and Ermiao Wan categorized formulas by HPLC[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2010, 16(13): 31-34. (in Chinese).
[8] Chinese Pharmacopoeia Commission. Chinese pharmacopoeia (First part)[S]. Beijing: China Medical Science Press, 2010. (in Chinese).
- 农业生物技术(英文版)的其它文章
- Improving the Heat Resistance of β-1,4 Glucanase by Introducing Disulfide Bonds
- Comparative Study on Biological and Commercial Characteristics of Mul-tiple Varieties of Broccoli (Brassica oleracea L. var. italica Plenck) and Vegetable Soybean (Glycine max (L.) Merr.)
- Effects of Bamboo Charcoal-based Biochar on Soil Enzyme Activity and Microbial Community Structure
- Summary of Short-vine Watermelon Breeding Practice
- Effects of Combined Application of Biochar-based Organic Fertilizer and Reduced Nitrogen Fertilizer on Soil Enzyme Activity and Yield of Purple Cabbage (Brassica oleracea var. capita rubra)
- Effects of Microelement Fertilizers on Main Economic Characters and Yield of Peanut

