Effects of Polygonatum odoratum Polysaccharide on Reproductive Hormones in Male Rats Fed a High-fat Diet
Yixin YAN, Gang ZHANG, Lingli BAI, Xin TANG, Mengjun YUAN, Yihong HU, Yan WANG, Chenzhong JIN


Abstract [Objectives] This study was conducted to investigate the effects of Polygonatum odoratum polysaccharide (POP) on organ relative weights and reproductive hormone levels in male rats fed a high-fat diet.
[Methods] Thirty healthy male Sprague-Dawley (SD) rats were randomly divided into two groups according to their body weight: 10 in normal control group (Group NC, n=10) and 20 in experimental group (n=20). The rats in experimental group were fed a high-fat diet for eight weeks before they were further randomly divided into two groups: high fat group (Group HF) and high fat +400 mg/(kg·d) POP group (Group HF+POP). In Group HF+POP, the rats were administered with POP for another six weeks, before their blood plasma was collected, and the relative weights of their testis and epididymis were calculated. The plasma levels of testosterone (T), estrogen (E2), follicle-stimulating hormone (FSH), cortisol (C) and luteinizing hormone (LH) were measured by radioimmunoassay, and the plasma levels of sex hormone-binding globulin (SHBG) and insulin-like growth factor-1 (IGF-1) were determined by enzyme-linked immunosorbent assay.
[Results] Compared with Group HF, POP could effectively inhibit rat obesity caused by high-fat diets, increase the relative weights of their testis and epididymis, plasma levels of LH, E2, FSH, T, SHBG and IGF-1, and reduce the plasma level of E2.
[Conclusions] Polygonatum odoratum polysaccharide (POP) is able to effectively regulate the level of reproductive hormones in high-fat diet fed rats, and helps to protect their reproductive function.
Key words Polygonatum odoratum polysaccharide; High-fat diet; Reproductive hormones; Obesity; SD rats
Received: December 6, 2022 Accepted: February 7, 2023
Supported by Scientific Research Fund of the Hunan Provincial Education Department of China (19A259); Natural Science Foundation of Hunan Province (2022JJ30312); National Innovation Experiment Program for University Students (201910553013); 2020 Innovation Experiment Program for College Students of Hunan University of Humanities, Science and Technology (2020-17).
Yixin YAN (1998-), female, P. R. China, with a bachelors degree in biotechnology.
*Corresponding authors.
Nowadays, the number of obese patients is increasing year by year, due to the general improvement in living standards and acceleration in the pace of life, and about 40% of adults worldwide are overweight or obese. Obesity is a chronic metabolic disease caused by various factors, and closely related to many diseases, such as cardiovascular disease, sleep apnea syndrome and malignant tumors. The prevalence of obesity is rising dramatically all over the world. According to Report on Nutrition and Chronic Diseases among Chinese Residents in 2020, 34.3% of adult Chinese are overweight and 16.4% are obese.
Recently, numerous studies have shown that obesity has significant adverse effects upon reproductive function. For example, obesity is associated with increased risk of prostate cancer, which reduces semen quality, and it also increases the risk of polycystic ovary syndrome, which eventually results in reproductive hormone disorders and subfertility[1-2]. The reasons why obesity can cause subfertility are summarized as follows: first, obesity can cause decreases in sperm concentration and motility[3-4]. A large number of data have shown that body mass index (BMI) is closely associated with semen quality, because increased BMI may reduce ejaculate volume, and increase the risk of azoospermia and oligospermia in obese men[4-5]. Second, obesity may cause reproductive endocrine disorders. The sex hormone imbalance and dysfunction of the hypothalamic-pituitary-gonadal axis in obese patients may eventually impair their reproductive function. For example, obesity will impact the secretion of reproductive hormones including testosterone (T), estrogen (E2), luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which then affects the function of testicular leydig cells and supporting cells, inhibits the release of sex hormones and interferes with sperm formation and maturation[5-6]. Among these sex hormones, T is an important male hormone, as it has physiological functions such as maintaining spermatogenesis, stimulating the development of reproductive organs and promoting nucleic acid synthesis[7]. Studies have found that obese male patients have significantly lower levels of T, LH, sex hormone-binding globulin (SHBG) and insulin-like growth factor 1 (IGF-1), and significantly higher level of E2 than non-obese males. Chavarro et al.[8] found that BMI has a negative correlation with total testosterone and SHBG levels, and a significantly positive correlation with E2 level. Too high E2 level can reduce spermatogenic cells, especially accelerate apoptosis of spermatogonia and spermatoblasts, and eventually affect the formation and development of sperm. In addition, high level of E2 inhibits the secretion of LH and FSH via its negative feedback to the hypothalamic-pituitary-gonadal axis[9]. Therefore, the initiation of spermatogenesis can only proceed when the reproductive hormones are at relatively steady levels. On the contrary, reproductive hormone disorders will cause abnormal spermatogenesis via a negative feedback manner.
Polygonatum odoratum polysaccharide (POP), a natural plant-based polysaccharide, is a highly active substance extracted from the root of medicinal and edible plant P. odoratum. Its main components are rhamnose, mannose, xylose and arabinose[10]. Polysaccharide has been proven to possess anti-aging, antibacterial, antitumor, anti-immunity, antioxidant, and blood lipid-regulating activities[11-14]. Previous studies on POP mostly focused on its extraction, biological activity and effects in regulating blood lipid metabolism[12, 15]. Our early findings also showed that POP can improve intestinal microbial community structure, inhibit the growth of harmful bacteria, and prevent obesity in rats fed a high-fat diet[16]. Unexpectedly, we found that POP can also distinctly increase rat testicular weight, which suggests that it can be used to improve reproductive abnormality caused by obesity. In addition, a lot of studies have shown that natural active polysaccharides can protect reproductive function. For example, Lycium chinense polysaccharide can reduce the reproductive cell apoptosis of female rats caused by 2,4-D, improve the serum T level and organ coefficient, sperm count and motility of male rats, and thus improve their reproductive performance[17-18]. Laminaria japonica polysaccharides have an obvious protective effect on reproductive function in rats with ionizing radiation-induced injury and in lead-poisoned rats[19-20]. Astragalus membranaceus polysaccharides can increase the level of reproductive hormones in hens during the late laying period, improve their ovarian function and egg-laying performance[21]. Therefore, this study was conducted to further explore the effects of POP on the level of reproductive hormones in male Sprague-Dawley (SD) rats fed a high-fat diet, so as to provide a theoretical basis for the rational utilization of POP and for the improvement of reproductive function of obese patients.
Materials and Methods
Materials, reagents and animals
Polygonatum odoratum polysaccharide (POP) was isolated and prepared from Polygonatum odoratum in our laboratory. It was extracted in alkali solution and precipitated in ethanol, purified through Sevag procedure. Its main components in molar ratio were n (rhamnose):n (mannose):n (xylose):n(arabose)=31.78:31.89:11.11:1.00[10]. The total sugar content in the sample was about 61%, measured by phenol-sulfuric acid assay.
Serum testosterone (T), estrogen (E2), follicle-stimulating hormone (FSH), cortisol (C), luteinizing hormone (LH), sex hormone binding globulin (SHBG) and IGF-1 kits were all purchased from Tianjin Jiuding Medical Bioengineering Co., Ltd.
Thirty specific pathogen-free (SPF), healthy, male SD rats (body weight 250-280 g) were purchased from Hunan Slac Laboratory Animal Co., Ltd.[SCXK(Xiang)2016-0002]. The general feed was bought from Hunan Slac Jingda Laboratory Animal Co., Ltd. (10% kcal fat, 3.6 kcal/g), and the high-fat feed from Beijing Botai Hongda Biotechnology Co., LTD. (34% kcal fat, 4.6 kcal/g)[16].
Methods
Experimental design and animal treatments
By referring to our early report[16], 30 rats were fed adaptive diet for one week before they randomly divided into two groups: 10 in normal control group (Group NC, n=10) and 20 in experimental group. The rats in Group NC were fed an ordinary diet, and those in experimental group were fed a high-fat diet. Eight weeks later, the rats in experimental group, with a body weight 20% greater than that of Group NC were considered as obese rats, which were further divided into two groups: 10 in Group HF (n=10), 10 in Group HF+POP (n=10), and fed a high fat diet for another six weeks, while the rats in group NC were still fed an ordinary diet. During this period, 400 mg/(kg·d) POP was as given by gavage feeding to each rat in Group HF+POP once every day at 9:00 am, while the same volume of distilled water was given in Group NC and Group HF.
Behavioral observations
Rat behaviors including morphological changes, such as body size, fur color and mental state, and abnormal behaviors, such as resistance to touch, sounds, startle, escape and slow response in each group were all observed and recorded once every other day.
Sample collection
At the end of the experiment, all the rats were fasted for 12 h before death. After being anesthetized with ether, their abdomen was disinfected with 75% ethanol, blood was sampled from the abdominal aorta, transferred to blood collection tubes containing heparin sodium, allowed to stand at room temperature for 30 min, centrifuged at 4 ℃ and 3 500r/min for15 min to separate plasma, which was then saved at -80 ℃ before analysis. After the blood samples were collected, the rats were immediately dissected, and their epididymis and testis were sampled, and cleaned with cold normal saline, wiped with absorbent paper and weighed.
Calculation of the relative weights of testis and epididymis
Each rat was weighed once a week, and the data had been reported previously[16]. Their testis and epididymis were accurately weighed immediately after dissection. Their relative weights were calculated according to Equation 1-1 as follows[22].
Relative weight (%)=Organ weight (g) / Body weight (g)×100(1)
Determination of reproductive hormone indices
The levels of plasma T, E2, FSH, C and LH were measured by radioimmunoassay, and the levels of SHBG and IGF-1 were detected by enzyme-linked immunosorbent assay (ELISA), respectively, following kit instructions.
Data analysis
The experimental data were statistically processed by SPSS 21.0 statistical software, and expressed as Mean±SEM, and analyzed using one-way ANOVA and Tukeys test at P<0.05.
Results and Analysis
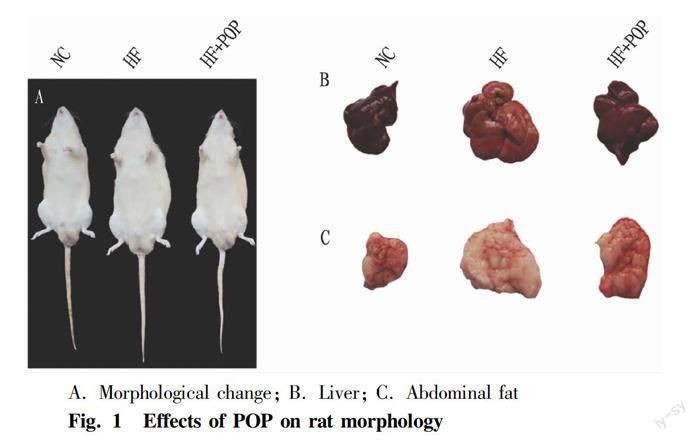
Effects of POP on morphological changes in obese rats
The rats in Group HF became more and more obese during the experimental period (Fig.1A), and exhibited some behavioral and anatomical abnormalities. For instance, they looked tired and moved less. Their livers turned grayish yellow (Fig. 1B), and abdominal fat mass obviously increased (Fig. 1C). However, all the abnormalities returned towards normal after being administered with POP for six weeks.
Effects of POP on epididymis and testis relative weights in obese rats
The results showed that the relative weights of epididymis and testis in Group HF were 33.05% and 40.6% lower than those in Group NC, proving that the high-fat diet significantly reduced the two indices of rats (P<0.01, Fig. 2). However, after the rats were given POP via gastric gavage for six weeks, the relative weights of their epididymis and testis were significantly increased, 15.46% and 27.48% higher than those in Group HP (P<0.01, Fig.2), indicating that POP can significantly improve the relative weights of epididymis and testis of rats fed a high-fat diet, so that the abnormalities in these organs can return towards normal.
Effects of POP on plasma LH, FSH, T, E2 and C levels in obese rats
Compared with Group NC, the high-fat diet decreased the plasma LH, FSH, and T levels in rats (Fig. 3A-C, P<0.01) very significantly, while showed no significant effect on plasm C level (Fig. 3E, P>0.05), and increased the plasm E2 level very significantly (Fig. 3D, P<0.01). However, after six weeks of POP administration, the plasma E2 level in obese rats was very significantly reduced (Fig.3F P<0.01), and the LH, FSH and T levels were very significantly increased (Fig. 3A-C, P<0.01), by 18.77%, 22.60% and 40.86%, respectively. But POP administration showed no significant effect on plasma C level in obese rats. All these results suggested that POP could improve the disorders in plasma hormone secretion caused by high-fat diet.
Effects POP on plasma SHBG and IGF-1 in obese rats
Compared with Group NC, the high-fat diet significantly decreased the plasma SHBG and IGF-1 levels of rats (Fig. 4, P<0.01). However, six weeks of POP administration significantly improved the plasma SHBG and IGF-1 levels of rats fed a high-fat diet (Fig. 4, P<0.05).
Conclusion and Discussion
El Salam have reported in 2018 that obesity is an enemy of male fertility[3], and numerous studies have proven that obesity caused by high-fat diets increases the risk of prostate cancer in men, resulting in decreased semen parameters and quality, reproductive endocrine metabolism disorders, spermatogenesis disorders, etc.[5,23-24], and reproductive hormones and sperm are secreted by testis. Obesity impacts negatively on male fertility by changing the levels of reproductive hormones. For example, high-fat diet-induced obesity will reduce plasma LH and FSH levels, inhibit LH and FSH binding to specific receptors in testis, resulting in a decrease in testicular T level, and thus leading to the apoptosis of spermatogenic cells via mitochondrial pathways[25]. In addition, abnormal lipid metabolism can inhibit the synthesis of SHBG and IGF-1 in plasma. And SHBG is responsible for regulating T activity in plasma, and T acts locally on the spermatogenic epithelium to regulate spermatogenesis. Obese patients have higher levels of aromatase, which can quickly convert T to E2, resulting in a decrease in total T level[26].
Obesity caused by high-fat diets is one of the important causes of subfertility. The level of sex hormones is an important measurement for reproductive ability. Among them, T, as an important androgen, has the physiological functions of maintaining spermatogenesis, stimulating the development of reproductive organs and promoting protein synthesis. Estrogen (E2) can regulate the spermatogenesis by regulating the secretion of hypothalamic gonadotropins. Follicle-stimulating hormone (FSH) and LH are glycoprotein hormones secreted by the pituitary gland, LH directly acts on receptors on the membrane of Leydig cells, to promote their proliferation, thereby stimulating the synthesis and secretion of T, which is needed for spermatogenesis. Follicle-stimulating hormone (FSH) mainly promotes the development of spermatogenic epithelium and spermatogenesis, which synergistically regulate reproductive function and promote sperm maturation[27]. Lower level of LH and FSH will inhibit T synthesis, resulting in a decrease in intratesticular T level[28]. Sex hormone-binding globulin (SHBG), as a carrier protein for T, regulates the activity of T. Insulin-like growth factor-1 (IGF-1) is mainly synthesized in liver and released into the bloodstream, and then regulates male sexual development and testicular endocrine function via the hypothalamic-pituitary-testicular axis[26].
Studies have proven that Laminaria japonica polysaccharides[29] can increase the relative weights of epididymis and testis in lead-poisoned rats, and increase the serum levels of T, FSH and LH. Lycium barbarum polysaccharides[30] can also increase serum T level, reduce serum E2 level and increase the relative weight of gonadal organs in semi-castrated rats. All these findings are basically consistent with the results of our study. This study also found that high-fat diet remarkably reduces the relative weights of testis and epididymis, the plasma levels of LH, E2, FSH, T, SHBG and IGF-1, and increases the plasma level of E2 of male rats, which suggests that high-fat diet has caused reproductive abnormalities in rats. However, the administration of POP increased the relative weights of epididymis and testis, the plasma levels of LH, E2, FSH, T, SHBG and IGF-1, and reduced the plasma E2 level, suggesting that POP promote testicular tissue regeneration, hormone secretion, and the regulation of hypothalamic-pituitary-gonadal axis to improve sperm quantity and quality, and spermatogenic function. However, the study of Zhang[31] reported that Schizandra sinensis polysaccharides at different dosages reduces the serum FSH and LH level and increases the T level in testicular tissue homogenate from the rats with cyclophosphamide induced spermatogenic disorder. The findings are inconsistent with the results of the present study, which may be due to the different animal models used in these two papers. Cortisol (C), a glucocorticoid hormone controlled by pituitary adrenocorticotropic hormones, can promote protein decomposition, and also has synergistic or antagonistic interactions with other hormones, such as insulin, growth hormones and testosterone[32]. But the plasma C level showed no obvious changes among the three treatments. Long-term high-fat diet can induce abnormal lipid metabolism and subfertility inn rats[33]. By investigating the effects of POP on reproductive hormones in obese rats fed a high-fat diet in the present study, we found that POP can help to improve the obesity-induced reproductive abnormalities, which provides a theoretical basis for applying POP in the treatment of infertility. And another important topic for future research to investigate is the effects of high-fat diet induced obesity on sperm morphology and motility in rats.
References
[1] JIAO WW, QIU WR, LU Y, et al. A review on the effects of obesity on male reproductive function[J]. World Journal of Integrated Traditional and Western Medicine, 2021, 16(3): 585-588.
[2] NIU YL, ZHAO JL. Research Progress on the Effect of Simple Obesity on Female Reproductive Function and Pregnancy Outcome[J]. Journal of Ningxia Medical University, 2022, 44(7): 750-754.
[3] MAA El SALAM. Obesity, an enemy of male fertility: a mini review[J]. Oman Med J, 33(1):3-6. 10.5001/omj.2018.02.
[4] CHAUDHURI GR, DAS A, KESH SB, et al. Obesity and male infertility: multifaceted reproductive disruption[J]. Middle East Fertility Society Journal, 2022, 27(1): 8.
[5] XIA DE, YANG X, WANG X, et al. Research progress on the effects of obesity on the reproductive system[J]. Journal of Jilin Medical College, 2017, 38(2): 135-137.
[6] MONTEAGUDO PT, FALCAO AA, VERRESCHI IT, et al. The imbalance of sex-hormones related to depressive symptoms in obese men[J]. The Aging Male, 2016, 19(1): 20-26.
[7] SHI L, NIU HZ, YAO XL, et al. Effects of Different Feeding Levels on Testis Development and the Expression of Steroidogenesis-Related Genes and Androgen Receptor (AR) in Sheep[J]. Acta Veterinaria Et Zootechnica Sinica, 2021, 52(5): 1317-1327.
[8] CHAVARRO JE, TOTH TL, WRIGHT DL, et al. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic[J]. Fertil Steril, 2010, 93(7): 2222-2231.
[9] LIU XC, LIU J. Influences of endocrine-disrupting chemicals on pituitary gonadotropins:a review[J]. Asian Journal of Ecotoxicology, 2022, 17(2): 1-19.
[10] CHEN Y, YIN L, ZHANG X, et al. Optimization of alkaline extraction and bioactivities of polysaccharides from rhizome of Polygonatum odoratum[J/OL]. Biomed Res Int, 2014, https:∥doi.org/10.1155/2014/504896.
[11] WANG Y, LIN YX, KANG JH, et al. Effects of Polygonatum odoratum polysaccharide on lipid metabolism in rats fed a high-fat diet[J]. Acta Nutrimenta Sinica, 2018, 40(4): 353-359.
[12] WTT, DU MY, ZHANG M. Research progress on extraction of polysaccharide from Polygonatum odoratum and its application in food[J]. Modern Agricultural Science and Technology, 2021(23): 176-179.
[13] LIU T, DENG BL, LI T, et al. Study on the network pharmacology of Chinese medicine containing polysaccharide with Polygonatum odoratum as an example[J]. World Science and Technology-Modernization of Traditional Chinese Medicine, 2022, 24(7): 2658-2668.
[14] ZHANG S, SHI Y, HUANG L, et al. Comparative transcriptomic analysis of rhizomes, stems, and leaves of Polygonatum odoratum (Mill.) Druce reveals candidate genes associated with polysaccharide synthesis[J]. Gene, 2020(744): 144626.
[15] XU S, BI J, JIN, W, et al. Determination of polysaccharides composition in Polygonatum sibiricum and Polygonatum odoratum by HPLC-FLD with pre-column derivatization[J]. Heliyon, 2022, 8(5): e09363.
[16] WANG Y, FEI Y, LIU L, et al. Polygonatum odoratum polysaccharides modulate gut microbiota and mitigate experimentally induced obesity in rats[J]. Int J Mol Sci, 2018, 19(11).
[17] WU L, WANG J, HUANG W, et al. Plant-microbe rhizosphere interactions mediated by pehmannia glutinosa root exudates under consecutive monoculture[J]. Sci Rep, 2015(5): 15871.
[18] HAAS BJ, PAPANICOLAOU A, YASSOUR M, et al. De novo transcript sequence reconstruction from RNA-seq using the trinity platform for reference generation and analysis[J]. Nat Protoc, 2013, 8(8): 1494-512.
[19] CHEN J, DUAN Y, HU Y, et al. Transcriptome analysis of atemoya pericarp elucidates the role of polysaccharide metabolism in fruit ripening and cracking after harvest[J]. BMC Plant Biol, 2019, 19(1): 219.
[20] KUNDAJE A, MEULEMAN W. Automated sequence-based annotation and interpretation of the human genome[J]. Nat Genet, 2022, 54(7): 916-917.
[21] GUAN LH, ZHANG LY, LIU HB, et al. The Effects of astragalus polysaccharide on production performance, reproductive hormones and blood physiological and biochemical indexes of laying hens[J]. Journal of the Chinese Cereals and Oils Association, 2015, 30(7): 70-75.
[22] LIU J, LUO Q, YANG ML, et al. effect of Laminaria japonica polysaccharides on reproductive function of male rats with chronic ionizing radiation damage[J]. Food Science, 2010, 31(7): 293-296.
[23] MO LF, YANG JY, LIU J, et al. Research progress of modifiable risk factors affecting semen quality in men[J]. Popular Science & Technology, 2021, 23(261): 83-86.
[24] KATIB A. Mechanisms linking obesity to male infertility[J]. Cent European J Urol, 2015, 68(1): 79-85.
[25] CUI L. Effects of trophic obesity combined with lipid metabolism abnormalities on reproductive function in male rats[D]. Jinan: Shandong University, 2017.
[26] JIA YF. Effect and mechanism of high-fat diet-induced obesity on hypothalamic-pituitary-testicular gonadal axis in male SD rats[D]. Beijing: Peking Union Medical College, 2017.
[27] ZHANG DY. Measurement of serum gonadotropin and sex hormone levels in infertile male patients by radioimmunoassay[J]. Jiangxi Journal of Medical Laboratory, 2000(3): 190-183.
[28] FUI MN, DUPUIS P, GROSSMANN M. Lowered testosterone in male obesity: mechanisms, morbidity and management[J]. Asian J Androl, 2014, 16(2): 223-231.
[29] WANG YY, FU ZH, MAO XH, et al. Effects of alginate on reproductive system of wistar rats with lead poisoning[J]. Journal of Qingdao University (Natural Science Edition), 2012, 25(1): 47-50.
[30] LUO Q, HUANG XL, LI ZN, et al. Effect of Lycium barbarum polysaccharides on sexual function and reproductive function of male rats[J]. Acta Nutrimenta Sinica, 2006, 28(1): 62-70.
[31] ZHANG Y, SHEN N, QI L, et al. Therapeutic effect of Schisandra polysaccharides on rats with cyclophosphamide spermatogenesis disorder and its effect on reproductive hormones[J]. Chinese Journal of Integrative Medicine, 2013, 33(3): 4.
[32] ZENG QM. Effects of exercise on sex hormone levels in male SD rats with diet-induced obesity[D]. Wuhan: Wuhan Sports University, 2009.
[33] LI NC, LING L, LI XY, et al. Protective function of aerobatic exercise combined with clear-fat-herbal on high fat diet-induced reproductive function suppression in rats[J]. Journal of Clinical Medicine in Practice, 2015, 19(7): 16-20.
- 农业生物技术(英文版)的其它文章
- Improving the Heat Resistance of β-1,4 Glucanase by Introducing Disulfide Bonds
- Comparative Study on Biological and Commercial Characteristics of Mul-tiple Varieties of Broccoli (Brassica oleracea L. var. italica Plenck) and Vegetable Soybean (Glycine max (L.) Merr.)
- Effects of Bamboo Charcoal-based Biochar on Soil Enzyme Activity and Microbial Community Structure
- Summary of Short-vine Watermelon Breeding Practice
- Effects of Combined Application of Biochar-based Organic Fertilizer and Reduced Nitrogen Fertilizer on Soil Enzyme Activity and Yield of Purple Cabbage (Brassica oleracea var. capita rubra)
- Effects of Microelement Fertilizers on Main Economic Characters and Yield of Peanut

